Open Journal of Urology
Vol.05 No.09(2015), Article ID:59376,5 pages
10.4236/oju.2015.59024
Semen Abnormality Patterns and Parameters in Male Partners of Infertile Couples in Dakar (Senegal)
Mama Sy Diallo1*, Abdoulaye Séga Diallo1, Pyrrhus Fotso1, Yoro Diallo2, Babacar Diao3, Oumar Faye1
1Laboratory of Cytogenetic and Reproductive Biology, University Teaching Hospital Aristide Le Dantec, Dakar, Senegal
2Department of Urology, Faculty of Health Sciences, University of Thies, Thies, Senegal
3Urology and Andrology Unit, University Teaching Hospital Aristide Le Dantec, Dakar, Senegal
Email: *mamatasy@yahoo.fr
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 July 2015; accepted 1 September 2015; published 4 September 2015
ABSTRACT
Introduction: Inter individual variation for semen on analysis is well known. Therefore, Semen profile is not the same in individuals from different geographic locations, environments, ways of life, and populations. It is important for a laboratory to have an idea of the semen profile of its population, especially for those who present with infertility. The aim of this study was to draw the pattern of semen abnormalities in male partner of infertile couple in Dakar, Senegal. Materials and Methods: A retrospective study of all the semen samples of male partners of infertile couples submitted for analysis in the laboratory of Cytogenetic and Reproductive Biology of Dakar at the University Teaching hospital from January 2013 to June 2014. Results: A total of 262 male semen analyses were reported. Analysis was performed according to WHO, 2010 manual for examination of human semen. Our study reveals that 80.9% of men in infertile couples present an abnormality in sperm pattern. The main one is oligo-astheno-terato-necrozoospermia (20.2%), followed by azoospermia, (14.1%), astheno-necrospermia (10.3%) and astheno-terato-necro-zoospermia (10.3%). Leucocytospermia was found in 57.8%. Teratozoospermia (80.9%) was the most associated abnormality, followed by necrozoospermia (76.2%). Low sperm count under 5 million per ml concerned 27.7% of the patients, and 11.2% patients had low ejaculate volume. Nearly 42.1% of the male partners are potential candidates for Assisted Reproductive Technology for male infertility. Conclusion: The high rate of semen abnormality in patients of infertile couples and consequently the high rate of potential candidates to Assisted Reproductive Technology for male infertility are important reasons for taking into account rigorously the male subject if we want to improve fertility rate in our context. The pattern of the specific abnormalities found is precious clues to guide management of these patients.
Keywords:
Semen Parameters, Male Infertility, ART

1. Introduction
Semen analysis is the primary assessment tool to evaluate potential male infertility. It is still a fundamental step for exploring testicular function and gives an orientation to the clinician and helps him establish a diagnostic. Moreover, after a treatment, it can be one of the monitoring tools to show if there is improvement or not for example after a varicocelectomy. Male infertility among couples trying to conceive is nearly 50% [1] . According to the precedent studies in our center, the prevalence is estimated to 66%, with nearly 33% of isolated male infertility and 33% of mix infertility with female implication [2] .
A good interpretation of the different sperm parameters correlated with medical history of the patient and his clinical file can be very informative and give the good clues for an adapted treatment. It gives orientation for further semen exploration like a migration survey test in Low sperm count value. So the aim of our study was to determine the pattern of abnormalities in the semen of male partners of couples dealing with infertility. The other goal of our study was to estimate the part of patients that should benefit from an Assisted Reproductive Technology (ART) procedure for male indication.
2. Materials and Methods
This is a retrospective study that included 262 patients who did semen analysis in the laboratory of Reproductive Biology and Genetics of the Dakar University Teaching Hospital, Aristide Le Dantec between January 2013 and June 2014. The laboratory is dedicated to analysis, research and teaching in the field of Cytology, Histology, Reproductive Biology and Human Genetics in a level 1 Hospital and treat about 6000 samples a year. All patients selected were married, followed for infertility, and were trying to conceive for at least one year. We did not consider results from patients who were single, married less than one year and those who did the exam just to know about their fertility before they get married.
