Journal of Biomaterials and Nanobiotechnology
Vol.06 No.04(2015), Article ID:60432,8 pages
10.4236/jbnb.2015.649025
Synthesis and Characterization of Fe3O4 Coated on APTES as Carriers for Morin-Anticancer Drug
Bassam Saif1, Congli Wang1, Dong Chuan2, Shaomin Shuang1
1School of Chemistry and Chemical Engineering, Shanxi University, Taiyuan, China
2Research Center of Environmental Science and Engineering, Shanxi University, Taiyuan, China
Email: smshuang@sxu.edu.cn
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 August 2015; accepted 18 October 2015; published 21 October 2015
ABSTRACT
Morin (MR) is an anticancer drug present in fruits and Chinese herbs. Fe3O4 magnetic nanoparticles (MNPs) coated on 3-aminopropyl triethoxysilane (APTES) were synthesized (MNPs-APTES) as carriers for MR. The characterization of drug delivery system was confirmed by Fourier Transform Infrared (FTIR), Transmission Electron Microscope (TEM), X-Ray Diffraction (XRD), dynamic light scattering (DLS), and vibrating sample magnetometer (VSM). The adsorbed APTES on the magnetite surface (MNPs-APTES) was examined by FTIR. The TEM image showed that the average particle size is obtained to be about 26.7 nm for MNPs-APTES. The MR loading and release behavior of MNPs-APTES were studied and the results showed that up to 60% of the adsorbed drug was released within 4 h. In summary, the MNPs-APTES nanocarriers are based on the results, promising for targeted morin drug delivery.
Keywords:
Magnetic Nanoparticles, Drug Delivery, Flavonoid, Morin

1. Introduction
With dramatic progress in biomaterials and biomedical applications, huge amount of new and more promising drugs are discovered and designed. Scientists and researchers are now trying to develop mechanisms that allow drugs to go directly to a targeted area of the human body. The success of chemotherapy depends on the drug, its dosage, as well as how it is delivered to its target [1] . Nowadays, nanoparticles (NPs) such as Fe3O4 magnetic nanoparticles (MNPs) have gained significant attention due to their potential biomedical applications such as immunoassay [2] , magnetic resonance imaging [3] , bioseparators [4] and targeted drug delivery via applying an external magnetic field [5] . At the same time, MNPs also have attractive properties, such as biocompatibility [6] and low toxicity [7] . In addition, magnetic nanoparticles are easily obtained via various techniques such as micro-emulsion, ultra sound irradiation technology, and co-precipitation [8] [9] . Fe3O4 core serves as a carrier for magnetic targeting, while silica coating such as 3-aminopropyltriethoxysilane (APTES) on the Fe3O4 NPs offers sites for further modifications [10] . This APTES can bind to MNPs by adsorption or covalent bonding (MNPs- APTES), and through the active amino group in its structure it is able to combine with anti-cancer drug morin (MR). Morin (3,5,7,2’,4’-pentahydroxyflavone, Figure 1), is a member of the flavonoids groups that has been reported as an important agent effective chemotherapeutic used for the treatment of cancer [11] . Furthermore, numerous reports suggest that morin has a wide range of therapeutic applications such as anti-inflammation [12] , antioxidant [13] , it induces apoptosis in hepatocellular carcinogenesis model [14] , and xanthine oxidase inhibition activity [15] . In this study, Fe3O4 magnetic nanoparticles were fabricated by co-precipitation method and coated with APTES. Then, this MNPs-APTES were used as drug carriers. FTIR, TEM, XRD, DLS and VSM were used to characterize the synthesized nanoparticles. MR drug is loaded onto it and its loading and in vitro drug release profile was studied.
2. Experimental
2.1. Chemicals
3-aminopropyltriethoxysilane (APTES) was obtained from Aladdin Company Inc. Ferric chloride hexa-hydrate (FeCl3∙6H2O) and ferrous chloride tetrahydrate (FeCl2∙4H2O) were purchased from Tianjin Guangfu Fine Chemical Industry Research Institute. Dimethylsulfoxide (DMSO) was purchased from Shanghai Chemical Reagent Co. Ltd. and morin was purchased from Tianjin Fuyu Fine Chemical Co. Ltd. Deionized water was used in all of the experiments.
