Journal of Behavioral and Brain Science
Vol.06 No.02(2016), Article ID:63841,8 pages
10.4236/jbbs.2016.62011
Neurophenotyping of Zebrafish Larvae that Are Preconditioned with Anesthetic Agent
Julie L. Mustard1, Matthew A. Strope1, Christopher S. Havey1, Lucy S. Wang1, Norbert W. Seidler1,2*
1Division of Basic Sciences, Department of Biochemistry, Kansas City University, Kansas City, MO, USA
2Department of Anesthesiology, University of Missouri/Saint Luke’s Hospital, Kansas City, MO, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 January 2015; accepted 22 February 2016; published 26 February 2016
ABSTRACT
Anesthetics evoke a stress-response, upregulating heat shock genes. This neuroprotective response to proteotoxic stress represents preconditioning, a process by which neuronal tissue, previously exposed to anesthetics, is protected against future insult. It presumes a sub-lethal injury, affecting protein unfolding. Our hypothesis is: preconditioning evokes molecular events that result in downstream changes that offer a selective advantage in terms of neuronal function. We focused on the neurobehavioral aspects which we neurophenotyped. Larval zebrafish were exposed to trifluoroethanol (TFE), an anesthetic mimetic, and tested for both individual and group behavioral markers of neuronal function. In bright/dark tests, we observed that TFE-exposed larvae spent more time in the dark area (typically an adult-like response) than control larvae. The response of TFE larvae to noise startle was directly opposite to that of controls. TFE larvae swam towards the source of the startle (into the bright zone), whereas control larvae swam away from the source of the startle (into the dark), typical of fear-response. The larvae also exhibited several differences in social behaviors, including synchronized schooling and shoaling behaviors. The TFE-group showed a greater number of synchronized events versus controls. The TFE-group also exhibited more shoaling events compared with controls. While the long-term effects have yet to be determined, these results shed light on the mechanism of anesthetic preconditioning. These complex zebrafish behaviors normally develop with age and therefore represent, in the TFE-exposed group, a pattern of accelerated maturation of neuronal function, which is the neurophenotype attributed to preconditioning.
Keywords:
Zebrafish, Anesthetics, Preconditioning, Neurophenotype, Trifluoroethanol

1. Introduction
As the receptor targets of volatile anesthetics continue to be investigated, there is growing interest in the unintended consequences of anesthetics and the molecular targets that elicit these effects. Clinically relevant questions include why select patient populations experience subtle adverse effects of anesthetics: individuals that are exposed to anesthetics very early in life may be at risk for later cognitive deficiencies [1] , and some elderly patients may suffer sudden impaired quality of life following surgery―a process called post-operative cognitive dysfunction. The molecular mechanisms that are associated with these unintended detrimental effects of anesthetics remain poorly understood.
Although seemingly contradictory, research in the area of preconditioning [2] [3] may offer clues to some of the underlying mechanisms of pathology. Anesthetic preconditioning, which is analogous to ischemic preconditioning, is a process by which neuronal tissue previously exposed to volatile anesthetics exhibits a selective advantage, especially against future cellular insult. The precise molecular mechanism by which this occurs also remains elusive.
We think that the adverse effects of anesthetics as well as the phenomenon of preconditioning both involve an early event that that can be described as cellular protein unfolding/aggregation. The difference between detrimental and beneficial processes may be in the level of cellular response or lack thereof. Our model of anesthetic preconditioning is that the initial exposure to an anesthetic agent produces a proteotoxic injury (i.e. protein unfolding/aggregation) that evokes a robust cytoprotective response, such as heat shock response [2] -[5] . The subtleties of this beneficial response in the CNS can be neurophenotyped.
