Soft Nanoscience Letters
Vol.2 No.3(2012), Article ID:19814,5 pages DOI:10.4236/snl.2012.23008
β-Galactosidase Leakage from Escherichia coli Points to Mechanical Damages Likely Cause of Carbon Nanotube Toxicity
![]()
1Department of Molecular Reproduction, Development and Genetics, Indian Institute of Science, Bangalore, India; 2Present Address: Department of Molecular and Cellular Developmental Biology, University of Texas at Austin, Austin, USA; 3Department of Biotechnology, PA College of Engineering, Mangalore, India; 4Department of Physics, Indian Institute of Science, Bangalore, India; 5Present Address: Department of Electrical and Computer Engineering (Biomedical Group), King Abdulaziz University, Jeddah, KSA.
Email: mhussain2@kau.edu.sa
Received March 24th, 2012; revised April 23rd, 2012; accepted May 4th, 2012
Keywords: carbon nanotubes; cytotoxicity; membrane damage; microbial strains; reporter geneassay
ABSTRACT
We show that the cytotoxic effect of carbon nanotubes (CNTs) on bacteria is mediated by mechanical damage to the cell wall and membrane. Two β-galactosidase-producing strains of Escherichia coli harboringgenomically integrated reporter gene constructs, namely pchbB:lacZ and prpoS:lacZ, were used for the purpose. We first verified that CNTs result in an inhibition of cell growth. Enzyme activity was determined using a reporter gene assay in which CNTs were used without the lysis buffer (containing detergent). β-galactosidase activity in the presence of CNTs alone measured several fold more than the controls used (without nanotubes). This suggests that CNTs damage the cell membrane in a manner similar to the detergent in the lysis buffer and render E. coli cell walls porous, causing cell contents including enzymes to leak out into the medium. Our results support the hypothesis that mechanical damage to bacterial cell membranes is the prevailing cause of CNT-cytotoxicity.
1. Introduction
The possible use of singleand multi-walled carbon nanotubes (CNTs) in bulk has raised questions regarding the likely biological outcomes of such use, whether beneficial (control of pathogens or diseased cells, effluent treatment) [1,2] or harmful (damage to healthy tissues and organs of plants and animals including humans, soil contamination) [3-6]. The broader issue of CNTs having an adverse effect on the environment remains fully to be addressed. This will become important if CNTs are mass produced (whether for medicinal or for other purposes) and become airborne. Therefore, exploring and understanding the mechanism of CNT cytotoxicity is critical. The mechanisms leading to the anti-bacterial activity of CNTs, which may differ between cell types and also between organs and tissues in human or animal models, need fully to be understood.
Carbon nanotube toxicity to a variety of human and other animal cell lines has been reported and reviewed [7-9]. More recently CNT toxicity studies have been carried out on microorganisms, especially Escherichia coli [10,11] and the physico-chemical determinants of carbon nanotube toxicity have been discussed at length [8,12]. The results demonstrate a definite toxic effect of CNTs in all these studies [10-12]. It has been suggested that the cytotoxic effect of CNTs on bacterial cells could have medicinal value by virtue of being bactericidal [13-16].
The generation of reactive oxygen species (ROS) and oxidative stress have been discussed as likely routes for CNT toxicity [6,17-19]. Physical membrane damage leading to the rupture of bacterial cells has also been invoked [10,11]. The present work attempts to test this hypothesis in a novel manner. Our approach involves the measurement of extracellular enzyme activity in a medium in which β-galactosidase producing E. coli strains are suspended. β-galactosidase is an enzyme that catalyses the hydrolysis of β-galactoside sugars including lactose and the galactoside analogue o-nitrophenyl-β-Dgalactopyranoside (ONPG). Normally the enzyme is produced and acts inside the cell. For it to act on an extracellular substrate, cells must be lysed (e.g., using a detergent, freezing and thawing, or by some other means) so as to enable the enzyme to emerge into the extracellular medium. We have made use of this principle to study extracellular hydrolysis when cells are incubated with CNTs but without lysis buffer. We find that both strains of E. coli demonstrate significant extracellular enzyme activity in the presence of CNTs alone, that is, in the absence of detergent-induced cell lysis.
2. Materials and Methods
Unless stated otherwise, all chemicals were purchased from HiMedia, India or Difco, USA and are of analytical grade.
