Advances in Chemical Engineering and Science
Vol.3 No.2(2013), Article ID:30497,5 pages DOI:10.4236/aces.2013.32017
Effect of Curing Poly(p-Phenylene Sulfide) on Thermal Properties and Crystalline Morphologies
Carbon Convergence Materials Research Center, Institute of Advanced Composites Materials, Korea Institute of Science and Technology, Wanju-gun, Republic of Korea
Email: *cnt@kist.re.kr
Copyright © 2013 Sungho Lee et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 10, 2013; revised February 10, 2013; accepted March 10, 2013
Keywords: Poly(p-Phenylene Sulfide); Thermal Curing; Non-Isothermal Crystallization; Cross-Linking; Crystalline Morphologies
ABSTRACT
Commercial poly(p-phenylene sulfide) (PPS) was thermally cured, which resulted in an increase of molecular weight due to cross-linking. Non-isothermal crystallization studies of samples cured for up to 7 days at 250˚C showed a monotonous increase of crystallization temperature compared to pure PPS. However, a further increase of curing time decreased the crystallization temperature. The change in the half-crystallization time (t1/2) was similar to the crystallization temperature. Thus, the cross-linking of PPS affected crystallization behaviors significantly. To a certain extent, crosslinks acted as nucleation agents, but excessive cross-linking hindered the crystallization. Morphologies observed by polarized optical microscopy suggested that thermal curing for as little as 1 day contributed to the spherulitic structure having a smaller size, that was not observed with pure PPS.
1. Introduction
High performance thermoplastics have been developed and commercialized to satisfy the increasing demand for polymeric materials with such properties as thermal stability, high strength and modulus, and chemical resistance. Poly(p-phenylene sulfide) (PPS) is one of the high performance polymers, having outstanding properties compared to conventional thermoplastics such as polyethylene and polypropylene [1]. Since Philips Petroleum Company commercialized PPS in 1967, the synthesis, processing, and applications have evolved to produce various grades of products with diverse properties [2-5].
One of the interesting characteristics of PPS is curability, which means that PPS undergoes chemical reactions on exposure to an oxygen-containing atmosphere at high temperatures, leading to an increase of viscosity due to cross-linking [6]. The extent of the viscosity increase is dependent on the temperature and time of such an exposure [6]. The virgin PPS is a linear material of molecular weight of about 20,000 - 25,000 g/mol, which is too low to be used as a molding resin, Therefore, an increase of molecular weight was necessary, and curability has been considered the most important characteristic in that the moderate molecular weight of virgin PPS could be conveniently increased by a post curing process after polymerization [7]. Ma et al. reported the curing mechanism of PPS using various analytical techniques, such as FT-IR, solid-state 13C-NMR, and rheometric measurements [8]. They concluded that the two most important reactions in the melt cure of PPS were cross-linking and chain extension and that the distribution of molecular weight became broader and shifted toward higher molecular weight with increasing cure time [8].
The change of the internal structure of PPS that results from the curing reactions inevitably causes a change in the crystallization behavior, which is closely related to the morphology developed during processing. In this paper, the effect of the curing of PPS on its thermal properties and crystalline morphologies was studied. A commercial PPS resin was thermally cured from 1 to 16 days at 250˚C. To investigate the change of molecular weight during curing, the intrinsic viscosities of as-received and cured PPS were measured. Non-isothermal crystallization kinetics were investigated by differential scanning calorimetry (DSC). Furthermore, polarized optical microscopy (POM) was used to observe the morphologies of pure and cured PPS.
2. Experimental
2.1. Materials
PPS powders (W316 grade, density and molecular weight of 1.35 g/cm3 and 35,000 g/mol, respectively) supplied by Kureha Chemical Industry Co., Ltd (Japan) were used for this study. The PPS powders were encased in a 20 mL vial and then the samples were cured at 250˚C for 1 to 16 days in an air atmosphere.