Vertebrate behavior is a function of the central processing of sensory stimuli and transmission of motor commands to muscles from descending neurons to networks in the spinal cord [6] . Behavior, as such, is a valid endpoint for summative neuronal function. If the hypothesis is that an anesthetic agent, as a mediator of preconditioning, promotes a cytoprotective heat shock response that enhances neuronal integrity, then monitoring behavioral endpoints is a valid assessment of the beneficial changes to neuronal function. We undertook the current project to begin to understand the preconditioned neurophenotype. We chose zebrafish (Danio rerio), since their genes exhibit markedly similar characteristics and homology to those found in humans, and therefore, this animal model is valuable in investigating development and mimicking human pathophysiology. This organism, originally native to rice paddies and estuaries in India and Burma, has been established as a recognized experimental model since the early 1970s. Its briefer lifespan and ease of husbandry make zebrafish a sought after research alternative to rodent models. The behaviors of zebrafish larvae are elementary and measureable, allowing for results that offer broader predictive value. Zebrafish larvae were exposed to 2,2,2-trifluoroethanol (TFE), an anesthetic mimetic. We looked at larval swimming, which involves a complex repertoire of kinematic component behaviors, such as turns and bursts [7] .
2. Material and Methods
Larval zebrafish (Danio rerio; wild-type AB) were bred using standard IACUC/IBC-approved protocols. Embryos were harvested and incubated in sterile petri dishes at a fixed temperature of 28˚C. After hatching, at three days post-fertilization (3 dfp), larvae were divided at random into treated and untreated groups (three larvae per group) immediately prior to treatment. An aliquot of 5 mM aqueous TFE was added by pipet into the group petri dish (containing embryo media), versus control, which received the same volume of vehicle (18 mega W water). Sub-millimolar final concentrations of TFE were used to promote a tissue-response without eliciting any anesthetic effects (i.e. sedative). Following TFE exposure (0.1 mM for 3 hr at 28˚C, allowing for full tissue saturation), larvae were serially transferred by pipet (to remove TFE) into intermediate and final sterile group petri dishes. The two groups of larvae were incubated (28˚C) until 5dpf, at which time they were transferred into group nursery tanks on a self-monitoring rack system (Mini Z-Hab, pentairaes.com). The larvae were maintained using standard feeding (Skretting Gemma 75 at 5 dpf 3x daily) and care (28˚C, 750 mS, pH = 7.3) protocols until humanely euthanized at completion of the study.
The larvae were tested for behavioral and social markers of neuronal function. Two types of testing were performed: a stimulus-response at 4dpf and passive observation at 6 dpf. All results were compared with two-tailed t-tests using 95% confidence levels for significance.
At 4 dpf, a bright/dark test, with and without startle (Figure 1), was performed in which a light source was positioned over a petri dish in order to generate delineated zones of bright light and dark (80% light at 6700 lux;
Figure 1. Bright-dark experimental chamber. Fiber-optic illuminator was used as the light source and positioned as illustrated. The lux was measured using a light meter. The chamber was a standard size petri dish. The location of the alarm is shown as a symbol to the left.
20% dark at 150 lux). The control and TFE-exposed larvae were tested as two separate groups. The number of larvae in the group that occupied each zone (bright and dark) of the dish was determined at timed intervals. Then at 4min, a brief (2s) startle noise was generated by a lab timer alarm positioned at the farthest end of the bright zone opposite the dark. The average number of larvae in each zone was again determined at timed intervals over the succeeding 2 min. The mean values were calculated and compared. Student’s t-tests (two-tailed) were used to determine differences employing a 95% confidence limit.
At 6 dpf, social behavior was passively observed under standard room lighting. In this study we developed a novel procedure for quantifying normal (generally accepted) social behaviors. Four types of schooling and shoaling behaviors were identified and measured by location and movement over a fixed star-pattern grid (Fig- ure 2). Center dwelling and thigmotaxis were also measured using a fixed grid that consisted of a center circle that represented 25% of the total area. Videos (5 min) were recorded at the same set distance above each nursery cylinder placed over a fixed grid.
3. Results and Discussion
Schooling was defined as a coordinated, or synchronized, behavior involving multiple larvae, which are in close proximity of each other (£ two body lengths), using the following criteria: for “single-direction schooling”, they must burst-swim in parallel for at least two bursts; and for “double-direction schooling”, they must burst-swim in parallel for at least two half-bursts in one direction, and then, simultaneously change direction (≥90˚) and burst-swim again in parallel for at least two half-bursts. Burst-swimming is defined as episodic forward movement approximately one body length at a time.