2.1. Carbon Nanotubes (CNTs)
Carbon nanotubes (CNTs) that were either single-walled (SWNT, diameter size 1 - 2 nm) or multi-walled (MWNT-1 and MWNT-2, diameter sizes < 10 nm and 10 - 30 nm respectively) were used (see Figure 1; all three types were purchased from Shenzhen Nanotechnologies Co. Ltd., China). CNTs suspensions in saline or in bacterial growth media were sterilised by autoclaving and sonicated for 2 hr using a bath sonicator. The suspensions were further sonicated for 20 min immediately before use. CNTs suspensions were handled in a laminar flow hood to ensure sterile conditions.
2.2. Cells
E. coli bacteria (strain K12) containing lacZ reporter constructs were used in all experiments. Cells were grown in “lysogeny broth” medium [20] at 37˚C and harvested at mid-exponential growth phase. We studied the effect of exposing 1 × 107 cells/ml in a well-aerated shaken suspension to CNTs for 1 h at 37˚C by dispersing CNTs as purchased, either in saline solution (0.9% or 0.154 M NaCl) or in culture media at two concentrations
 (a) (b) (c)
(a) (b) (c)
Figure 1. Suspensions of CNTs with smaller size are visually clearer than the bigger size. (a) SWNT (diameter 1 - 2 nm); (b) MWNT-1 (diameter < 10 nm) and (c) MWNT-2 (diameter 10 - 30 nm).
(10 and 25 μg/ml). The duration of 1 hr was determined based on time series experiments that were conducted by incubating cells for 15 min, 1 hr, 5 hr and overnight (12 hr) in order to determine the duration required to obtain a 50% loss of viability. We mixed washed bacterial cells with 10 or 25 μg of CNTs in suspension and placed the mixture in culture wells along with controls. This was followed by incubation for 1 hr in a shaker, harvesting the cells, diluting them and plating them on nutrient plates for taking viable counts. Colonies were counted after overnight incubation and compared with controls in the absence of CNTs. Duplicate samples were analysed and experiments were repeated twice.
2.3. Enzyme Assay
We carried out β-galactosidase activity measurements on two strains of E. coli harbouring the reporter gene constructs pchbB:lacZ (chb-lacZ is a derivative of JM101, where LacZ is driven by the chb promoter and is integrated on the chromosome at att site; [21] or prpoS:lacZ) rpoS-lacZ is derived from NC122 where LacZ is driven by the rpoS promoter and integrated in the genome [22]. β-galactosidase inside the cell cleaves lactose to glucose and galactose, both of which serve as carbon (energy) sources. The synthetic compound o-nitrophenyl-β-D-galactoside (ONPG) is also recognized as a substrate and cleaved to yield galactose and o-nitrophenol which has a yellow color that can be monitored at 420 nm. When the concentration of the substrate ONPG is in significant excess over that of β-galactosidase, the rate of production of o-nitrophenol is proportional to the concentration of the enzyme. Thus the appearance of yellow color can be used to determine the enzyme activity that escapes cells after lysis (data not shown) or after CNT treatment.
Cells (reporter strains pchbB:lacZ and prpoS:lacZ) were grown in minimal medium till they reached an OD of 0.6. They were then harvested by spinning down for 2 mins at 10,000 g and resuspending in 1 ml. 100 μl of the suspension was incubated with nanotubes (25 μg) for 1 hr in a 96 well microtitre plate kept shaking in a gyrorotary shaker at 250 rpm. A set with no nanotubes was treated as the control. Cells were pelleted and resuspended in 800 μl of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, pH 7.0) followed by lysis, which was induced by adding 10 μl of 1% sodium dodecyl sulphate (SDS) detergent and 20 μl chloroform for. The last step was circumvented for cells that were incubated with CNTs. The mixture was vortexed for 10 seconds for efficient lysis. The β-galactosidasereaction was initiated by adding 200 μl of ONPG (4 mg/ml freshly made in Z buffer). Tubes were incubated at 28˚C till colour develops and the reaction was stopped by adding 500 μl of 1 M sodium carbonate. Enzyme activity was computed in Miller units [23] according to the formula in Equation (1) below.
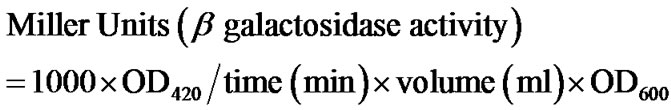 (1)
(1)
We performed two sets of experiments as follows. In the first set, a 100 µl suspension of cells carrying the pchbB:lacZ reporter construct was incubated with CNTs (25 μg) for 1 hr. Thereafter ONPG was added followed by incubation at 28˚C for 75 minutes during which colour developed. Cells with no CNTs were used as controls. An identical set of experiment was conducted using E. coli carrying the prpoS:lacZ reporter construct.