2.2. Characterization
The intrinsic viscosities of pure and cured PPS were obtained using a Brookfield viscometer with a Thermosel system and a S18 spindle (DV-II + Pro, Brookfield Co., UK). Samples were dissolved in 1-chloro naphthalene for preparing 4 wt% polymer solutions at 210˚C. The intrinsic viscosities were calculated using the Solomon-Ciuta relation as follows [9]:
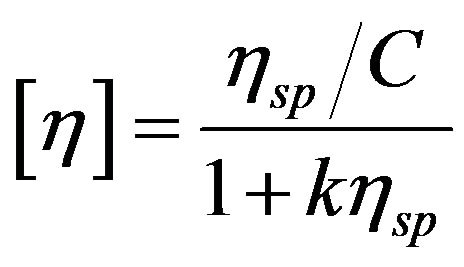
where k is 0.302 for PPS as a material property, C is the concentration of polymer in 1-chloro naphthalene, [η] is the intrinsic viscosity, and ηsp is the specific viscosity. The viscosity-average molecular weight was calculated using the Mark-Houwink-Sakurada (MHS) equation as follows:
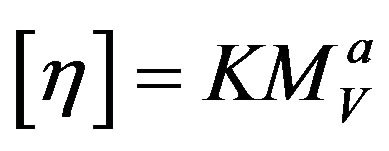
where K and a are 8.91 × 10−5 and 0.747 for PPS, respectively [9].
Thermal analysis was performed in a differential scanning calorimeter (DSC Q20, TA Instruments, USA). Samples were heated up to 350˚C at a rate of 10˚C/min and held at that temperature for 10 min to erase the thermal histories of the samples. Subsequently, samples were cooled down to 50˚C at a rate of 10˚C/min and then heated up to 350˚C again at a rate of 10˚C/min to study the effect of curing on the non-isothermal crystallization. The crystalline morphologies were examined in an Olympus BX-60 optical microscope (Olympus, Japan) with cross-polarizers. The samples were loaded on a hot stage (Mettler Toledo FP82HT, USA), and heated up to 350˚C at a rate of 10˚C/min. Samples were squeezed to be a thin film with holding at 350˚C for 10 min. Then, samples were cooled down to 50˚C at a rate of 10˚C/min, and the crystalline morphologies of the samples observed.
3. Results and Discussion
3.1. Molecular Weight
The intrinsic viscosities of as-received and thermally cured PPS at 250˚C were obtained using the SolomonCiuta relation. Since samples cured for more than 7 days did not dissolve in 1-chloro naphthalene, intrinsic viscosities [η] of pure samples and samples cured for 1, 3, or 5 days were measured. Note that the curing took place below the melting temperature (280˚C from the manufacturer), so that a solid-state cure was applied in this study. Figure 1 displays the viscosity-average molecular weights, which were calculated using the Mark-Houwink-Sakurada (MHS) equation. As-received PPS showed a viscosity-average molecular weight of ~28,000 g/mol. The 1 day-cured sample revealed a significant increase in the viscosity-average molecular weight (~40,000 g/mol), which increased up to ~58,000 g/mol with a further increase of curing time (5 days). Thus, as expected, the thermal curing led to an increase of molecular weight. It is known that PPS has three different types of chain structure, linear, branched, and cross-linked, depending on the conditions of polymerization and thermal treatment [10]. The cross-linking structure in cured PPS has been investigated; it involves chain extension, thermal cross-linking, and oxidative cross-linking (Scheme 1) [10]. In our system, it is evident that a cross-linking structure was formed by thermal curing in the presence of oxygen.
3.2. Non-Isothermal Crystallization Behavior
The crystallization behavior of pure and cured PPS was investigated using non-isothermal DSC (Figure 2). In the
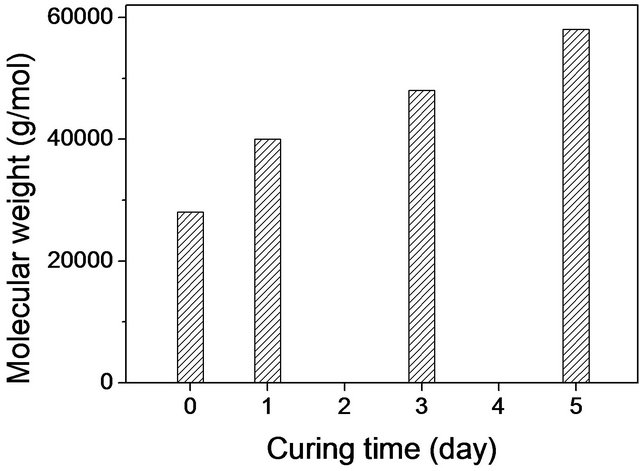
Figure 1. Viscosity-average molecular weights of pure PPS and samples cured for up to 5 days.