Shoaling was defined as a non-synchronized behavior involving either two crisscrossing larvae or three clustered larvae, using the following criteria: for “cross-shoaling”, the swim path of the two larvae must cross one another (note: the directions can range from being perfectly anti-parallel to perpendicular), and they must be within one body length of each other; and for “group-shoaling”, the three fish must dwell in close proximity, which was scored when they simultaneously occupied one of the nine designated sections visible as an underlying star-pattern grid (Figure 2). This grid contained eight equally sized sections positioned in a clockwise fashion surrounding a similarly sized section in the center. Criteria for scoring the social behaviors were used by five different experimenters that viewed the videos. Three experimenters were blinded as to the treatment group. The scores were averaged and presented as the average number of schooling (or shoaling) events per group.
Center-dwelling and thigmotaxis were measured using a grid (dashedline, Figure 2) that contained a center circle that defined 25% of the total two-dimensional swim area for the larvae. Each individual larva was tracked and the amount of time spent in the center circle was recorded and calculated as a fraction of total time. The inverse of these measured values represent thigmotaxis. Two independent experimenters recorded the times, which were averaged and compared.
In the bright/dark test, we observed that control larvae appeared to avoid the dark (Figure 3), an expected behavior in zebrafish larvae. If larvae location in the test dish (80% bright zone) were determined by random activity, a value of 2.40 (dashedline in Figure 3) would represent the average number found in the bright zone (n = 3; location factor, 0.80). The controls exhibited 2.82, significantly higher than if random movement dictated location (one-sample t-test gives 95% interval of 2.67 to 2.98; p < 0.001).
Light preference is typical of zebrafish larvae. However, it is not so much a function of phototaxis that is directional movement based on light cues and due to retinal photoreceptors [8] , as it is a function of dark photokinesis that is heightened activity driving larvae in a stochastic fashion out of dark zones effectively “trapping” them in brighter zones as a consequence of reduced activity. This behavioral feature is dependent on deep brain (i.e. hypothalamus in pre-optic region) photoreceptors [9] . In contrast to controls, we observed TFE-exposed larvae exhibited a preference for the dark. TFE-exposed larvae showed an average number of 1.99 in the bright zone (one-sample t-test gives 95% interval of 1.69 to 2.29; p < 0.001), significantly less than the predicted average of 2.40 based on random movement. This behavioral preference for the dark is called scototaxis and
Figure 2. Underlying fixed grids. The grids were placed to fit under stationary nursery cylinders and larvae were passively filmed to measure social behaviors. The solidlines represent the grid to study shoaling and the dashedline is the grid used for determining center-dwelling.
Figure 3. Adult-like behavior exhibited in TFE-exposed larvae. Bright/dark experiments were performed without startle. The dashed line indicates the reference value for random-directed behavior. Data for the bars are given as M ± SEM. Asterisk, p < 0.0001.
represents an adult neurophenotype [10] . Some have argued that scototaxis is a feature of anxiety [11] . When we tested for thigmotaxis, we did not observe a difference between controls and TFE-exposed larvae (data not shown), suggesting that TFE exposure did not have an anxiogenic effect.
We think that the preference for the dark in TFE-exposed larvae may be due to suppression of dark photokinesis and other maturation of the cognitive-driven regions of the brain. Increased exploratory behavior is recognized as a measure of decreased anxiety in many species [12] . When we assessed the number of different sections occupied by the swimming larvae in petri dishes using the star-pattern grid described above, we did not see a difference (data not shown), suggesting that on the exploratory axis (i.e. representative of anxiety) there was no difference between controls and TFE-exposed larvae. We, therefore, cannot conclude dark preference by the TFE-exposed larvae is due to anxiogenic effect.