2.1. Methods
Our laboratory is a place for the traineeship of students from the Biological Doctoral school of Dakar Universitary Medecine School. Our patients did give their consent for this work. We obtained the university’s ethics committee agreement.
Sample collection was done following abstinence from ejaculation for 3 days, and mainly by masturbation in a room dedicated, in the laboratory. The semen analysis was performed in accordance with the method of semen analysis described in the last WHO guidelines [3] to determine vitality we used eosin-nigrosin staining [3] . The results were interpreted using the reference values of the same guide. Those values were the followings:
・ Semen volume: 1.5 ml or more;
・ pH: 7.2 or more;
・ Sperm concentration: 15 million spermatozoa per ml or more;
・ Leucocytospermia: more than one million of leucocytes per ml;
・ Progressive motility: 32% or more motile;
・ Total motility (percentage of progressive motility and non progressive motility): 40% or more motile;
・ Vitality 58% or more live spermatozoa;
・ Sperm morphology (percentage of normal forms): 32% or more in David scale.
2.2. Statistical Analysis
The data was analyzed using the SPSS 14.0 statistical software for Windows and graphical representations were obtained after exportation of the different data into excel version 2010. Some data were compared for frequencies, means, with Pearson Chi-square (X2) with levels of significance set to less than 0.05 (p < 0.05).
3. Results
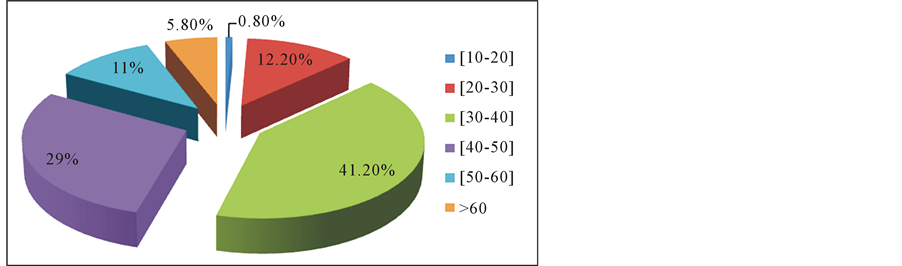
Two hundred and sixty two patients met the criteria. The mean age of the patients was 38.0 ± 7.1 years .The repartition of the patients by group age can be visualized by Figure 1. The mean duration of infertility was 5.3 years (ranges 1 - 5.7). Primary infertility represented more than half of the patients in this sample (57.4%). In the medical history, we found that 113 patients presented in the past a pathology that could be related to infertility. Among them 48 of them were treated for an infection of the genital tract and 46 of them were followed for varicocele. The other patients (n = 149) came for analysis for the first time and the etiology of their infertility was under establishment (Table 1).
The mean length of abstinence before the exam was 5.8 days (ranges 2 - 7) and 17.6% collected the semen by coïtus interruptus.
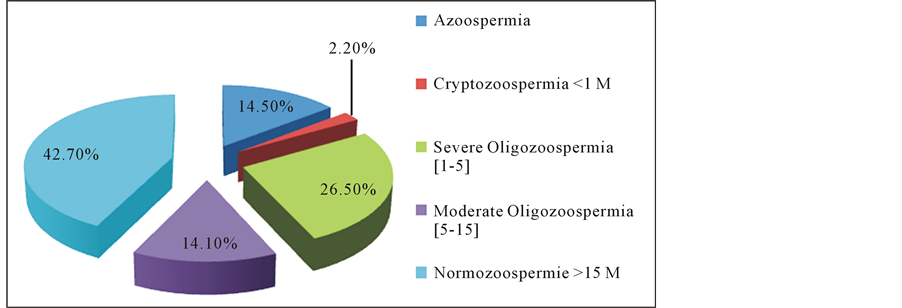
The analysis of the different semen parameters showed that concerning the volume, 11.9% of patients presented with hypospermia. The prevalence of azoospermia was estimated to 14.5% in our series. We found oligozoospermia in more than one third of the patients and the sperm count was under 5 million for almost 27.7% of the patients (Figure 2). Seventy-six point two patients (76.2%) presented with necrozoospermia (Table 2) and 74.4% with low motility (n = 194). We found leucocytospermia in 57.8% of the samples. Only 19.1% of the spermocytogramms presented normal forms after evaluation of the morphology according to the David criteria (WHO, 2010).