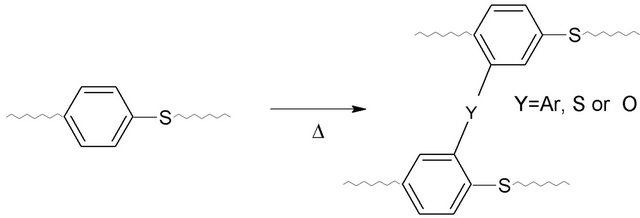
Scheme 1. Crosslinking mechanism of PPS during the curing process.
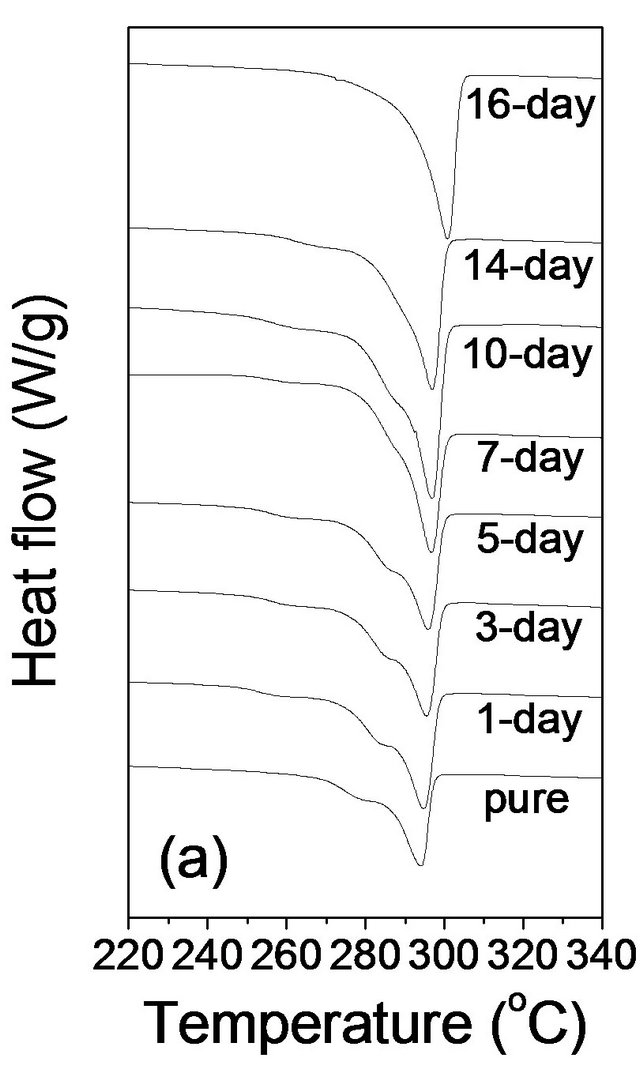
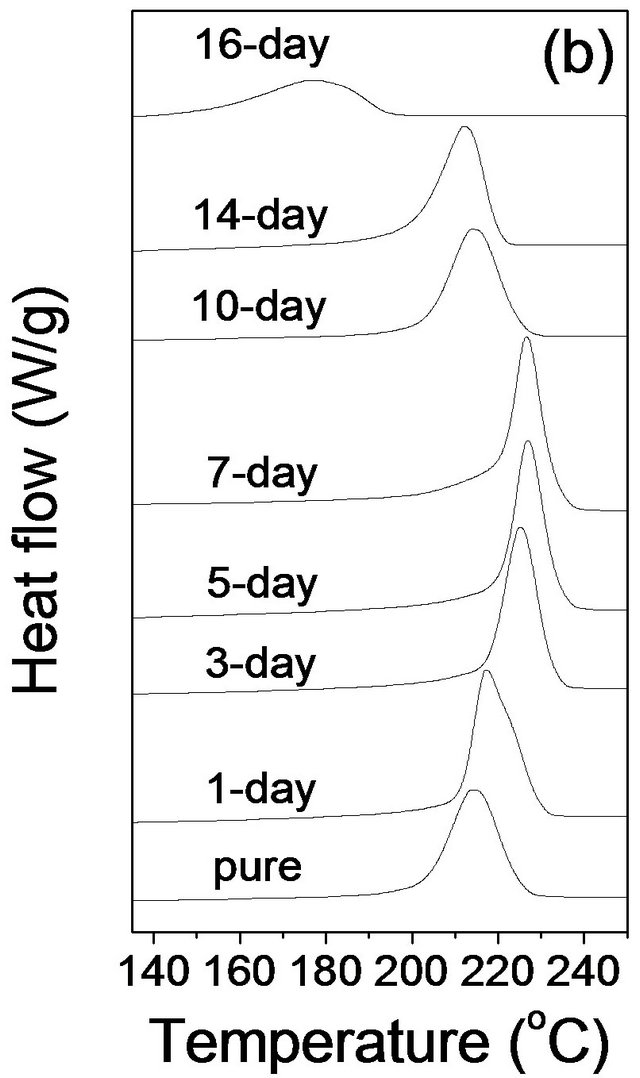
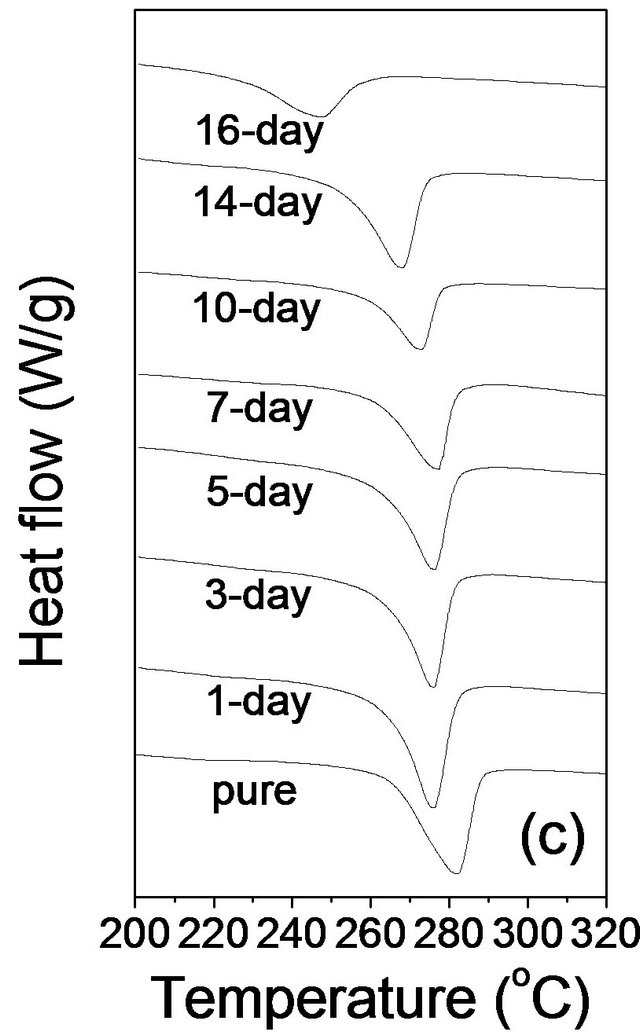
Figure 2. DSC thermograms of pure PPS and cured samples in (a) the first heating, (b) the first cooling, and (c) the second heating scan at a rate of 10˚C/min.
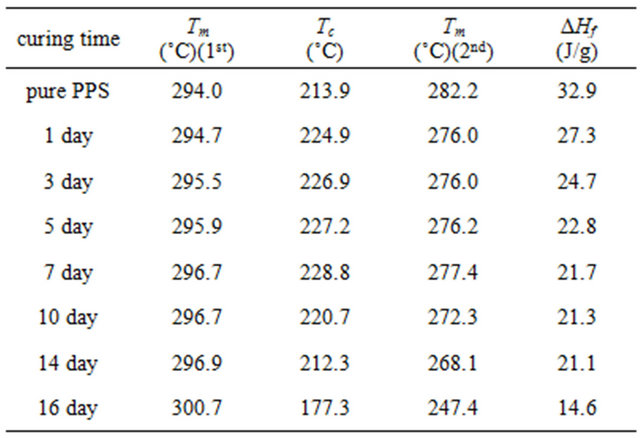
first heating scan curve in Figure 2(a), pure PPS showed a shoulder at ~280˚C and the main melting temperature (Tm) with a heating rate of 10˚C/min was found to be at 294.0˚C. A significant change of Tm was not observed for the 1 day cured PPS (294.7˚C). However, the shoulder shifted to ~283˚C. It is interesting that another weak shoulder, at ~260˚C, was observed only for the cured PPS samples; it could be the result of re-crystallization during solid-state curing in the oven at 250˚C. This shoulder and the main Tm did not change for PPS samples cured for up to 14 days. The sample cured for 16 days showed Tm of 300.7˚C without any shoulder.