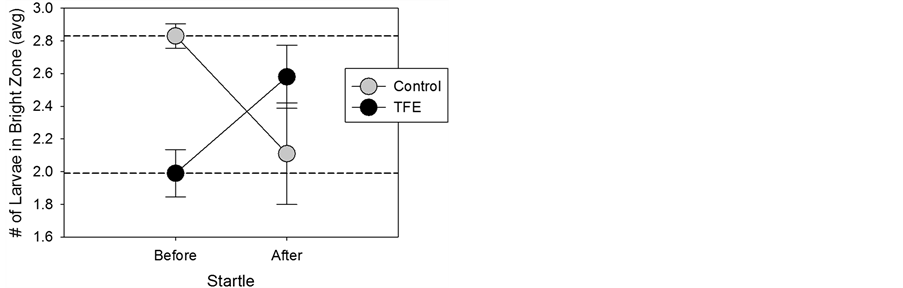
Following a sudden alarm-like sound (i.e. startle stimulus), we observed that the control larvae moved from the bright zone to the dark (Figure 4). The startle response of TFE-exposed larvae was directly opposite that of controls. TFE-exposed larvae swam towards the source of the startle and remained in the bright zone.
The startle response is an autonomous reflex that can be evoked by a stimulus that is sudden and unexpected. This is seen in larvae as a coordinated series of defined escape behaviors that appear as early as 2 dpf, such as larvae rapidly bend and swim away from an abrupt stimulus [13] , events that can be seen milliseconds after a brief tone or vibration. We examined larvae behavior over a 120s period following startle. We interpreted the responses of the control larvae as representing fear-directed neuronal signals that suppress dark photokinesis. The startle response behavior of TFE larvae, on the other hand, appears antithetical to an anxiety-driven response and may reflect a maturation of cognitive processes, similar to food-seeking.
TFE-exposed larvae also exhibited several differences in social behaviors (Figure 5), including single-direc- tion schooling and shoaling (two larvae: cross-shoaling; three larvae: group-shoaling). The TFE-group showed 5.6 ± 1.21 (M ± SEM) single-direction schooling events per 5 min period versus controls (3.0 ± 1.26; p < 0.001). The TFE-group exhibited 12.4 ± 1.36 cross-shoaling events per 5 min period compared with controls (5.0 ± 1.58; p < 0.01).
Larvae at this developmental stage typically show avoidance behavior of conspecifics. Larvae at 7 dpf have no social preference for being near each other [14] [15] . Shoaling behaviors develop later [16] . We know that older zebrafish (i.e. juveniles and adults) spend most of their time in cohesive groups (or shoals). Shoaling does not imply that there is a synchronization of swimming directions, only that there is aggregation of individuals. Preference for shoaling appears to be a learned phenomenon [17] and therefore a function of cognitive processes. Schooling behavior refers to the coordinated movement of the individuals (i.e. single-direction schooling). Our data is consistent with the hypothesis that TFE-induced preconditioning offers a selective advantage to brain (i.e. neural circuitry), perhaps accelerating maturation of the developing brain, enhancing the ability of larvae to process information.
Figure 4. Lack of fear response exhibited in TFE-exposed larvae. Bright/dark experiments were performed with data collected before and after startle. The two dashed lines represent the before startle values for control and TFE. Data are given as M ± SEM. In both groups, the “after startle” value differed significantly from their respective “before startle” values (p < 0.05).
Figure 5. Mature neurophenotype exhibited in TFE-ex- posed larvae. Observational data of social activity was recorded as the number of events in a 5min period. Data are given as M ± SEM. Asterisks, p < 0.05; hashtag, p = 0.054.
The literature shows us that anesthetic preconditioning improves cognitive performance following traumatic brain injury [18] . Our lab previously demonstrated that isoflurane increases expression of chaperone genes [2] . Chaperone expression is linked to enhanced cognitive performance [19] . While the long-term effects have yet to be determined, the results of the current study may shed light on the mechanism of anesthetic preconditioning. These complex zebrafish behaviors normally develop with age and therefore represent, in the TFE-exposed group, a pattern of accelerated maturation of neuronal function, which we attribute to the preconditioning effect of TFE.