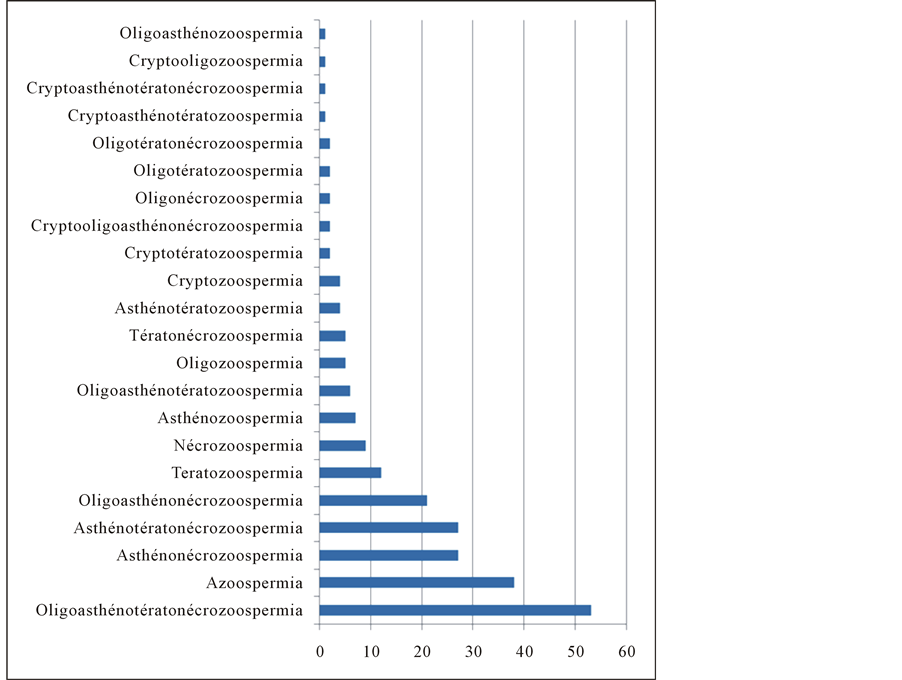
The most common profile of abnormality, in our study is oligo-astheno-terato-necrozoospermia (n = 53), followed by azoospermia (n = 38) then by astheno-necrozoospermia (n = 27) and astheno-terato-necrozoospermia
Table 1. Main pathologies related to infertility found in the past medical history.
Table 2. Patients distribution according to semen vitality.
Figure 1. Patient repartition by age group.
Figure 2. Patient distribution according semen concentration.
Figure 3. Patterns of the semen abnormalities (N = 217).
(n = 27). Teratozoospermia (80, 9%) was the most associated abnormality, followed by necrozoospermia (76.2%) (Figure 3).
4. Discussion
The mean age of our patients was 38.7 years (±7.1) and seemed to increase a little if we refer to the past studies in our laboratory, with Diao study a mean age of 33.2 (±4.1) (p < 0.05) [2] . This finding is similar to the findings reported by Prisant [4] (32.7 years) in France and also in the Urology center of Grand Yoff in Dakar the past years [5] . In West Africa, we found almost the same data in Burkina Faso [6] . But In Nigeria with Ikéchebelu [7] , we found that a younger group was mainly involved and they were 25 to 32 years old. That was also the case in David series [8] with a mean age of 29 years old.
The length of abstinence must be taken into account when analyzing the sperm. According to the findings by several authors, that length is responsible for many variations, in the same subject [9] [10] . When the length is too long, semen quality can decline. In our study the length of abstinence was 8.8 days and is similar to the data in Prisant’ publication [4] . This length is between the minimal and the maximal length recommended by WHO in the last laboratory manual, even if the best is to respect about 3 days [3] .
Actually, Hamamah [10] reports that volume and sperm density increase by 0.4 ml and 13 million per ml respectively per day of abstinence. But at the same time, there is a decreasing of motility and of the number .We proposed coitus interruptus only when patients failed with masturbation.
The medical history of our patients revealed that 43.1% of them were followed or had a pathology that could explain abnormal semen.