The PPS samples were held at 350˚C for 10 min after the first heating scan to remove the prior thermal history and then slowly cooled at 10˚C/min. In the first cooling, the crystallization temperature (Tc) of pure PPS was 213.9˚C. There was a significant increase of Tc for the 1 day cured sample (224.9˚C), and then a continuous further increase for samples cured for up to 7 days (228.8˚C). It is interesting to note that the further curing resulted in a decrease of crystallization temperature with the 16 days cured sample showing a Tc of 177.3˚C. It is likely that the curing induced cross-linking structure led to a higher Tc in the system because cross-linking structure acted as a nucleating agent. However, the further increase of curing time beyond 7 days caused the cross-linking structure to become dominant, which would hinder the formation of a crystalline structure.
Inoue and Suzuki described the effects of the crosslinking of ethylene-propylene-diene terpolymer (EPDM) particles on the crystallization of the polypropylene (PP) matrix [11]. The cross-linked EPDM/PP composites showed higher crystallization temperatures for the PP than occurred in the uncross-linked EPDM/PP composites, indicating that cross-linked EPDM played the role of a nucleating agent. More interestingly, a further increase in cross-linked EPDM content led to a decrease in the crystallization temperature of PP. We suggest that the cross-linking of PPS similarly acted as nucleating agents to change the crystallization behaviors of uncross-linked PPS.
The second heating scans were also obtained to investigate the melting behavior after the first controlled cooling in DSC because the first heating scans reflect the thermal history experienced during the thermal curing and initial processing. The second heating scans are presented in Figure 2(c), and the measured transition temperatures (Tm) and heats of fusion (∆Hf) are summarized in Table 1. In contrast to the first heating scans, only one melting peak was observed with all samples, which is evidence that the first melting peaks reflected the thermal history including the processing and curing. The melting peak in the second heating scan for pure PPS was found to be 282.2˚C. The 1 day cured sample showed a melting peak of 276.0˚C, 6.2˚C lower than that of pure PPS, and there was no significant difference of Tm in the second heating scans up to the 7 days curing sample. However, the values decreased with a further increase of curing time. From the second heating scans, the crystallinities of
Table 1. Transition temperatures (in the 1st heating, 1st cooling, and 2nd heating) and heat of fusion of pure PPS and cured samples in the second heating scans at a cooling rate of 10˚C/min and a heating rate of 10˚C/min.
the samples were determined by dividing ∆Hf of crystallization by the 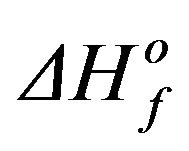 value of 80.4 J/g for PPS [12]; the values were found to be 40.9%, 34.0%, 28.4%, 21.3%, and 14.6% for pure PPS and samples cured for 1, 5, 10, and 16 days, respectively. It is evident that the crystallinity decreased as the curing time increased, presumably as a result of the cross-linking structure.
value of 80.4 J/g for PPS [12]; the values were found to be 40.9%, 34.0%, 28.4%, 21.3%, and 14.6% for pure PPS and samples cured for 1, 5, 10, and 16 days, respectively. It is evident that the crystallinity decreased as the curing time increased, presumably as a result of the cross-linking structure.
The non-isothermal crystallization kinetics of pure PPS and the cured samples were analyzed. The relative degree of crystallinity, X, at crystallization time t can be obtained by the following equation:
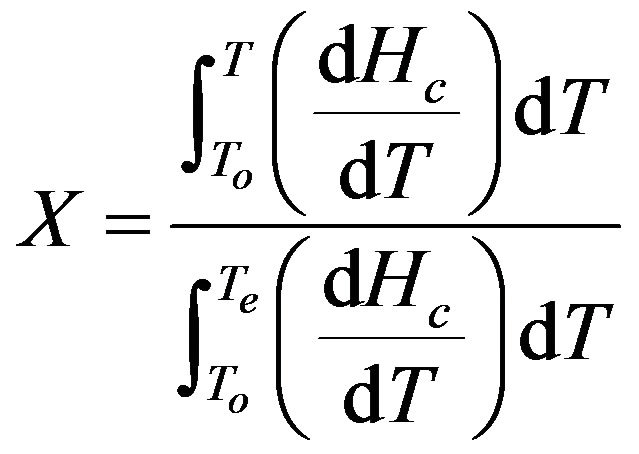
where To and Te are the onset and end crystallization temperatures, respectively, and dHc/dT is the heat flow rate. In non-isothermal crystallization, the time t is related with the temperature T as follows [13,14]:
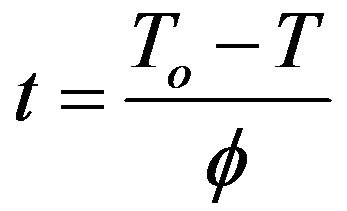
where T is the temperature at time t, To is the temperature at which the crystallization begins (t = 0) and ![]() is the cooling rate. Using the above two equations, the X vs. t plots of pure PPS and the cured samples in Figure 2(b) are shown in Figure 3. The half-crystallization times (t1/2) calculated from the X vs. t plots such as those in Figure 3 are summarized in Table 2. It was apparent for the pure samples and those cured for up to 7 days that a higher curing time resulted in less time being needed to reach a given relative degree of crystallinity, indicating a faster crystallization process. However, a further increase of curing time decreased t1/2. From these results, it is noteworthy that even though thermally induced crosslinking led to a faster crystallization, excessive crosslink-ing hindered the crystallization, as was observed in the first cooling thermograms (Figure 2(b)).