Biophysical in vitro studies show that the hydrophobic interactions that are in large part responsible for the native state of biologically active proteins are disrupted by TFE [20] . Our lab demonstrated that TFE significant altered the native structure of albumin [21] .
The molecular targets that pertain to the unintended consequences of anesthetics, and in particular preconditioning, are thought to be different [2] [3] than those involved in eliciting the pharmacology of anesthesia. We chose TFE, the fluorinated equivalent of ethanol, in part because it is fully miscible in water. TFE exhibits properties of volatile fluorinated ethers, which are not miscible in water and therefore difficult to use with aquatic models, such as zebrafish larvae. The CNS effect of ethanol is similar to a number of sedative/hypnotic agents (i.e. benzodiazepines, neural steroids and volatile anesthetics), in that ethanol specifically enhances the activity of g-aminobutyric acid (GABA) A-type receptors [22] and inhibits the activity of N-methyl-D-aspartate (NMDA) receptors [23] . TFE exhibits five-fold greater potency than ethanol at inhibiting NMDA-activated currents [24] . Furthermore, TFE is an analog of TCE (trichloroethanol), the active metabolite of chloral hydrate and directly responsible for the anesthetic effects of chloral derivatives. While the anesthetic concentration of TCE is in the low millimolar range, the concentration of TFE associated with anesthesia has not been established, but it would be presumably less than that of TCE based on its comparatively weaker effects on NMDA-receptors [24] . In the current study, we used sub-millimolar concentrations of TFE that did not elicit any anesthetic effects (i.e. sedative) on zebrafish larvae. Halogenation may appear to be a crucial molecular feature. Diverse halogenated compounds, such as isoflurane, are typically used as inhalational anesthetics, and TFE, in fact, is a metabolite of isoflurane.
We hypothesize that preconditioning with anesthetics evokes molecular events that result in downstream changes (i.e. specific genetic expression) that protect and improve neuronal function. Anesthetics evoke a stress- response that includes upregulation of heat shock genes [2] . Exposure of SH-SY5Y cells to isoflurane causes an immediate increase in the expression of 45 different chaperone genes; and even after 24 hr, 60% of these remain upregulated. Several studies have suggested that preconditioning involves a heat shock response [25] .
This neuroprotective response to proteotoxic stress represents preconditioning. It presumes that the exposure to anesthetics contribute to a sub-lethal injury, likely impacting protein unfolding [3] , involving increased expression of α-crystallin, an inducible neuronal chaperone that inhibits aggregation of unfolded proteins. We chose zebrafish larvae to delineate the neurophenotype associated with preconditioning. Our results confirm that brief exposure to low levels of anesthetics enhances neuro-behavioral performance.
Nonetheless, our working model suggests the first event upon exposure is an injury and this injury may be exacerbated at higher levels (or prolonged exposure) leading to detrimental effects. We are currently investigating the threshold between active cytoprotection and permanent injury, due to the limitations of having only one concentration utilized in this study.
4. Conclusion
The complex zebrafish behaviors that were studied in this paper normally develop with age and therefore represent, in the TFE-exposed group, a pattern of accelerated maturation of neuronal function. These mature behaviors include the following: a greater preference for the dark and movement towards the startle (relative to control and developmental expectations), and schooling/shoaling social behaviors typical of older fish. This preconditioned neurophenotype is attributed to the sub-lethal proteotoxic injury by lower levels of anesthetics that evoke a neuroprotective stress-response, upregulating heat shock genes. This process not only protects against future neuronal cellular insult, but also creates a selective cognitive advantage.
Acknowledgements
Support for this project was in part from funding provided by the Office of Research and Sponsored Programs at Kansas City University of Medicine and Biosciences. The authors gratefully acknowledge the support and advice of Diana Baumann and staff at the Reptile &Aquatics Facility at Stowers Institute for Medical Research.
