Agricultural Sciences
Vol.5 No.3(2014), Article ID:43020,7 pages DOI:10.4236/as.2014.53025
Physicochemical characterization of chia (Salvia hispanica) seed oil from Yucatán, México
Facultad de Ingeniería Química, Universidad Autónoma de Yucatán, Mérida, México; *Corresponding Author: bancona@uady.mx
Copyright © 2014 Maira Rubi Segura-Campos et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Maira Rubi Segura-Campos et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 18 December 2013; revised 21 January 2014; accepted 7 February 2014
KEYWORDS
Chía; Salvia hispanica; Oil; Physicochemical Characteristic
ABSTRACT
A physicochemical characterization of oil from chia seeds was carried out. Proximate composition analysis showed that fat and fiber were the principal components in the raw chia flour. Physical characterization showed that chia oil has a relative density from 0.9241, a refraction index of 1.4761 and a color with more yellow than red units. Chemical characterization showed that chia oil registered an acidity index of 2.053 mg KOH/g oil, a saponification index of 222.66 mg KOH/g oil, a content of unsaponifiable matter of 0.087%, an Iodine index of 193.45 g I/100 g oil and a peroxide index of 17.5 meq O2/kg oil. Chia oil showed a higher content of α and β linolenic and palmitic acids. Chia oil is the vegetable source with the highest content of essential fatty acids.
1. INTRODUCTION
Salvia hispanica L., whose common name is chia, is an annual herbaceous plant belonging to the Lamiaceae or Labiatae family. In pre-Columbian times, it was one of the basic foods of several Central American civilizations, less important than corn and beans, but more important than amaranth. Tenochtitlan, the capital of the Aztec Empire, received 5 - 15,000 tons of chia annually as a tribute from conquered nations [1]. The seed was also used as an offering to the Aztec gods, and because of its religious use, it essentially disappeared for 500 years [2]. Wild and domesticated chia differs little. Currentlyonly Salvia hispanica, and not other species of the genus Salvia, can be grown domestically. To prevent the misidentification of Salvia hispanica and other species of Salvia, a clear understanding of the morphological and genotypical differences among them had been proposed as solutions [3]. Owing to the fact that it can grow in arid environments, it has been highly recommended as an alternative crop for the field crop industry.
Chia seed is composed of protein (15% - 25%), fats (30% - 33%), carbohydrates (26% - 41%), high dietary fiber (18% - 30%), ash (4% - 5%), minerals, vitamins, and dry matter (90% - 93%). It also contains a high amount of antioxidants [4]. Chia seed is traditionally consumed in Mexico, the southwestern United States, and South America, but it is not widely known in Europe. However, in 2009 the European Union approved chia seeds as a novel food, allowing them to comprise up to 5% of a bread product’s total matter [5]. Today, chia is mostly grown in Mexico, Bolivia, Argentina, Ecuador, Australia, and Guatemala, and it has been demonstrated that the species has great potential as a future crop plant [6]. The plant produces small white and dark seeds. Most of the chia population that is commercially grown today contains a low percentage of white seeds. Their shapes are oval and in general, the white seeds are somewhat larger than the black ones. Ixtaina et al. [4] reported length, width and thickness values of 2.11, 1.32 and 0.81 mm for dark seeds and 2.15, 1.40 and 0.83 mm for white seeds, respectively.
Nowadays, chia seeds are being reintroduced to western diets in order to improve human health. In this respect, chia seeds have been investigated and recommended due to their high levels of proteins, antioxidants, dietary fiber, vitamins and minerals but particularly due to their oil content with the highest proportion of α-linolenic acid (ω-3) compared to other natural sources known to date [6]. Chia seeds contain up to 39% of oil, which has the highest known content of α-linolenic acid, up to 68% [7] compared to 57% in flax [8]. Chia is one of the most efficient omega-3 (n-3) sources for enriching foods [9]. Chia seeds and meal have not shown any of the problems associated with other n-3 sources such as flaxseed or marine products in terms of fishy flavor, animal weight loss and digestive problems, etc. [2].