is the cooling rate. Using the above two equations, the X vs. t plots of pure PPS and the cured samples in Figure 2(b) are shown in Figure 3. The half-crystallization times (t1/2) calculated from the X vs. t plots such as those in Figure 3 are summarized in Table 2. It was apparent for the pure samples and those cured for up to 7 days that a higher curing time resulted in less time being needed to reach a given relative degree of crystallinity, indicating a faster crystallization process. However, a further increase of curing time decreased t1/2. From these results, it is noteworthy that even though thermally induced crosslinking led to a faster crystallization, excessive crosslink-ing hindered the crystallization, as was observed in the first cooling thermograms (Figure 2(b)).
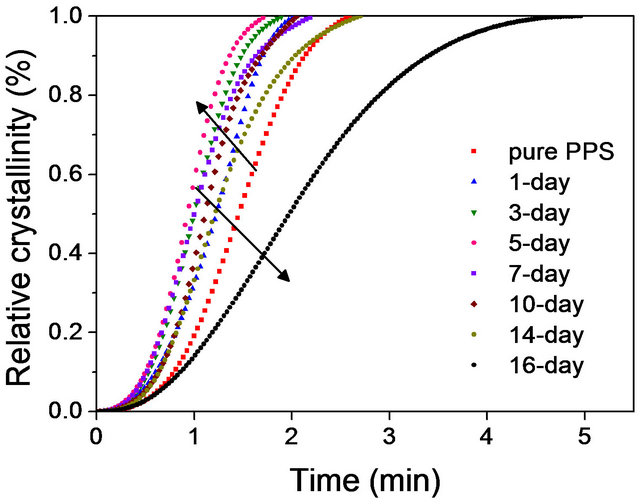
Figure 3. Relative crystallinity with time for non-isothermal crystallization of pure PPS and cured samples at a cooling rate of 10˚C/min.
Table 2. The half-time of crystallization (t1/2) of pure PPS and cured samples at a cooling rate of 10˚C/min.
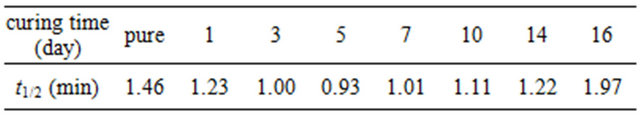
3.3. Polarized Optical Microscopy
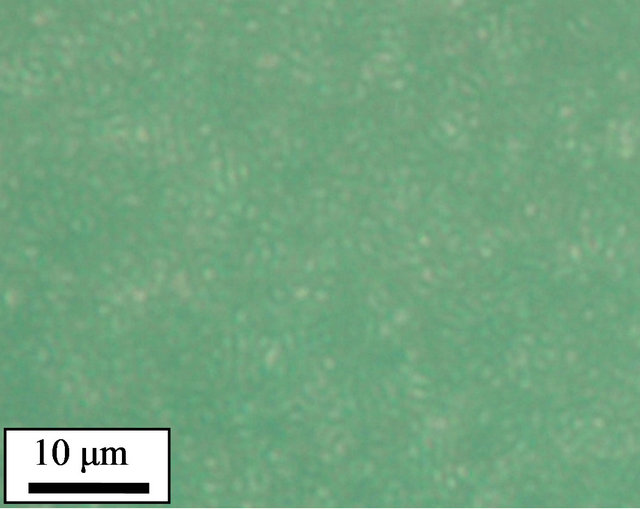
The crystalline morphologies of pure and cured PPS were observed using polarized optical microscopy (POM). To mimic the thermal history of the non-isothermal study in DSC, the samples were loaded on a hot stage and heated up to 350˚C at a rate of 10˚C/min before being held at 350˚C for 10 min. Subsequently, the samples were cooled down to 50˚C at a rate of 10˚C/min, and the crystalline morphologies of the samples observed. The pure PPS (Figure 4(a)) showed spherulites whose size was 40 - 50 μm. However, the 1 - 5 days cured samples did not show clear spherulites. Instead, a large number of small crystals were observed (Figures 4(b)-(d)). It was reported that cross-linked EPDM particles acting as nucleating agents resulted in a decrease in the dimensions of PP spherulites [11]. The POM results for our system confirmed that cross-linked PPS induced by thermal curing also acted as a nucleating agent to decrease the crystalline size of PPS.