Cite this paper
Julie L.Mustard,Matthew A.Strope,Christopher S.Havey,Lucy S.Wang,Norbert W.Seidler, (2016) Neurophenotyping of Zebrafish Larvae that Are Preconditioned with Anesthetic Agent. Journal of Behavioral and Brain Science,06,99-106. doi: 10.4236/jbbs.2016.62011
References
- 1. Hudson, A.E. and Hemmings, H.C. (2011) Are Anaesthetics Toxic to the Brain? British Journal of Anaesthesia, 107, 30-37.
http://dx.doi.org/10.1093/bja/aer122 - 2. McClintick, C.A., Theisen, C.S., Ferns, J.E., Fibuch, E.E. and Seidler, N.W. (2011) Isoflurane Preconditioning Involves Upregulation of Molecular Chaperone Genes. Biochemical and Biophysical Research Communications, 411, 387-392.
http://dx.doi.org/10.1016/j.bbrc.2011.06.156 - 3. Ferns, J.E., Theisen, C.S., Fibuch, E.E. and Seidler, N.W. (2012) Protection against Protein Aggregation by Alpha-Crystallin as a Mechanism of Preconditioning. Neurochemical Research, 37, 244-252.
http://dx.doi.org/10.1007/s11064-011-0601-4 - 4. Pattin, A.E., Ochs, S., Theisen, C.S., Fibuch, E.E. and Seidler, N.W. (2010) Isoflurane’s Effect on Interfacial Dynamics in GAPDH Influences Methylglyoxal Reactivity. Archives of Biochemistry and Biophysics, 498, 7-12.
http://dx.doi.org/10.1016/j.abb.2010.04.001 - 5. Baker, M.R., Benton, S.K., Theisen, C.S., McClintick, C.A., Fibuch, E.E. and Seidler, N.W. (2011) Isoflurane’s Effect on Protein Conformation as a Proposed Mechanism for Preconditioning. Biochemistry Research International, 2011, Article ID: 739712.
http://dx.doi.org/10.1155/2011/739712 - 6. Orger, M.B., Kampff, A.R., Severi, K.E., Bollmann, J.H. and Engert, F. (2008) Control of Visually Guided Behavior by Distinct Populations of Spinal Projection Neurons. Nature Neuroscience, 11, 327-333.
http://dx.doi.org/10.1038/nn2048 - 7. Budick, S.A. and O’Malley, D.M. (2000) Locomotor Repertoire of the Larval Zebrafish: Swimming, Turning and Prey Capture. Journal of Experimental Biology, 203, 2565-2579.
- 8. Burgess, H.A., Schoch, H. and Granato, M. (2010) Distinct Retinal Pathways Drive Spatial Orientation Behaviors in Zebrafish Navigation. Current Biology, 20, 381-386.
http://dx.doi.org/10.1016/j.cub.2010.01.022 - 9. Fernandes, A.M., Fero, K., Arrenerg, A.B., Bergeron, S.A., Driever, W. and Burgess, H.A. (2012) Deep Brain Photoreceptors Control Light-Seeking Behavior in Zebrafish Larvae. Current Biology, 22, 2042-2047.
http://dx.doi.org/10.1016/j.cub.2012.08.016 - 10. Serra, E.L., Medalha, C.C. and Mattioli, R. (1999) Natural Preference of Zebrafish (Danio rerio) for a Dark Environment. Brazilian Journal of Medical and Biology Research, 32, 1551-1553.
http://dx.doi.org/10.1590/S0100-879X1999001200016 - 11. Maximino, C., Marques de Brito, T., Dias, C.A., Gouveia Jr., A. and Morato, S. (2010) Scototaxis as Anxiety-Like Behavior in Fish. Nature Protocols, 5, 209-216.
http://dx.doi.org/10.1038/nprot.2009.225 - 12. Crawley, J. and Goodwin, F.K. (1980) Preliminary Report of a Simple Animal Behavior Model for the Anxiolytic Effects of Benzodiazepines. Pharmacology Biochemistry and Behavior, 13, 167-170.
http://dx.doi.org/10.1016/0091-3057(80)90067-2 - 13. Eaton, R.C., Farley, R.D., Kimmel, C.B. and Schabtach, E. (1977) Functional Development in the Mauthner Cell System of Embryos and Larvae of the Zebra Fish. Journal of Neurobiology, 8, 151-172.