Marcelli [11] assesses in his studies that an etiology could be found in nearly 40% of infertility in general. The main reported pathology when patients were already followed up in our series was varicocele (42.5%) and it is also the first pathology in men infertility with a prevalence of about 40% [12] .
This study indicates that abnormal semen quality is implicated in about (80.9%) of male partners of couples seeking remedy for their inability to conceive. We found a percentage of 84.3% in the series of Niang in Senegal [5] . According to our past data, in our center, this percentage is increasing from 62.8% to 80.9% (p < 0.05) [2] . This relative increasing of abnormalities in the semen, despite lower reference values introduced by WHO in 2010 could be related to the decline of sperm, described in literature in the past years [3] . This was illustrated by several studies [13] -[15] .
In this study, the pattern of abnormalities show that the prevalence of oligoasthenoteratonecrozoospermia is the highest (20.2%) followed by azoospermia (14.5%) and astheno-necrozoospermia (10.3%) and astheno- necrozoospermia (10.3%). But, according to Pontonnier [16] , oligoasthenoteratozoospermia, is the first abnormality generally found in the general population) and especially in varicocele. The importance of patients followed for varicocele in our series could explain this finding and also the fact that we found that 37.2% of our patients had in the past an infection of the genital tract. Moreover, necrozoospermia and leucocytospermia are well known as to be stigmatisms of infection in the sperm [10] . In our series we found that necrozoospermia was the most associated abnormality and concerned 76.6% of patients. Indeed, the prevalence of leucocytospermia was 57.8%. This finding is important to guide the clinician and systematize sperm culture in our infertile patients during their medical checkup for infertility.
Male genital tract infection is an important etiological factor leading to deterioration of spermatogenesis, impairment of sperm function and/ or obstruction of seminal tract [17] .
Azoospermia is also very important in our findings with a rate of 14.5%. This is little more than the double, compared to the data in Owolabi study (6.2%) [17] . This can be the result of endocrinal, or genetic disorder but is also a consequence after genital tract infection and varicocele [10] . An abnormality in ejaculation has to be researched, especially in our series where the prevalence of hypospermia is 11.9%. Before concluding definitively to an azoospermia, anejaculation, incomplete ejaculation and retrograde ejaculation have to be identified.
Severe oligozoospermia (under 5 million per ml) concerned almost quarter of our patients (27.7%).
It seems that if we gather, the data concerning azoospermia (14.4%), and low sperm density (27.7%), at least 42.1% of the patients should be potential candidates to an Assisted Reproductive Procedure for male infertility. This number could increase after sperm survey and migration test within the patients that presented with moderate oligozoospermia or severe teratozoospermia in our series.
This study indicates that genetic testing such as karyotyping, research of micro deletion on Y chromosome are justified in our context. Sperm culture is important and survey and migration tests should be proposed to patients.
5. Conclusion
Analysis of semen parameters in our center, according to WHO 2010 in our laboratory reveals that 80.9% of men in infertile couple present an abnormality in sperm. The main one is oligo-astheno-terato-necrozoospermia (20.2%), followed by azoospermia (14.1%), astheno-necrozoospermia (10.3%) and asthenoteratonecrozoospermia (10.3%). Leucocytospermia was found in 57.8% of the cases. Low sperm count under 5 million per ml concerned 27.7% of the patients. Nearly 42.1% of the male partners are potential candidates for ART for male infertility. More than ever, a multidisciplinary team must be set up to be able to care for our patients, who present a particular profile, need further exploration and access to Assisted Reproductive Technology.
Conflict of Interest
There is no conflict of interest.
Cite this paper
Mama SyDiallo,Abdoulaye SégaDiallo,PyrrhusFotso,BabacarDiao,YoroDiallo,OumarFaye, (2015) Semen Abnormality Patterns and Parameters in Male Partners of Infertile Couples in Dakar (Senegal). Open Journal of Urology,05,155-160. doi: 10.4236/oju.2015.59024
References
- 1. Coccuzza, M., Cocuzza, M.A., Bragais, F.M.P. and Agarwal, A. (2008) The Role of Varicocele Repair in the New Era of Assisted Reproductive Technology. Clinics, 63, 395-404.
http://dx.doi.org/10.1590/s1807-59322008000300018 - 2. Diao, B., Faye, O. and Fal, P.A. (2006) Profil Spermiologique de l’époux dans les couples infertiles en milieu négro-africain au Sénégal. Andrologie, 16, 247-252. http://dx.doi.org/10.1007/BF03034863
- 3. WHO (2010) Laboratory Manual for the Examination and Processing of Human Semem. WHO Press, 5, 113.