3. Results
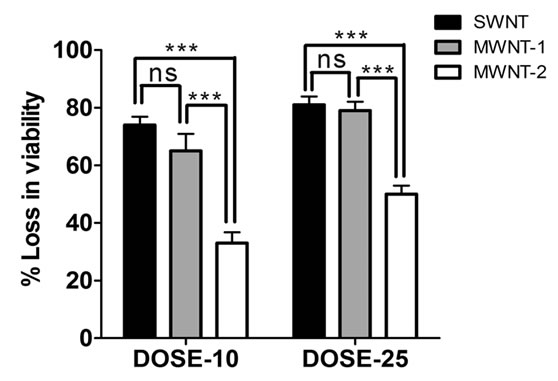
In order to pick suitable conditions for further experimentation, we carried out preliminary studies on the antibacterial activity of single-walled nanotubes (SWNTs) and two different sizes of multi-walled nanotubes (MWNTs) CNT cytotoxicity to E. coli cells depended on the type of CNTs used, their size and dose (Figure 2).
SWNTs exhibited stronger antibacterial activity than
 (a)
(a)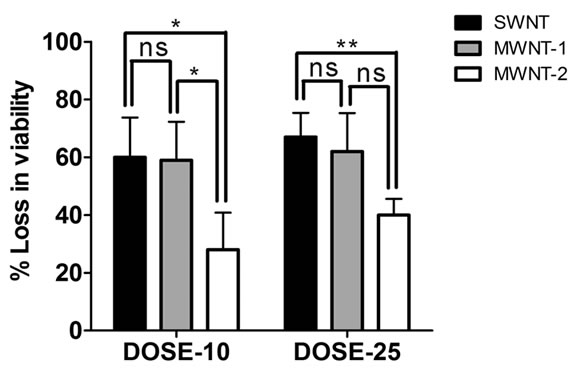 (b)
(b)
Figure 2. Suspended type of experiments was conducted in (a) saline, as well as in (b) LB culture medium by suspending CNTs at two different doses; 10 μg/ml and 25 μg/ml. Data obtained using saline suspension is more pronounced as compared to LB suspension. SWNT reduced viability to a greater level than MWNTs and a dose of 25 μg/ml increased the efficacy of the nanotubes to reduce viability (significance values calculated using one-way ANOVA).
MWNTs (see Figure 2 legend for details) and the magnitude of cytotoxicity varied for two different sizes of MWNTs (Figure 2). These observations support the findings that CNT size (diameter) plays an important role in the inactivation of E. coli K12 bacteria [11]. Studies have also indicated that CNT size and surface area are important characteristics from a toxicological point of view [24,25]. A plausible reason for this is that as the size of CNTs decreases, their specific surface area increases, leading to an increased opportunity for interaction and uptake by living cells.
The results shown in Figure 2 are the results of exposing bacterial cells to CNTs for one hour. To test whether an exposure of the order of one hour is required for the bactericidal effect of CNTs, we decided to expose cells for durations ranging from 15 min to overnight (12 hr). Since a CNT concentration of 25 μg/ml and saline suspension conditions yielded a pronounced cytotoxic effect, we retained those conditions. The extended timeseries experiments showed that there was a size-dependent loss of viability with time (Figure 3). The loss of viability was higher in the presence of smaller SWNTs as compared to larger MWNTs. This finding is in line with studies showing that SWNTs exhibit significant cytotoxicity to human and animal cells whereas MWNTs exhibit a milder toxicity [3-5]. A one-hour exposure was sufficient to cause nearly 50% loss of viability by all types of CNTs used (Figure 3).
Using the same conditions, we carried out β-galactosidase assays and quantified enzyme activity in the extracellular medium. The graph shown in Figure 4 indicates the trend in enzyme activity measured in terms of Miller Units.
