Soft Nanoscience Letters
Vol.3 No.4(2013), Article ID:37809,4 pages DOI:10.4236/snl.2013.34015
A Crystalline Material Made from Oxidation of Vanadium Nitride via Foam Route
![]()
1Plasma Technology Research Center, National Fusion Research Institute, Daejeon, South Korea; 2Department of Bio-Electronic Physics, Kwangwoon University, Seoul, South Korea.
Email: *ychong@nfri.re.kr
Copyright © 2013 Yong Cheol Hong, Han Sup Uhm. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 18th, 2013; revised August 18th, 2013; accepted August 25th, 2013
Keywords: Vanadium Nitride; Organic-Inorganic Composite; Foam
ABSTRACT
Facile method of synthesizing organic-inorganic solid foam in complex structure is presented. The synthesis method is based on the use of neutral surfactant (1-hexadecylamine, C16H33NH2) as structure directing agent and inorganic salt (vanadium nitride, VN) as precursor. The foam containing C16H33NH2 has been synthesized by evolution of oxygen gas produced spontaneously from hydrogen peroxide through a viscous VN gel. The foam from the precursor of 0.5 g VN nanopowder grows gradually, reaching about 0.3 liters 10 minutes after excess addition of H2O2 and stirring. The ultralight, crystalline material made via foam processing was observed by XRD, SEM, TEM, and FTIR.
1. Introduction
Inorganic-organic materials, due to their large, controllable pore sizes, high surface areas, and easy functionalization for applications in biology, chemistry, and material sciences, have been studied over the past few years in order to investigate their novel properties [1,2]. Generally, surfactants with cationic, anionic, and neutral charges and amphiphilic block copolymers have been utilized as structure directing agents. Most research in this field has been focused on oxides as a framework constituent. There have been several reports [3-5] relating to the synthesis of mesostructured metal oxides, such as zirconium, niobium, titanium, tantalum, vanadium, hafnium, and tungsten oxides. These mesostructured materials have been synthesized via surfactant template [1], polymer template [5], ligand-assisted [6], and nanoreplicated routes [3]. Since vanadium oxides, nitrides, and phosphates are extremely important in catalysis and in advanced materials applications, considerable efforts have been devoted to the synthesis of vanadium substituted zeolites and mesoporous silicate molecular sieves [7,8] as well as pure vanadium oxides and vanadophosphates. Despite considerable successes, it remains a challenge for chemists and material scientists to develop simple and efficient routes to advanced functional materials. Recently, a novel, simple method for the synthesis of transition-metal oxide foams was reported by Livage and co-workers [9]. This method produces a macroporous inorganic-organic solid, a composite of vanadium pentoxide (V2O5) and 1-hexadecylamine (C16H33NH2), with the latter intercalated in between the oxide layers. Here, we report on the synthesis of a crystalline material, so-called organic-inorganic solid foam, made from vanadium nitride precursor via foam route. The material was characterized using XRD, SEM, TEM, and FTIR.
2. Experimental
In previous reports, V2O5 was used as the vanadium source, and short-chain amines or long-chain ammonium salts were used as templates or intercalates [10-12]. Here, we used the vanadium nitride (VN) nanopowder synthesized from our research group as the vanadium source and 1-hexadecylamine (Aldrich) as the template. The VN nanopower with the average size of ~30 nm was synthesized by injecting VCl4 aerosol bubbled by argon gas into a microwave plasma reactor at atmospheric pressure. The synthesis method and procedure were reported in our previous article [13,14]. In a typical synthesis of the organic-inorganic solid foam, 2.8 g of C16H33NH2 was dissolved in 4.2 ml acetone and 0.5 g (or 7.7 × 10−3 mol) of VN nanopowder was added to the mixture. A pasty and viscous material was formed and then a solution of hydrogen peroxide (70 ml, 30%) was added as oxidant. Oxygen gas evolves in this step as H2O2 decomposes and voluminous greenish black foam creates spontaneously and exothermically, reaching about 0.5 liters 10 minutes after H2O2 addition, showing typical VN color. This means that VN does not oxidize yet, and C16H33NH2 between the VN layers does not intercalate. In order to oxidize VN after the completion of the black foam, the foam was crushed and stirred with the mixture solution below the foam before 10 ml of H2O2 in excess was added. Again, oxygen gas evolved and voluminous bright yellow foam was formed, meaning the production of vanadium oxide foam.