http://dx.doi.org/10.1002/neu.480080207 - 14. Pelkowski, S.D., Kapoor, M., Richendrfer, H.A., Wang, X., Colwill, R.M. and Creton, R. (2011) A Novel High- Throughput Imaging System for Automated Analyses of Avoidance Behavior in Zebrafish Larvae. Behavioural Brain Research, 223, 135-144.
http://dx.doi.org/10.1016/j.bbr.2011.04.033 - 15. Creton, R. (2009) Automated Analysis of Behavior in Zebrafish Larvae. Behavioural Brain Research, 203, 127-136.
http://dx.doi.org/10.1016/j.bbr.2009.04.030 - 16. Buske, C. and Gerlai, R. (2011) Shoaling Develops with Age in Zebrafish (Danio rerio). Progress in Neuro-Psycho-pharmacology & Biological Psychiatry, 35, 1409-1415.
http://dx.doi.org/10.1016/j.pnpbp.2010.09.003 - 17. Engeszer, R.E., Ryan, M.J. and Parichy, D.M. (2004) Learned Social Preference in Zebrafish. Current Biology, 14, 881-884.
http://dx.doi.org/10.1016/j.cub.2004.04.042 - 18. Umschweif, G., Alexandrovich, A.G., Trembovler, V., Horowitz, M. and Shohami, E. (2013) The Role and Dynamics of Β-Catenin in Precondition Induced Neuroprotection after Traumatic Brain Injury. PLoS ONE, 8, e76129.
http://dx.doi.org/10.1371/journal.pone.0076129 - 19. Sunyer, B., Diao, W.F., Kang, S.U., An, G., Boddul, S. and Lubec, G. (2008) Cognitive Enhancement by SGS742 in OF1 Mice Is Linked to Specific Hippocampal Protein Expression. Journal of Proteome Research, 7, 5237-5253.
http://dx.doi.org/10.1021/pr800594b - 20. Zhou, N.E., Kay, C.M. and Hodges, R.S. (1992) Synthetic Model Proteins. Positional Effects of Interchain Hydrophobic Interactions on Stability of Two-Stranded Alpha-Helical Coiled-Coils. The Journal of Biological Chemistry, 267, 2664-2670.
- 21. Craig, H.D., Eklund, J.D. and Seidler, N.W. (2008) Trifluoroethanol Increases Albumin’s Susceptibility to Chemical Modification. Archives of Biochemistry and Biophysics, 480, 11-16.
http://dx.doi.org/10.1016/j.abb.2008.09.009 - 22. Mehta, A.K. and Ticku, M.K. (1988) Ethanol Potentiation of Gabaergic Transmission in Cultured Spinal Cord Neurons Involves Gamma-Aminobutyric Acid-Gated Chloride Channels. The Journal of Pharmacology and Experimental Therapeutics, 246, 558-564.
- 23. Weight, F.F. (1992) Cellular and Molecular Physiology of Alcohol Actions in the Nervous System. International Review of Neurobiology, 33, 289-348.
http://dx.doi.org/10.1016/S0074-7742(08)60694-7 - 24. Peoples, R.W. and Weight, F.F. (1998) Inhibition of Excitatory Amino Acid-Activated Currents by Trichloroethanol and Trifluoroethanol in Mouse Hippocampal Neurons. British Journal of Pharmacology, 124, 1159-1164.
http://dx.doi.org/10.1038/sj.bjp.0701949 - 25. Amour, J., Brzezinska, A.K., Jager, Z., Sullivan, C., Weihrauch, D., Du, J., et al. (2010) Hyperglycemia Adversely Modulates Endothelial Nitric Oxide Synthase during Anesthetic Preconditioning through Tetrahydrobiopterin- and Heat Shock Protein 90-Mediated Mechanisms. Anesthesiology, 112, 576-585.
http://dx.doi.org/10.1097/ALN.0b013e3181cded1f
NOTES

*Corresponding author.