4. Conclusion
Commercial PPS was thermally cured, resulting in an increase of its molecular weight due to cross-linking as compared to the original sample; the viscosity-average molecular weight of samples cured for 5 days was ~58,000 g/mol. The crystallization temperature (Tc) of pure PPS was found to be 213.9˚C by non-isothermal DSC studies. The values of Tc increased monotonously for samples cured for up to 7 days (228.8˚C), but a further increase in curing time up to 16 days led to a decrease of Tc down to 177.3˚C. In addition, the change of the half-crystallization time (t1/2) was similar to that of the crystallization temperature. Pure PPS, and samples cured for 7 days and 16 days, showed half-crystallization times of 1.46, 1.01, and 1.97 min, respectively. Thus, the cross-linking of PPS affected the crystallization behaviors significantly; a certain amount of cross-linking structure acted as a nucleation agent, but excessive cross-linked hindered the crystallization. Crystalline morphologies observed by polarized optical microscopy suggested that thermal curing for as little as 1 day contributed to the formation of smalller size crystalline structures, that the larger spherulites were not observed for pure PPS.
5. Acknowledgements
This work was supported by a grant from Korea Institute
 (a)
(a)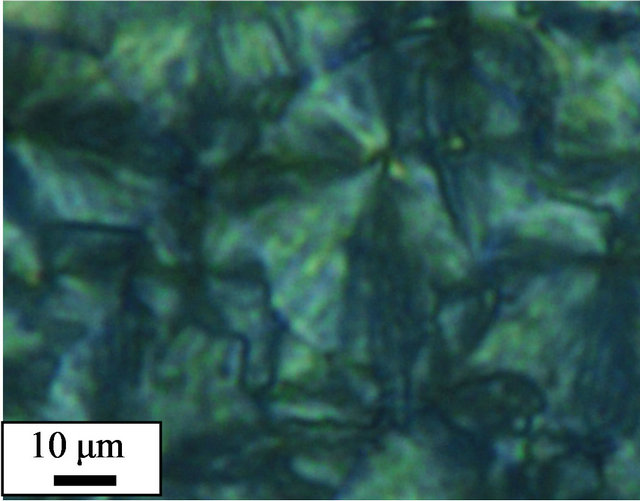 (b)
(b)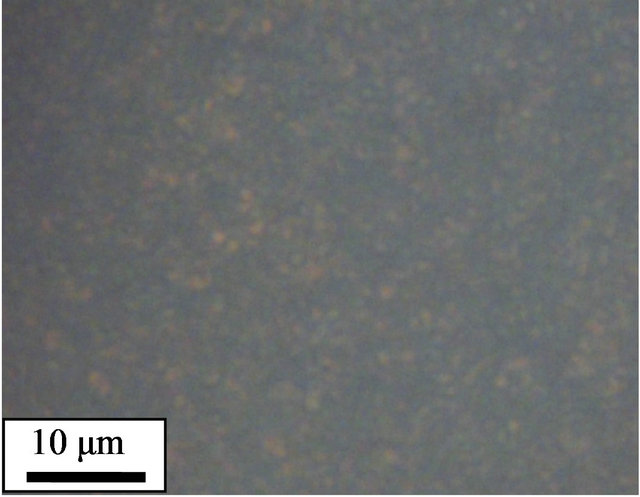 (a)
(a)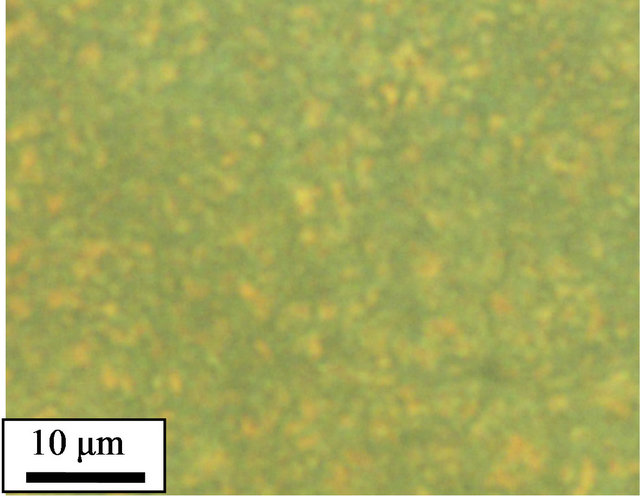 (b)
(b)
Figure 4. Polarized optical micrographs of (a) pure PPS, and samples cured for (b) 1 day, (c) 3 days, and (d) 5 days.