3. Results and Discussion
The volume of the foam using 0.2 g VN nanopowder increases gradually reaching about 0.3 liters 10 minutes after stirring, as shown in Figure 1(a). This material is very light, as only 0.2 g of VN is needed to generate the large volume of foam. Figure 1(b) shows typical XRD patterns of the resultant phases. The XRD pattern of the solid material is similar to that of the mesostructured vanadium oxide reported in Ref. 11 and exhibits a series
 (a)
(a)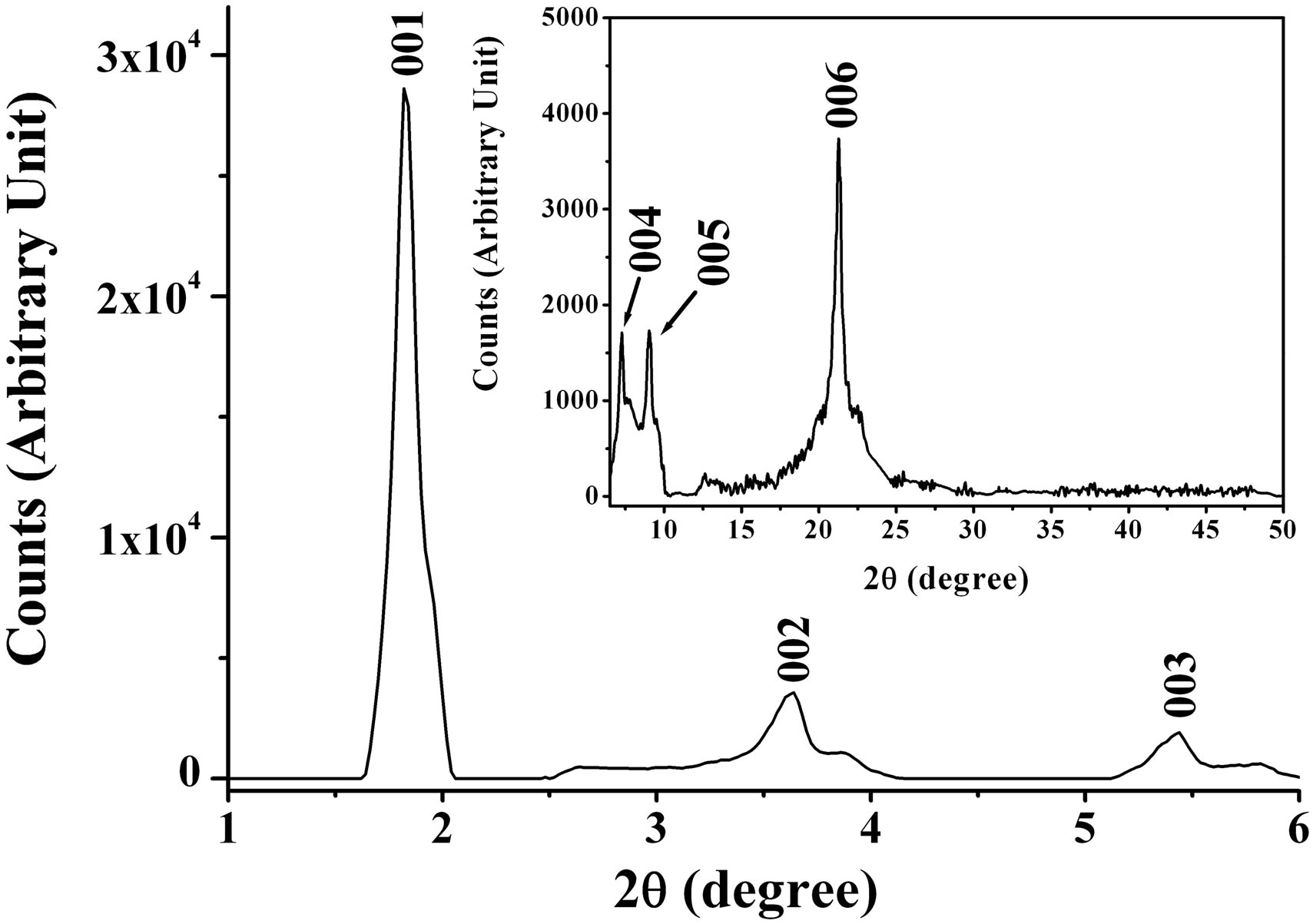 (b)
(b)
Figure 1. (a) Pictures before (left) and after (right) 0.5 g of VN nanopowder reacts with H2O2 in the presence of C16H33NH2 dissolved in acetone; (b) XRD pattern of the foam.