Exposure to nanotubes significantly increased the enzyme activity with respect to the control. SWNTs led to a
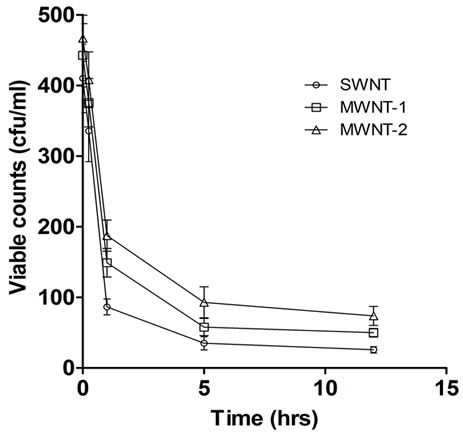
Figure 3. Plot of a set of five experimental readings of viable bacterial count with time of exposure to CNTs. Experiments were conducted in saline suspension and a dose of 25 mg/ml of CNTs was used based on the dose response result shown in Figure 2. SWNT was found to be more potent than MWNT-1 (p < 0.01) and MWNT-2 (p < 0.001) when exposed for an hour (values calculated using two-way ANOVA).
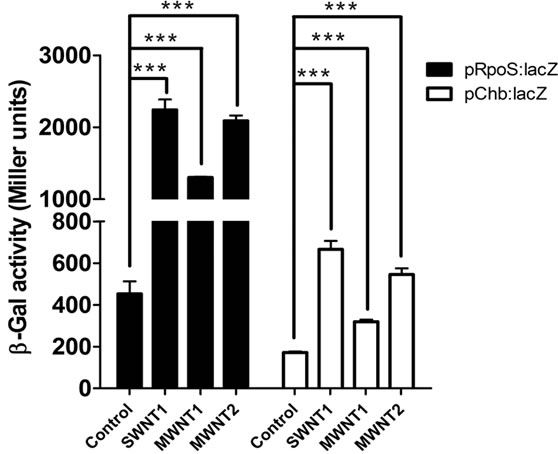
Figure 4. β-gal assay results. Left panel are from prpoS: lacZ strains while the results on the right are from pchbB: lacZ strains. MWNT (bigger size) records lesser change in enzyme activity as compared to SWNT (smaller size).
higher change in enzyme activity as compared to MWNTs (Figure 4).
4. Discussion
There is a significant increase in extracellular enzyme activity measured after incubation with CNTs alone— that is, in the absence of detergent-induced lysis (Figure 4). This implies that CNT incubation must have led to a release of active enzyme protein from the bacteria. The efficacy of the CNTs is correlated to their size (diameter), with SWNTs causing the highest loss in viability (also change in enzyme activity) followed by MWNT-1 and lowest by MWNT-2, a trend that is attributable to a decrease in surface-to-volume ratio from SWNTs to MWNT- 2. SWNT caused about 1.2 fold increase in cell lysis over MWNT-1 and 2.5 fold increase over MWNT-2. The enzyme activity measured in strain prpoS:lacZ was much higher than in strain pchbB:lacZ, which is almost certainly due to the different strength of the two promoters (the rpoS promoter being stronger than the chbB promoter); [26,27].
Kang S. and colleagues have found that single-walled CNTs (SWNTs) can pierce bacterial cell walls [10,11]. Specifically, by using highly purified SWNTs with a narrow diameter distribution, they demonstrated through SEM imaging that direct contact with SWNTs can cause severe morphological changes of cell walls and DNA microarray data suggested cell membrane damage validating the cytotoxicity assay showing a loss of viability up to 80% in E. coli bacteria [10-12]. They hypothesized that SWNT aggregates caused irrecoverable damage to E. coli by physical damage to the outer membrane of the cells that led to the release of intracellular content. The induction of oxidative stress may be yet another factor behind SWNT antibacterial activity [28]. Our work provides additional evidence in favour of the hypothesis that physical damage to bacterial cells is an important cause of CNT toxicity. We have worked with CNTs that are in suspension and presumably mobile; under conditions of deposition (when CNTs are not in suspension rather are settled on the substrate in static condition) or when CNT composites (when CNTs are used in combination of another materials, CNTs are not suspended but are static in such cases as well) are used, the outcomes are equivocal: most studies show no cytotoxicity (reviewed in [9]).
5. Acknowledgements
A.K.S. thanks the Department of Science and Technology, New Delhi for financial support. M.A.H. thanks Indian Academy of Sciences, Bangalore for summer fellowship. VN was supported by the University Grants Commission. This article was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah. The authors, therefore, acknowledge with thanks DSR technical and financial support.