2.2. Preparation of Fe3O4 Nanoparticles (MNPs)
The co-precipitation method was used for the synthesis of Fe3O4 nanoparticles [16] . In the typical experimental procedure, 8.11 g of iron (III) chloride hexahydrate (FeCl3∙6H2O), 5.96 g of iron (II) chloride tetrahydrate (FeCl2∙4H2O) were added into three-necked flask contains 200 mL deionized water previously heated to 85˚C. The system was purged with N2 and the mixture of the iron solution was stirred followed by the slow addition of ammonia (NH3∙H2O) to bring the pH 9. During the reaction process, the temperature was maintained at 85˚C and (pH 9). The precipitate was washed several times with deionized water and ethanol. The as-prepared Fe3O4 powder was obtained after 24 h drying in vacuum condition at 40˚C. This can be explained as:
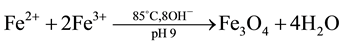
2.3. Preparation of APTES Coated MNPs (MNPs-APTES)
APTES coated MNPs were synthesized using Cao et al. method [17] . Briefly, 0.3 g of Fe3O4 was dispersed into a mixture of 4 mL deionized water and 600 mL absolute ethanol by ultrasonic vibration for 30 min. Then, 1.2
Figure 1. Chemical structure of morin.
mL of APTES was added into the mixture under constant mechanical stirring for 7 h. The resulting functionalized MNPs-APTES nanoparticles were isolated by a magnet, then washed with ethanol and dried at 40˚C under vacuum for 24 h. The schematic representation of the preparation procedure of MNPs-APTES is illustrated in Scheme 1.
2.4. Morin (MR) Drug Loading and Release Studies
The drug loading was measured using UV-V is spectrophotometry. The anti-cancer drug (MR) loading was carried out by dispersing 50 mg of magnetic nanoparticles coated APTES in (5 mL DMSO with 20 mL ethanol) solution containing MR (12 - 81 mg/mL). The solutions stirring at room temperature for 24 h allow partitioning of the drug into the MNPs-APTS. Then, the particles were magnetically separated from the solution by an external magnet, and MR content in the solution was determined at 388 nm. The drug loading was determined as the difference between the initial MR concentration and the MR concentration in the supernatant. The drug loaded magnetic nanoparticles were then magnetically separated and dried. The MR release study was obtained by investigated the dried drug loaded nanoparticles (10 mg) in 250 mL PBS (pH 5.2 and 7.4) at 37˚C for 4 h under stirring. At given time intervals, 4 mL samples were withdrawn from the incubation medium and the amount of drug release was estimated at 388 nm by UV-Vis spectrophotometry.
2.5. Characterization
The Fourier transformed infrared spectroscopy (FTIR, 8400 S Shimadzu) spectra were obtained using the KBr
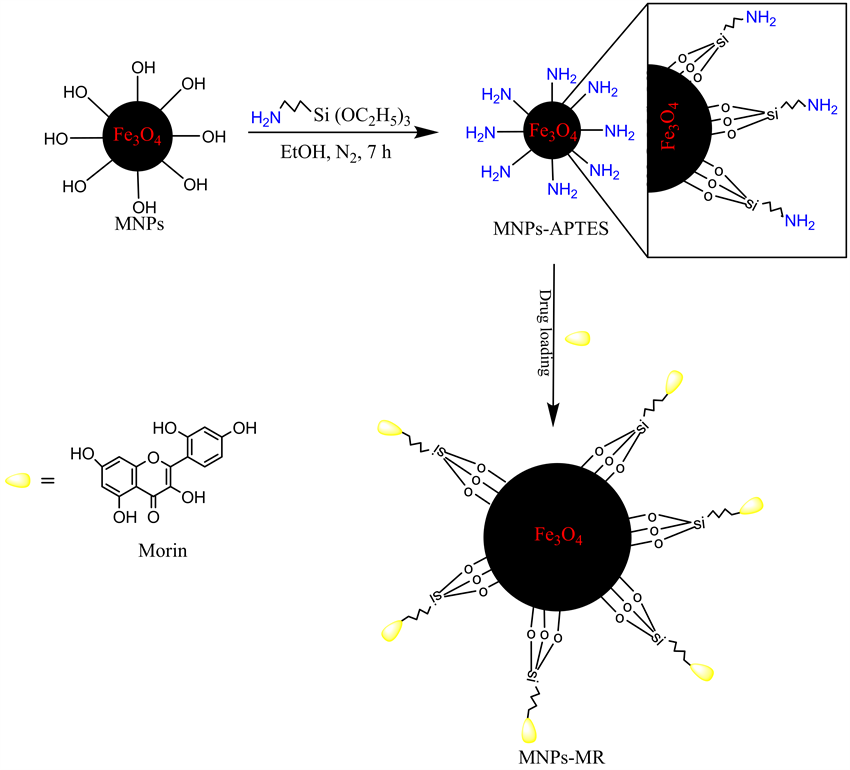
Scheme 1
pellet method. The particle size and morphology of the magnetic nanoparticles (MNPs and MNPs-APTES) were determined by transmission electron microscopy (TEM, JEM-1011). Magnetic nanoparticles were also analyzed by X-Ray diffraction (XRD, Rigaku D max-2400). The hydrodynamic diameter measured by dynamic light scattering (DLS, Nano-ZS 90) for MNPs-APTES. Finally, the magnetic properties were investigated with vibrating sample magnetometer (VSM, Lakeshore).