of 00l reflections with the most intense and lowest order reflection that we could observe occurred at a basal distance, d, 47.5 Å, compared to d = 33.4 Å for the marcroporous crystalline V2O5 foam [9]. The most intense reflection means the distance between the planes of solid material, explaining that the basal distance of solid material is not that of both VN and C16H33NH2. This basal distance does not correspond to that noted for C16H33NH2 molecules intercalated in vanadium oxide gels in which the alkyl chains are perpendicular to the oxide planes [9,15]. In the XRD pattern of the VN black foam mentioned above, the basal distance was observed to be 41.6 Å. Also, this is not consistent with that assigned for C16H33NH2 molecules intercalated perpendicularly to the vanadium nitride planes. Here, the increase of the basal distance can basically be described as an intercalation process of water into the layers of the vanadium oxide and nitride in which C16H33NH2 molecules were intercalated. The basal distance d increased by steps of about 2.8 Å corresponding to the van der Walls diameter of a water molecule. For example, the difference Δd of the basal distances between the vanadium oxide foam in this article and the V2O5 foam reported in Ref. [9] is about 14.1 Å, suggesting that five water layers have been intercalated.
The SEM and TEM images of the synthesized foam are showed in Figure 2. SEM image of the foam in Figure 2(a) exhibits that it is very porous and has an irregular surface morphology. The inset of Figure 2(a) is a magnified view of a large pore. Large pores reaching a few micrometers due to oxygen evolution are observed. The pattern of the foam structure resembles a lamellar structure found in the synthesis of carbon nitrides via a solvent-free route at low temperature [16]. Mechanical properties of the porous foam are very poor. The foam is brittle and can easily be crushed into a powder. In the synthesis of the vanadium oxide foam from VN gels, the amine is directly intercalated into vanadium oxide layers, forming van der Waals intercalation between organic molecules and the inorganic species during the foam progress. It is believed that C16H33NH2 molecules cocondense with anionic inorganic clusters in the gels to create lamellar structures, as shown in Figures 2(b) and (c).
Vanadium nitride reacts with hydrogen peroxide to give VN·nH2O or partially oxidized VNOx·nH2O gels that have a layered structure composed of negatively charged vanadium nitride or oxynitride. These gels can intercalate organic molecules such as protonated longchain alkylamines, forming a greenish black foam. In the foaming process presented in this work, therefore, both gelation and intercalation simultaneously occur when the VN nanopowder is added to the C16H33NH2 molecules and H2O2 solutions. The excess addition of H2O2 in the black foam causes the formation of bright yellow vanadium oxide foam, as shown in Figure 1(a), oxidizing VN and intercalating C16H33NH2 molecules into vanadium oxide layers.