REFERENCES
- A. S. Brady-Estévez, T. H. Nguyen, L. Gutierrez and M. Elimelech, “Impact of Solution Chemistry on Viral Removal by a Single-Walled Carbon Nanotube Filter,” Water Research, Vol. 44, 2010, pp. 3773-3780. doi:10.1016/j.watres.2010.04.023
- A. S. Brady-Estévez, M. H. Schnoor, C. D. Vecitis, N. B. Saleh and M. Elimelech, “Multiwalled Carbon Nanotube Filter: Improving Viral Removal at Low Pressure,” Langmuir, Vol. 26, No. 18, 2010, pp. 14975-14982. doi:10.1021/la102783v
- D. B. Warheit, B. R. Laurence, K. L. Reed, D. H. Roach, G. A. M. Reynolds and T. R. Webb, “Comparative Pulmonary Toxicity Assessment of Single-Wall Carbon Nanotubes in Rats,” Toxicological Sciences, Vol. 77, No. 1, 2004, pp. 117-125. doi:10.1093/toxsci/kfg228
- C. W. Lam, J. T. James, R. McCluskey and R. L. Hunter, “Pulmonary Toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days after Intratracheal Instillation,” Toxicological Sciences, Vol. 77, No. 1, 2004, pp. 126- 134. doi:10.1093/toxsci/kfg243
- G. Jia, H. F. Wang, L. Yan and X. Wang, “Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, MultiWall Nanotube, and Fullerene,” Environmental Science & Technology, Vol. 39, No. 5, 2005, pp. 1378-1383. doi:10.1021/es048729l
- S. K. Manna, S. Sarkar, J. Barr, K. Wise, E. V. Barrera, O. Jejelowo, A. C. Rice-Ficht and G. T. Ramesh, “Single Walled Carbon Nanotube Induces Oxidative Stress and Activates Nuclear Transcription Factor-kB in Human Keratinocytes,” Nano Letters, Vol. 5, No. 9, 2005, pp. 1676- 1684. doi:10.1021/nl0507966
- K. Aschberger, H. J. Johnston, V. Stone, R. J. Aitken, S. M. Hankin, S. A. Peters, C. L. Tran and F. M. Christensen, “Review of Carbon Nanotubes Toxicity and Exposure—Appraisal of Human Health Risk Assessment Based on Open Literature,” Critical Reviews in Toxicology, Vol. 40, No. 9, 2010, pp. 759-790. doi:10.3109/10408444.2010.506638
- H. J. Johnston, G. R. Hutchison, F. M. Christensen, S. Peters, S. Hankin, K. Aschberger and V. Stone, “A Critical Review of the Biological Mechanisms Underlying the in Vivo and in Vitro Toxicity of Carbon Nanotubes: The Contribution of Physico-Chemical Characteristics,” Nanotoxicology, Vol. 4, No. 1, 2010, pp. 207-246. doi:10.3109/17435390903569639
- M. A. Hussain, M. A. Kabir and A. K. Sood, “On the Cytotoxicity of Carbon Nanotubes,” Current Science, Vol. 96, No. 5, 2009, pp. 664-673.
- S. Kang, M. Pinault, L. D. Pfefferle and M. Elimelech, “Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity,” Langmuir, Vol. 23, No. 17, 2007, pp. 8670-8673. doi:10.1021/la701067r
- S. Kang, M. Herzberg, D. F. Rodrigues and M. Elimelech, “Antibacterial Effects of Carbon Nanotubes: Size Does Matter!” Langmuir, Vol. 24, No. 13, 2008, pp. 6409- 6413. doi:10.1021/la800951v
- S. Kang, M. S. Mauter and M. Elimelech, “Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity,” Environmental Science & Technology, Vol. 42, 2008, pp. 7528-7534. doi:10.1021/es8010173
- J. D. Schiffman and M. Elimelech, “Antibacterial Activity of Electrospun Polymer Mats with Incorporated Narrow Diameter Single-Walled Carbon Nanotubes,” ACS Applied Materials & Interfaces, Vol. 3, 2011, pp. 462-468. doi:10.1021/am101043y
- S. Aslan, C. Z. Loebick, S. Kang, M. Elimelech, L. D. Pfefferle and P. R. Van Tassel, “Antimicrobial Biomaterials Based on Carbon Nanotubes Dispersed in Poly(lactic-co-glycolic acid),” Nanoscale, Vol. 2, No. 9, 2010, pp. 1789-1794. doi:10.1039/c0nr00329h