3. Results and Discussion
3.1. FTIR Studies
FT-IR spectra of MNP, APTES-MNP and morin binded on APTES-MNP are shown in Figure 2. The strong absorption at 584 cm−1 in curves a, b and c are attributed to the stretch of Fe-O [18] , the peak at 3425 cm−1 is attributed to the stretching vibrations of OH adsorbed on the surface of the Fe3O4 nanoparticle (Figure 2(a)). APTES is absorbed on the magnetite nanoparticles surfaces by Fe-O-Si bands, the coating of APTES is established by the presence of stretching vibration of CH2 bonds on aminopropyl group appeared at 2922 and 2850 cm−1 which confirmed the binding of APTES molecules at the surface of magnetite (APTES-MNP) [19] , and the bands around 1089 and 1193 cm−1 corresponded to the presence of SiO-H and Si-O-Si groups in Figure 2(b). [17] . Additionally, the peaks at 1623 and 3403 cm−1 showed the presence of the N-H stretching vibrations and bending mode of free-NH2 group, respectively. In Figure 2(c), the appearance of new bands at 1353, 1451, 1506, 1606, 1653, 3475 and 3296 cm−1 in the FTIR graph indicates the presence of morin. A band appeared at 1228 cm−1 due to the formation of C-N bond between NH2 group in APTES and OH group present on the morin with the elimination of water.
3.2. TEM Studies
The morphology of the MNPs and MNPs-APTES were characterized using a transmission electron microscope (TEM) images shown in Figure 3. Figure 3(a) showed that the MNPs possess spherical morphology with an average diameter of about 14.2 nm in size. After surface coating with APTES, the TEM micrograph on Figure 3(b) clearly observed the successful synthesis of MNPs-APTES and, the particle size was around 26.7 nm. In addition, the particle size of MNPs-APTES was obtained as 44 nm using dynamic light scattering (DLS)
Figure 2. FTIR spectra of (a) MNPs, (b) MNPs-APTES, and (c) MNPs-MR.
Figure 3. TEM of MNPs (a) and MNPs-APTES (b).
measurements.
3.3. XRD Studies
Figure 4 shows the XRD patterns of MNPs and MNPs-APTES. The diffraction peaks at (2θ = 30.29˚, 35.69˚, 43.30˚, 53.69˚, 57. 38˚, 64.8˚ and 74.58˚) which corresponds to (220), (311), (400), (422), (511), (400), and (533) can be easilyindexed to a cubic spinel structure of magnetite according to the standard XRD pattern of MNPs (JPCDS card, file No. 77 - 1545) [20] . It was also an evident that the APTES coating of the MNPs did not lead to a phase change of MNPs. The average crystallite size of MNPs-APTES is estimated about 30 nm according to linewidth of the (311) plane refraction peak using Scherrer’s formula, D = (0.94λ/B cosθ)
Where D is the average crystalline diameter, 0.94 is the Scherrer constant, λ the X-ray wavelength (λ = 0.154 nm), B is half width of XRD diffraction lines and θ is the Bragg’s angle in degree. Accordingly, the average crystallite size was in good agreement with the TEM analysis.
3.4. Magnetic Measurements
Figure 5 shows the magnetization curve of MNPs-APTES at room temperature. The S-shape of MNPs-APTES exhibited zero coercivity and permanence indicating its superparamagnetism with a saturation magnetization (Ms) value 41.5 emu/g, which can be ascribed to the existence of APTES on surface of MNPs. Therefore, This MNPs-APTES having magnetic response could be carry drugs to targeted locations under an external magnetic field. The determined magnetic separation time is about 15 s.
Figure 4. XRD of MNPs (a) and MNPs-APTES (b).
Figure 5. Magnetisation curve of MNPs-APTES at room temperature.