The FTIR sectrum of the foam in Figure 3 shows strong absorption at 2956, 2919, 2850, and 1468 cm−1, which could be assigned respectively to the stretching and bending modes of the different C-H vibrations in the C16H33NH2. Two absorption bands at 3426 and 1637 cm−1, which could be attributed to the stretching and bending modes of O-H vibrations, respectively, confirms the intercalation of water molecules into vanadium oxide layers of the foam crystalline. The observation of O-H peaks in FTIR supports that five water layers have been intercalated into vanadium oxide layers, being consistent with XRD data. Absorption bands below 1000 cm−1 could be indexed to various vibrations of V-O and V=O. In particular, the band around 960 cm1 could be assigned to the V=O vibration in compared with crystalline V2O5, revealing the shift to lower energy by some additional
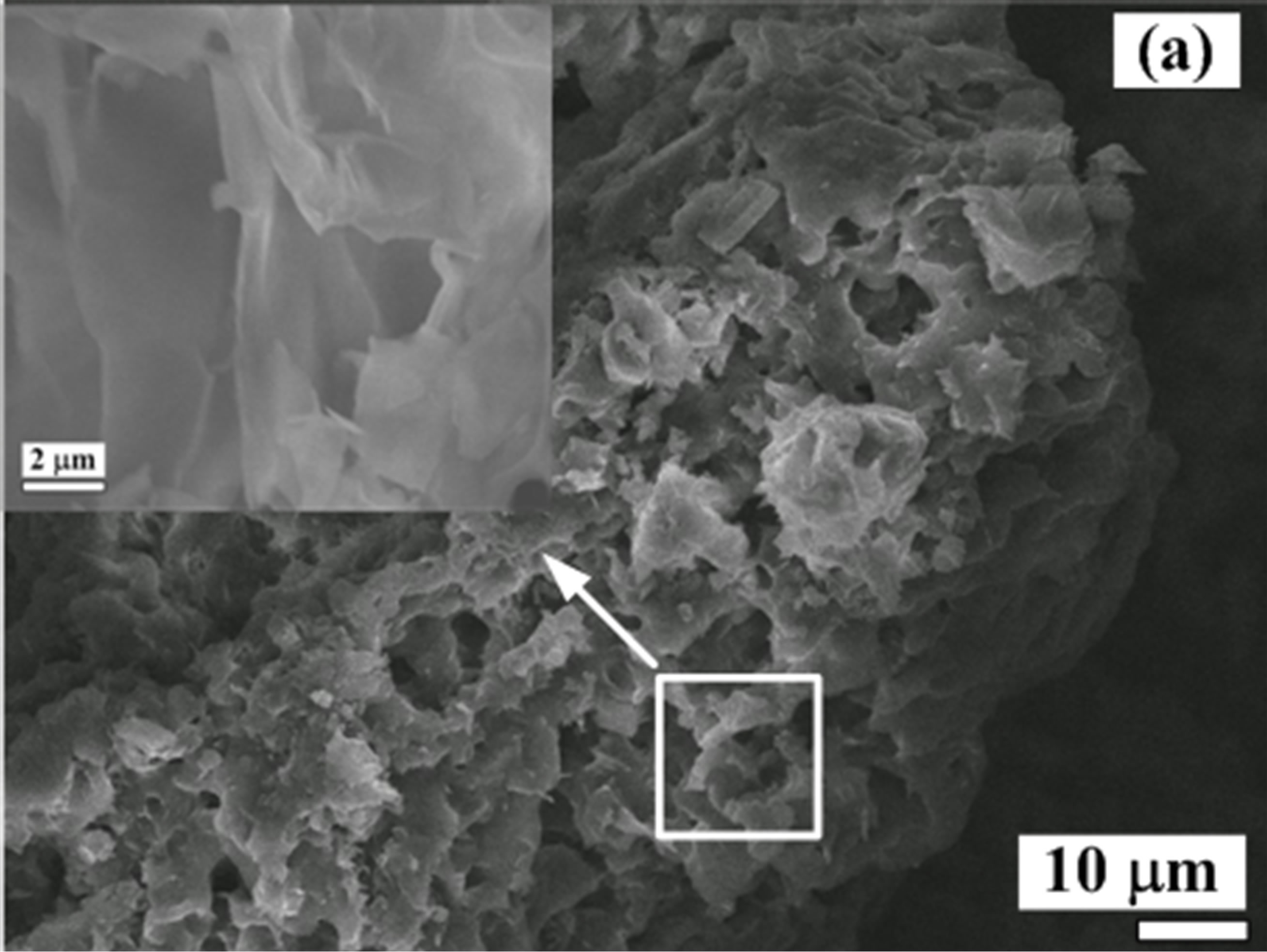
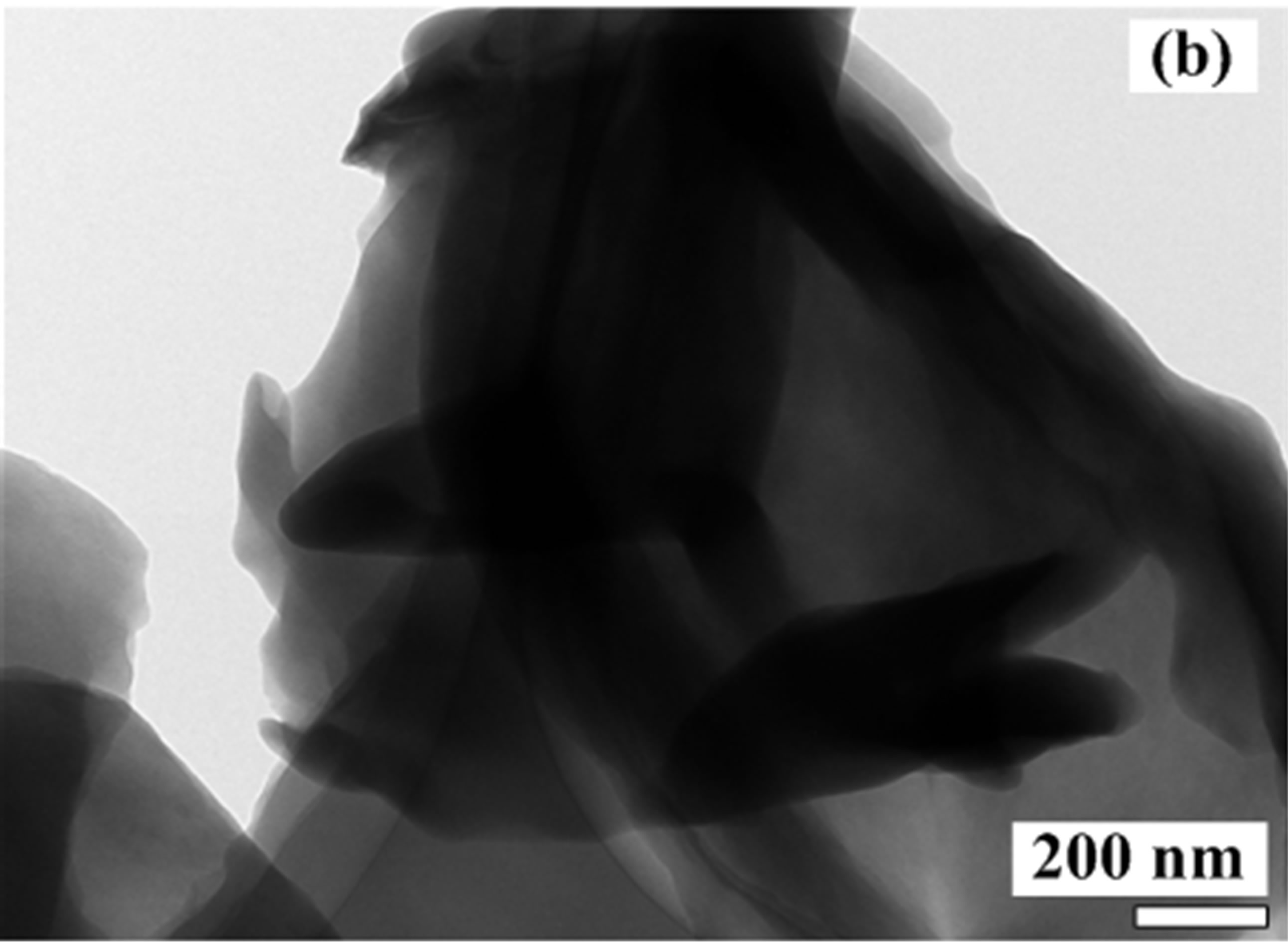
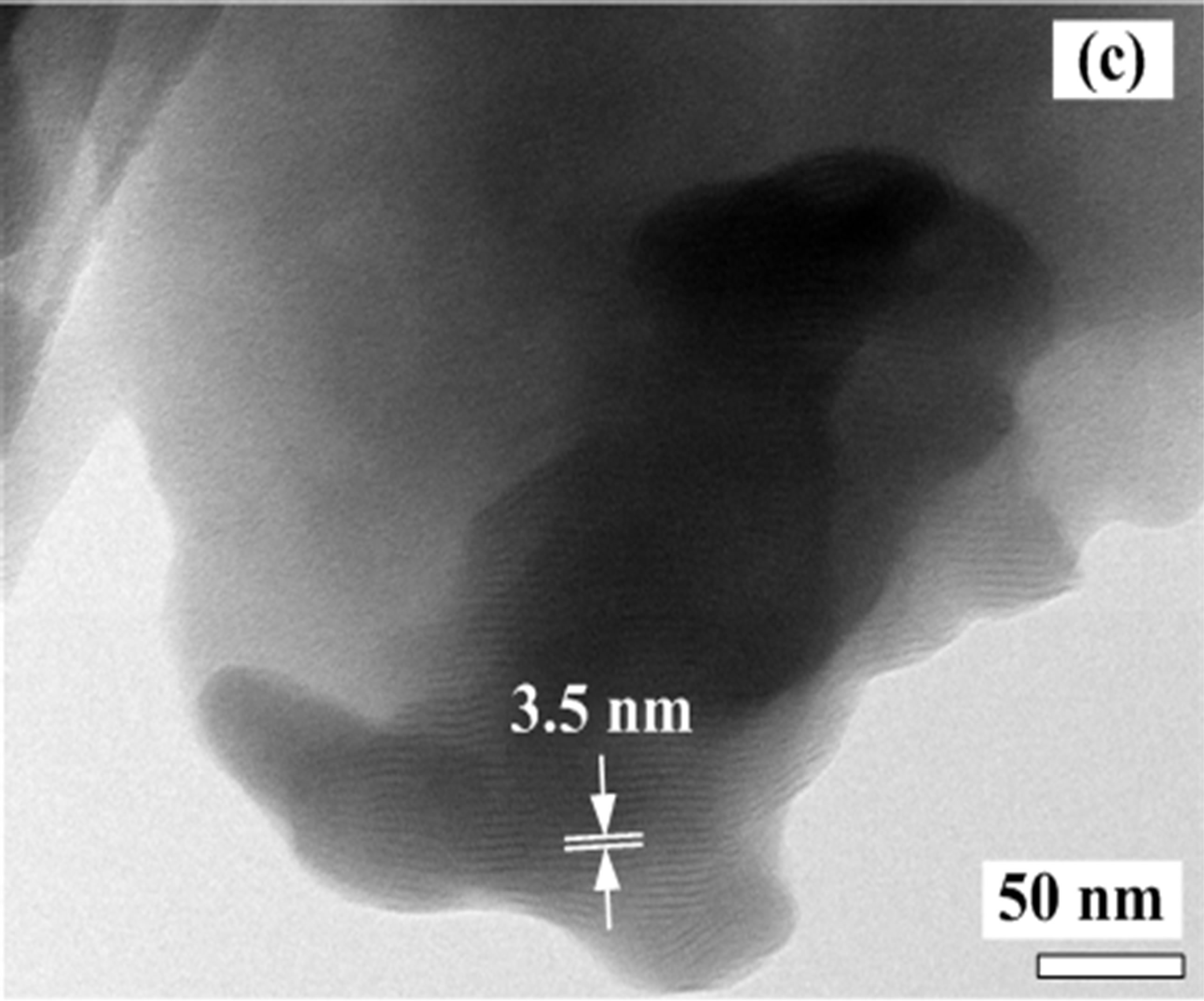
Figure 2. (a) SEM and (b); (c) TEM images of the synthesized foam with a lamellar structure.