- D. F. Rodrigues and M. Elimelech, “Toxic Effects of Single-Walled Carbon Nanotubes in the Development of E. coli Biofilm,” Environmental Science & Technology, Vol. 44, No. 12, 2010, pp. 4583-4589. doi:10.1021/es1005785
- T. Akasaka, M. Matsuoka, T. Hashimoto, S. Abe, M. Uo and F. Watari, “The Bactericidal Effect of Carbon Nanotube/Agar Composites Irradiated with Near-Infrared Light on Streptococcus Mutans,” Materials Science and Engineering B, Vol. 173, No. 1-3, 2010, pp. 187-190. doi:10.1016/j.mseb.2010.01.001
- A. Nel, T. Xia, L. Madler and N. Li, “Toxic Potential of Materials at the Nanolevel,” Science, Vol. 311, No. 5761, 2006, pp. 622-627. doi:10.1126/science.1114397
- A. A. Shvedova, V. Castranova, E. R. Kisin, D. Schwegler-Berry, R. Murray, V. Z. Gandelsman, A. Maynard and P. Baron, “Exposure to Carbon Nanotube Material: Assessment of the Biological Effects of Nanotube Materials Using Human Keratinocyte Cells,” Journal of Toxicology and Environmental Health, Part A, Vol. 66, 2003, pp. 1909-1926. doi:10.1080/713853956
- K. Pulskamp, S. Diabate and H. F. Krug, “Carbon Nanotubes Show No Sign of Acute Toxicity but Induce Intracellular Reactive Oxygen Species in Dependence on Contaminants,” Toxicology Letters, Vol. 168, No. 1, 2007, pp. 58-74. doi:10.1016/j.toxlet.2006.11.001
- G. Bertani, “Studies on Lysogenesis. I. The Mode of Phage Liberation by Lysogenic Escherichia coli,” Journal of Bacteriology, Vol. 62, No. 3, 1951, pp. 293-300.
- J. Plumbridge and O. Pellegrini, “Expression of the Chitobiose Operon of Escherichia coli Is Regulated by Three Transcription Factors: NagC, ChbR and CAP,” Molecular Microbiology, Vol. 52, No. 2, 2004, pp. 437-449. doi:10.1111/j.1365-2958.2004.03986.x
- H. E. Schellhorn and H. M. Hassan, “Transcriptional Regulation of katE in Escherichia coli K-12,” Journal of Bacteriology, Vol. 170, No. 9, 1988, pp. 4286-4292.
- J. Miller, “Experiments in Molecular Genetics,” Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, 1972.
- F. Tian, D. Cui, H. Schwarz, G. G. Estrada and H. Kobayashi, “Cytotoxicity of Single-Wall Carbon Nanotubes on Human Fibroblasts,” Toxicology in Vitro, Vol. 20, No. 7, 2006, pp. 1202-1212. doi:10.1016/j.tiv.2006.03.008
- Y. Sato, A. Yokoyama, K. Shibata, Y. Akimoto, S. Ogino, Y. Nodasaka, T. Kohgo, K. Tamura, T. Akasaka, M. Uo, K. Motomiya, B. Jeyadevan, M. Ishiguro, R. Hatakeyama, F. Watari and K. Tohji, “Influence of Length On Cytotoxicity of Multi-Walled Carbon Nanotubes against Human Acute Monocytic Leukemia Cell Line THP-1 in Vitro and Subcutaneous Tissue of Rats in Vivo,” Molecular BioSystems, Vol. 1, 2005, pp. 176-182. doi:10.1039/b502429c
- A. H. Kachroo, A. K. Kancherla, N. S. Singh, U. Varshney and S. Mahadevan, “Mutations that Alter the Regulation of the Chb Operon of Escherichia coli Allow Utilization of Cellobiose,” Molecular Microbiology, Vol. 66, No. 6, 2007, pp. 1382-1395.
- M. Ranjna, M. Sudha and M. Subramony, “Enhanced Expression of the Bgl Operon of Escherichia coli in the Stationary Phase,” FEMS Microbiology Letters, Vol. 288, No. 1, 2008, pp. 131-139. doi:10.1111/j.1574-6968.2008.01346.x
- C. D. Vecitis, K. R. Zodrow, S. Kang and M. Elimelech, “Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes,” ACS Nano, Vol. 4, No. 9, 2010, pp. 5471-5479. doi:10.1021/nn101558x