of Science and Technology Institutional program and the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Knowledge Economy, Republic of Korea.
REFERENCES
- J. K. Fink, “5-Poly(phenylene sulfide),” In: J. K. Fink, Ed., High Performance Polymers, William Andrew Inc., Burlington, 2008, pp. 175-207. doi:10.1016/B978-081551580-7.50006-7
- J. T. Edmond and H. W. Hill Jr., “Production of Polymers from Aromatic Compounds,” US Patent 3354129, 1967.
- L. C. Lopez and G. L. Wilkes, “Poly(p-Phenylene Sulfide) —An Overview of an Important Engineering Thermoplastic,” Journal of Macromolecular Science Part C: Polymer Reviews, Vol. 29, No. 1, 1989, pp. 83-151. doi:10.1080/07366578908055165
- H. W. Hill Jr. and D. G. Brady, “High Performance Polymers and Composites,” J. I. Kroschwitz, Ed., John Wiley & Sons, New York, 1991, p. 551.
- P. Cebe, “Review of Recent Developments in Poly(phenylene Sulphide),” Polymer & Polymer Composites, Vol. 3, 1995, p. 239.
- H. W. Hill Jr. and D. G. Brady, “Properties, Environmental Stability, and molding Characteristics of Polyphenylene Sulfide,” Polymer Engineering & Science, Vol. 16, No. 12, 1976, pp. 831-835. doi:10.1002/pen.760161211
- D. G. Brady, “The Crystallinity of Poly(phenylene sulfide) and Its Effect on Polymer Properties,” Journal of Applied Polymer Science, Vol. 20, No. 9, 1976, pp. 2541-2551. doi:10.1002/app.1976.070200921
- C. M. Ma, L. Hsiue, W. Wu and W. Liu, “Rheological and Morphological Properties of Thermal-Aged Poly(phenylene Sulfide) Resin,” Journal of Applied Polymer Science, Vol. 39, No. 6, 1990, pp. 1399-1415. doi:10.1002/app.1990.070390615
- C. J. Stacy, “Molecular Weight Distribution of Polyphenylene Sulfide by High Temperature Gel Permeation Chromatography,” Journal of Applied Polymer Science, Vol. 32, No. 3, 1986, pp. 3959-3969. doi:10.1002/app.1986.070320314
- R. T. Hawkins, “Chemistry of the Cure of Poly(p-phenylene Sulfide),” Macromolecules, Vol. 9, No. 2, 1976, pp. 189-194. doi:10.1021/ma60050a001
- T. Inoue and T. Suzuki, “Selective Crosslinking Reaction in Polymer Blends. III. The Effects of the Crosslinking of Dispersed EPDM Particles on the Impact Behavior of PP/EPDM Blends,” Journal of Applied Polymer Science, Vol. 56, No. 9, 1995, pp. 1113-1125. doi:10.1002/app.1995.070560911
- D. G. Brady, “The Crystallinity of Poly(phenylene Sulfide) and Its Effect on Polymer Properties,” Journal of Applied Polymer Science, Vol. 20, No. 9, 1976, pp. 2541- 2551. doi:10.1002/app.1976.070200921
- P. Cebe, “Non-Isothermal Crystallization of Poly(ether Etherketone) Aromatic Polymer Composite,” Polymer Composites, Vol. 9, No. 4, 1988, pp. 271-279. doi:10.1002/pc.750090405
- C. R. Herrero and J. L. Acosta, “Effect of Poly(epichlorhydrin) on the Crystallization and Compatibility Behavior of Poly(ethylene oxide)/Polyphosphazene Blends,” Polymer Journal, Vol. 26, 1994, pp. 786- 796. doi:10.1295/polymj.26.786
NOTES
*Corresponding author.