3.5. Drug Loading and Release Studies
The drug loading and release behavior of MNPs-APTES are shown in Figure 6(a) and Figure 6(b). In Figure 6(a) loading profile, the percentage of loaded MR increased and reached the maximal value of 48.7% for 78 mg/mL MR. This due to the conjugated of hydroxyl group in MR to the active amino group in the surface of MNPs-APTES (Figure 1). The drug released profile of MR from MNPs-APTES was studied in PBS with a pH of (5.2 and 7.4) at 37˚C, respectively. As seen in Figure 6(b), approximately 68.2% MR was released from MNPs-APTES within 4 h at pH 5.2 whereas, 19.6% of drug was released at pH 7.4. Thus, the drug release at
Figure 6. Morin loading on MNPs-APTES (a) and morin release from MNPs-APTES (b).
pH 5.2 was much faster than that at pH 7.4. It may due to the protonation of amine groups under this environment.
4. Conclusion
The novelty of this study is the successful binding of MR to MNPs-APTES. Characterization of the drug delivery system was carried out by FTIR, TEM, XRD, DLS, and VSM techniques. Spherical iron oxide nanoparticles were synthesized by co-precipitation technique and coated with APTES solution. The TEM analysis revealed that the average particle size increased to 26.7 nm after APTES coating. Moreover, the mean particle size of MNPs-APTES obtained from DLS was larger than that obtained from TEM analysis. The drug loading and release behavior of MNPs-APTES showed that the maximal value of the drug loading capacity was approximately 48.7% and the in vitro release behavior of drug presented that 68.2% and 19.6% were released in acidic and neutral buffered solutions at pH (5.2 and 7.4), respectively within 4 h. Thus, MNPs-APTES can be used as a potential carrier targeted morin anticancer drug delivery applications.
Acknowledgements
The authors would like to thank the financial support of the National Natural Foundations of China (No.21175086 and No.21175087), School of International Education and Exchange and Shanxi University.
Cite this paper
BassamSaif,CongliWang,DongChuan,ShaominShuang, (2015) Synthesis and Characterization of Fe3O4 Coated on APTES as Carriers for Morin-Anticancer Drug. Journal of Biomaterials and Nanobiotechnology,06,267-275. doi: 10.4236/jbnb.2015.649025
References
- 1. Manju, S., Sharma, C.P. and Sreenivasan, K. (2011) Targeted Coadministration of Sparingly Soluble Paclitaxel and Curcumin into Cancer Cells by Surface Engineered Magnetic Nanoparticles. Journal of Materials Chemistry, 21, 15708-15717. http://dx.doi.org/10.1039/c1jm12528a
- 2. Iida, H., Takayanagi, K., Nakanishi, T. and Osaka, T. (2007) Synthesis of Fe3O4 Nanoparticles with Various Sizes and Magnetic Properties by Controlled Hydrolysis. Journal of Colloid and Interface Science, 314, 274-280. http://dx.doi.org/10.1016/j.jcis.2007.05.047
- 3. Weissleder, R., Bogdanov, A., Neuwelt, E. and Papisov, M. (1995) Long Circulating Iron Oxides for MR Imaging. Advanced Drug Delivery Reviews, 16, 321-334. http://dx.doi.org/10.1016/0169-409X(95)00033-4
- 4. Jain, T.K., Roy, I., De, T.K. and Maitra, A. (1998) Na-nometer Silica Particles Encapsulating Active Compounds: A Novel Ceramic Drug Carrier. Journal of the American Chemical Society, 120, 11092-11095. http://dx.doi.org/10.1021/ja973849x
- 5. Sang, Q., Luan, L., Feng, S., Yan, H. and Liu, K. (2012) Using a Bifunctional Polymer for the Functionalization of Fe3O4 Nanoparticles. Reactive and Functional Polymers, 72, 198-205. http://dx.doi.org/10.1016/j.reactfunctpolym.2012.01.003
- 6. Katz, E., Weizmann, H. and Willner, I. (2005) Magneto Switchable Reactions of DNA Monolayers on Electrodes: Gating the Processes by Hydrophobic Magnetic Nanoparticles. Journal of the American Chemical Society, 17, 9191-9200. http://dx.doi.org/10.1021/ja0517771
- 7. heng, G.F., Zhao, J., Tu, Y.H., He, P.G. and Fang, Y.Z. (2005) Sensitive DNA Electrochemical Biosensor Based on Magnetite with a Glassy Carbon Electrode Modified by Multi-walled Carbon Nanotubes in Polypyrrole. AnalyticaChimstry Acta, 533, 11-16.