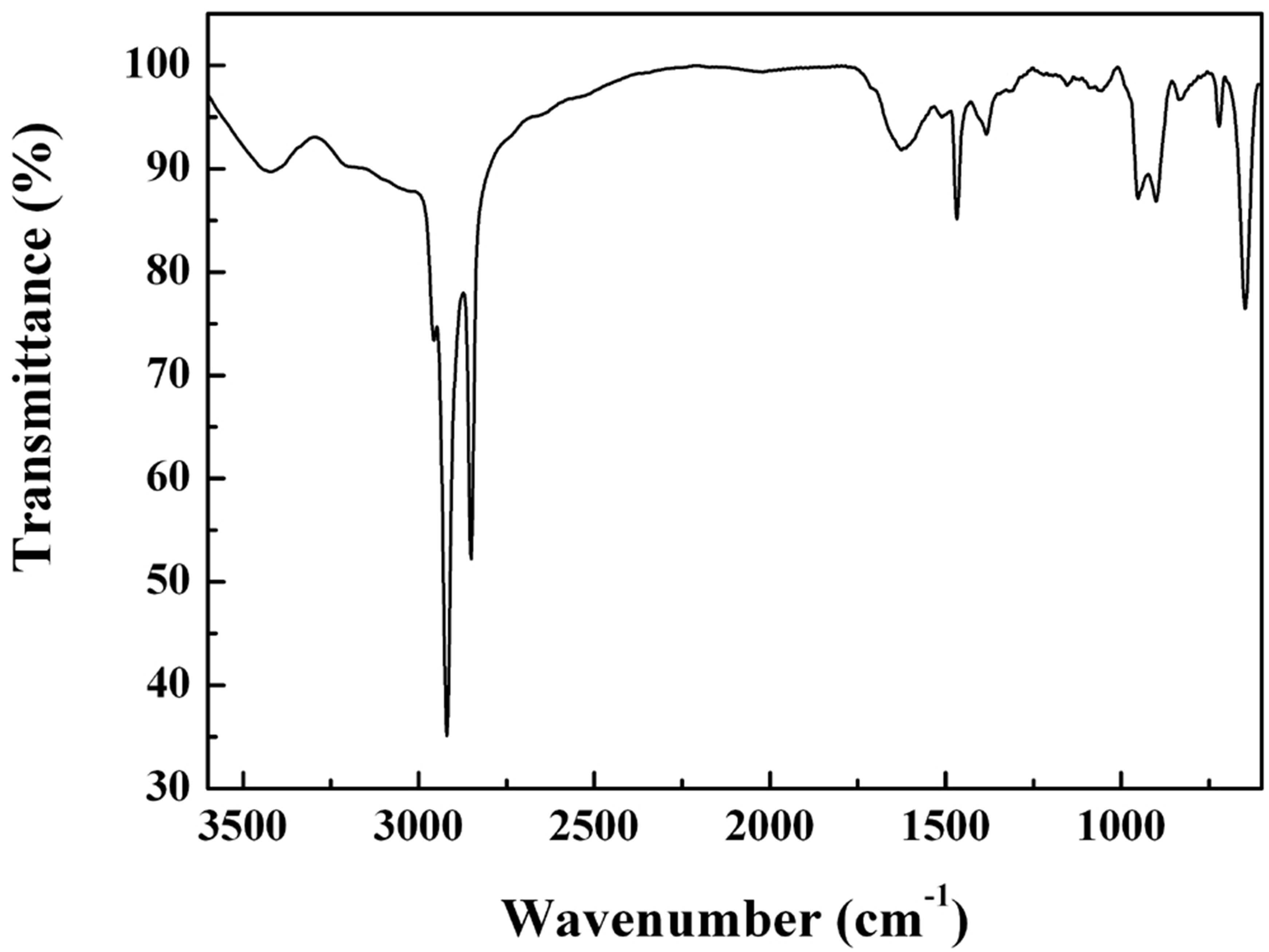
Figure 3. FTIR spectrum collected on the foam.
bonding either to the oxygen or to the vanadium at the vacant bonding position following the intercalation of C16H33NH2.
4. Conclusion
Facile method of synthesizing organic-inorganic solid foam in complex structure was presented, based on the use of C16H33NH2 as structure directing agent and VN as precursor. The foam route in this work showed that amines were simply intercalated between the vanadium oxide planes via VN foaming process, showing that the experimental requirements are not critical. This method indicates that metal nitrides can be used for the synthesis of new inorganic-organic material via foam route as like metal oxides, expecting the applications to mesoporous molecular sieves and catalysts because of their large surface area and pore diameter.
5. Acknowledgements
This work was supported by R&D Program through the National Fusion Research Institute of Korea (NFRI) funded by the Government funds and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2010-0029422) in part.