- 4. Prisant, N., Cohen, B.P. and Amar, E. (2011) Tératozoospermie: Mythe ou réalité? Etude d’une cohorte de 101 404 examens de sperme. Gynécologie Obstétrique & Fertilité, 39, 136-140.
http://dx.doi.org/10.1016/j.gyobfe.2010.11.005 - 5. Niang, L., Ndoye, M. and Labou, I. (2009) Profil épidémiologique et clinique de l’infertilité masculine à l’hôpital général de Grand-Yoff, Sénégal: A propos de 492 cas. Andrologie, 19, 103-107.
- 6. Diafouka, F., Gbassi, K.G. and Djanhan, Y. (2006) Intérêt du spermogramme et du dosage séminal de l’orosomucoïde dans l’exploration de l’hypofertilité masculine. Journal des Sciences Pharmaceutiques et Biologiques, 7, 50-56.
- 7. Ikechebelu, J.I., Adinma, J.I., Orie, E.F. and Ikegwuonu, S.O. (2003) High Prevalence of Male Infertility in South-Eastern Nigeria. Journal of Obstetrics & Gynaecology, 23, 657-659.
http://dx.doi.org/10.1080/01443610310001604475 - 8. David, G., Bisson, J.P., Czyglik, F., Jouannet, P. and Gernignon, C. (1975) Anomalies morphologiques du spermatozoïde humain. Journal de Gynécologie Obstétrique et Biologie de la Reproduction, S1, 17-36.
- 9. Devaux, A. (2010) Valeurs limites du spermogramme: Comment les interpréter ? Quelle conduite adopter? Gynéco-logie Obstétrique & Fertilité, 38, 16-17.
http://dx.doi.org/10.1016/S1297-9589(10)70008-4 - 10. Hammamah, S. and Barthelemy, C. (1997) Spermogramme et tests de fécondance: Intérêt Et Limites. Journées de Techniques Avancées, Guadeloupe.
- 11. Marcelli, F., Robina, G. and Rigota, J.M. (2009) Prise en charge de l’infertilité masculine. Progrès en Urologie, 19, 260-264. http://dx.doi.org/10.1016/j.purol.2008.10.027
- 12. Muratorio, C., Meunier, M. and Sonigo, C. (2013) Varicocèle et infertilité: Où en sommes-nous en? Gynécologie Obstétrique & Fertilité, 41, 660-666. http://dx.doi.org/10.1016/j.gyobfe.2013.09.012
- 13. Auger, J., Kunstmann, J.M., Czyglik, F. and Jouannet, P. (1995) Decline in Semen Quality among Fertile Men in Paris during the Past 20 Years. The New England Journal of Medicine, 332, 281-285.
- 14. Chakroun, F.N., Abid, N., Rebai, A., Sellami, A., Ben, A.B., Guermazi, M., et al. (2009) Semen Quality Decline among Men in Infertile Relationships: Experience over 12 Years in the South of Tunisia. Journal of Andrology, 30.
- 15. Geoffroy, S.C., Loundou, A.D., Romain, F., Achard, V., Courbiere, B., et al. (2012) Decline of Semen Quality among 10932 Males Consulting for Couple Infertility over a 20-Year Period in Marseille, France. Asian Journal of Andrology, 14, 584-590. http://dx.doi.org/10.1038/aja.2011.173
- 16. Pontonnier, F. and Bujan, L. (1993) Comment Reconnaître et classer une infécondité masculine. Rev Prat, 43, 941-947.
- 17. Owolabi, A.T., Fasubaa, O.B. and Ogunniyi, S.O. (2013) Semen Quality of Male Partners of Infertile Couples in Ile-Ife, Nigeria. Nigerian Journal of Clinical Practice, 16, 37-40.
NOTES

*Corresponding author.