- 8. Zhu, A.P., Yuan, L.H. and Liao, T.Q. (2008) Suspension of Fe3O4 Nanoparticles Stabilized by Chitosan and Ocar-boxymethylchitosan. International Journal of Pharmaceutics, 350, 361-368. http://dx.doi.org/10.1016/j.ijpharm.2007.09.004
- 9. Deng, H., Li, X.L., Peng, Q., Wang, X., Chen, J.P. and Li, Y.D. (2005) Monodisperse Magnetic Single-Crystal Ferrite Microspheres. Angewandte Chemie International Edition, 44, 2782-2785. http://dx.doi.org/10.1002/anie.200462551
- 10. Smith, E.A. and Chen, W. (2008) How to Prevent the Loss of Surface Functionality Derived from Amino Silan. Langmuir, 24, 12405-12409. http://dx.doi.org/10.1021/la802234x
- 11. Rice-Evans, C., Miller, N.J. and Paganga, G. (1996) Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radical Biology & Medicine, 20, 933-956. http://dx.doi.org/10.1016/0891-5849(95)02227-9
- 12. Fang, S.H., Hou, Y.C., Chang, W.C., Hsiu, S.L., Chao, P.D. and Chiang, B.L. (2003) Morin Sulfates/Glucuronides Exert Anti-Inflammatory Activity on Activated Macrophages and Decreased the Incidence of Septic Shock. Life Sciences, 74, 743-756. http://dx.doi.org/10.1016/j.lfs.2003.07.017
- 13. Hanasaki, Y., Ogawa, S. and Fukui, S. (1994) The Correlation between Active Oxygens Scavenging and Antioxidative Effects of Flavonoids. Free Radical Biology & Medicine, 16, 845-850. http://dx.doi.org/10.1016/0891-5849(94)90202-X
- 14. Sivaramakrishnan, V. and Devaraj, S.N. (2010) Morin Fosters Apoptosis in Experimental Hepatocellular Carcinogenesis Model. Chemico-Biological Interactions, 183, 284-292. http://dx.doi.org/10.1016/j.cbi.2009.11.011
- 15. Yu, Z., Fong, W.P. and Cheng, C.H.K. (2006) The Dual Actions of Morin (3,5,7,2’,4’-Pentahydroxyflavone) as a Hypouricemic Agent: Uricosuric Effect and Xanthine Oxidase Inhibitory Activity. Journal of Pharmacology and Experimental Therapeutics, 316, 169-175. http://dx.doi.org/10.1124/jpet.105.092684
- 16. Liu, X., Ma, Z., Xing, J. and Liu, H. (2004) Preparation and Characterization of Amino-Silane Modified Superparamagnetic Silica Nanospheres. Journal of Magnetism and Magnetic Materials, 270, 1-6. http://dx.doi.org/10.1016/j.jmmm.2003.07.006
- 17. Cao, H., He, J., Deng, L. and Gao, X. (2009) Fabrication of Cyclodextrin Functionalized Superparamagnetic Fe3O4/Amino-Silane Core-Shell Nanoparticles via Layer-by-Layer Method. Applied Surface Science, 255, 7974-7980. http://dx.doi.org/10.1016/j.apsusc.2009.04.199
- 18. Ma, M., Zhang, Y., Yu, W., Shen, H.Y., Zhang, H.Q. and Gu, N. (2003) Preparation and Characterization of Magnetite Nanoparticles Coated by Amino Silane. Colloids and Surface A, 212, 219-226. http://dx.doi.org/10.1016/S0927-7757(02)00305-9
- 19. Yamaura, M., Camilo, R.L., Sampaio, L.C., Macedo, M.A., Nakamura, M. and Toma, H.E. (2004) Preparation and Characterization of (3-Aminopropyl) Triethox-ysilane-Coated Magnetite Nanoparticles. Journal of Magnetism and Magnetic Materials, 279, 210-217. http://dx.doi.org/10.1016/j.jmmm.2004.01.094
- 20. Das, M., Mishra, D., Maiti, T.K., Basak, A. and Pramanik, P. (2008) Bio-Functionalization of Magnetite Nanoparticles Using an Aminophosphonic Acid Coupling Agent: New, Ultradispersed, Iron-Oxide Folate Nanoconjugates for Cancer-Specific Targeting. Nanotechnology, 19, Article ID: 415101. http://dx.doi.org/10.1088/0957-4484/19/41/415101