REFERENCES
- Y. Y. Lyu, S. H. Yi, J. K. Shon, S. Chang, L. S. Pu, S. Y. Lee, K. Char, G. D. Stucky and J. M. Kim, “Highly Stable Mesoporous Metal Oxides Using Nano-Propping Hybrid Gemini Surfactants,” Journal of the American Chemical Society, Vol. 126, No. 8, 2004, pp. 2310-2311. http://dx.doi.org/10.1021/ja0390348
- C. T. Kresge, M. E. Leonowiez, W. J. Roth, J. C. Vartuli and J. S. Bexk, “Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism,” Nature, Vol. 359, 1992, pp. 710-712. http://dx.doi.org/10.1038/359710a0
- J. D. Wang, D. Li, J. K. Liu, X. H. Yang, J. L. He and Y. Lu, “One-Step Preparation and Characterization of Zinc Phosphate Nanocrystals with Modified Surface,” Soft Nanoscience Letters, Vol. 1, No. 1, 2011, pp. 81-85. http://dx.doi.org/10.4236/snl.2011.13015
- A. Corma, “From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis,” Chemical Reviews, Vol. 97, No. 6, 1997, pp. 2373-2419. http://dx.doi.org/10.1021/cr960406n
- P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, “Generalized Syntheses of Large-Pore Mesoporous Metal Oxides with Semicrystalline Frameworks,” Nature, Vol. 396, No. 6707, 1998, pp. 152-155. http://dx.doi.org/10.1038/24132
- D. Antonelli and J. Y. Ying, “Synthesis of Hexagonally-Packed Mesoporous TiO2 by a Modified Sol-Gel Method,” Angewandte Chemie International Edition, Vol. 35, No. 4, 1996, pp. 426-430. http://dx.doi.org/10.1002/anie.199604261
- G. G. Janauer, A. Dobley, J. Guo, P. Zavalij and M. S. Whittingham, “Low Temperature Synthesis of Lamellar Transition Metal Oxides Containing Surfactant Ions,” Chemistry of Materials, Vol. 8, No. 8, 1996, pp. 2096- 2101. http://dx.doi.org/10.1021/cm960111q
- J. S. Reddy, P. Liu and A. Sayari, “Vanadium Containing Crystalline Mesoporous Molecular Sieves Leaching of Vanadium in Liquid Phase Reactions,” Applied Catalysis, Vol. 148, No. 1, 1996, pp. 7-21. http://dx.doi.org/10.1016/S0926-860X(96)00222-0
- G. T. Chandrappa, N. Steunou and J. Livage, “Materials Chemistry: Macroporous Crystalline Vanadium Oxide Foam,” Nature, Vol. 416, No. 6882, 2002, pp. 702-703. http://dx.doi.org/10.1038/416702a
- P. Liu, I. L. Moufrakovski, J. Liu and A. Sayari, “Mesostructured Vanadium Oxide Containing Dodecylamine,” Chemistry of Materials, Vol. 9, No. 11, 1997, pp. 2513- 2520. http://dx.doi.org/10.1021/cm970067u
- V. Luca, D. J. MacLachlan, J. M. Hook and R. Withers, “Synthesis and Characterization of Mesostructured Vanadium Oxide,” Chemistry of Materials, Vol. 7, No. 12, 1995, pp. 2220-2223. http://dx.doi.org/10.1021/cm00060a002
- Y. Zhang, C. J. O’Connor, A. Clearfield and R. C. Haushalyer, “An Organically Templated Layered Vanadium Oxide: Hydrothermal Synthesis, Single-Crystal Structure, and Magnetic Properties of (H3N(CH2)3NH3)[V4O10],” Chemistry of Materials, Vol. 8, No. 3, 1996, pp. 595-597. http://dx.doi.org/10.1021/cm9503846
- D. H. Shin, C. U. Bang, Y. C. Hong and H. S. Uhm, “Preparation of Vanadium Pentoxide Powders by Microwave Plasma-Torch at Atmospheric Pressure,” Materials Chemistry and Physics, Vol. 99, No. 2-3, 2006, pp. 269-275. http://dx.doi.org/10.1016/j.matchemphys.2005.10.026
- Y. C. Hong, D. H. Shin and H. S. Uhm, “Production of Vanadium Nitride Nanopowders from Gas-Phase VOCl3 by making use of microwave plasma torch,” Materials Chemistry and Physics, Vol. 101, No. 1, 2007, pp. 35-40. http://dx.doi.org/10.1016/j.matchemphys.2006.02.009
- A. Bouhaouss and P. Aldebert, “Intercalation d'ions Alkylammonium et d’alkylamines a Longues Chaines dans les Gels de V2O5,” Materials Research Bulletin, Vol. 18, No. 10, 1983, pp. 1247-1256. http://dx.doi.org/10.1016/0025-5408(83)90028-4
- Q. Guo, Q. Yang, C. Yi, L. Zhu and Y. Xie, “Synthesis of Carbon Nitrides with Graphite-Like or Onion-Like Lamellar Structures via a Solvent-Free Route at Low Temperatures,” Carbon, Vol. 43, No. 7, 2005, pp. 1386-1391. http://dx.doi.org/10.1016/j.carbon.2005.01.005
NOTES
*Corresponding author.