A correlation between high-saturated fatty acids (SFA) and low polyunsaturated fatty acid (PUFA) intake and diseases such as cardiovascular diseases, diabetes, and metabolic syndrome were widely reported [10]. Besides, the additive effect of α-linolenic acid (ALA) and n-3 long-chain PUFA was observed to exhibit cardio-protective effects in women [11], which led to consequent human clinical studies of chia on disease risk factors. To date, four clinical trials have been carried out. Among these trials, only that of Nieman et al. [12] showed no health benefits from chia seed. This difference could be due to the treatment durations employed and also the actual biochemical components of the dietary chia seed used in the various studies. Nevertheless, later studies [10] demonstrated the benefits of chia to human health. Today, chia is still an essential element in the diet of the inhabitants of Mexico and several Central American countries, becoming an increasingly popular food and is common in supermarkets and health food stores around the world. The objective of the present study was to determine the physicochemical properties of oil from chia (Salvia hispanica) seeds cultivate in Yucatán, México and show its advantages over those grown in other countries.
2. MATERIALS AND METHODS
2.1. Materials
Chia (S. hispanica, L.) seeds were obtained in the Yucatan State of Mexico. Reagents were of analytical grade and purchased from J.T. Baker (Phillipsburg, NJ, USA), Sigma (Sigma Chemical Co., St. Louis, MO, USA), Merck (Darmstadt, Germany) and Bio-Rad (Bio-Rad Laboratories, Inc. Hercules, CA, USA).
2.2. Chia Seed Proximal Composition
Flour was produced from 2 kg of chia seed by first removing all the impurities and damaged seeds, crushing the remaining sound seeds (Moulinex DPA139, Zapopan, Jalisco, México) and then milling them (Krups 203 mill, México D.F., México). Standard AOAC [13] procedures were used to determine nitrogen (method 954.01), fat (method 920.39), ash (method 925.09), crude fiber (method 962.09), and moisture (method 925.09) contents in the milled seeds. Nitrogen (N2) content was quantified with a Kjeltec Digestion System (Tecator, Höganäs, Skåne län, Sweden) using cupric sulfate and potassium sulfate as catalysts. The protein content was calculated as nitrogen × 6.25. Fat content was obtained from a 1-h hexane extraction. The ash content was calculated from the sample weight after burning at 550˚C for 2 h. The moisture content was measured based on sample weight loss after oven drying at 110˚C for 2 h. The carbohydrate content was estimated as a nitrogen-free extract (NFE) by the difference from the sum of the protein, fat, ash and crude fiber content.
2.3. Chia Oil Extraction by the Soxhlet Method
The Franz von Soxhlet extractor method described by AOAC [13] was used for the extraction and determination of the percentage oil yield and percentage oil recuperation. Crushed seeds weighing 650 g were wrapped in a weighed filter paper and placed in the thimble, and approximately 2.5 L of normal hexane was poured into a weighed round-bottomed flask. The hexane was heated to boil with an electro thermal heater for 4 h of continuous extraction. The defatted sample was removed and the solvent recovered. The flask and its oil content were further dried in the oven at 60˚C for 30 min and cooled in a desiccator. The flask and its content were reweighed to determine the weight of the oil. The experiment was repeated two more times to get an average. The percentage oil yield was obtained by expressing the oil weight as a percentage of the weight of the sample. The percentage oil recuperation was obtained by expressing the oil weight as a percentage of the weight of oil in the seed.
2.4. Physicochemical Characterization of Chia Oil
The physicochemical properties of the chia oil were determined using the official methods and recommended practices of the “Norma Oficial Mexicana” (NMX) among others. Physical properties such as relative density, refractive index and color were determined following the NMX-F-75-1987 [14], the Kirk et al. [15] method and the NMX-F-116-1987 [16], respectively. Chemical properties was determined as follows: Acidity was evaluated following NMX-F-101-1987 [17]; the saponification index was determined according to NMX-F-174-S-1981 [18]; the Iodine index was evaluated using an adaptation of the original method ofNMX-F-152-S-1981 [19] and Kirk et al. [15]; the peroxides index was determined according to NMX-F-154-1987 [20]; the saponificable matter was evaluated according to the procedure described by Hart and Fischer [21]. Lipid extraction wasdone according to the Bligh and Dyer [22] method; phospholipid quantification was done following the Rouser [23] method and the fatty acid profile was done as follows: the saponification and derivatization of lipids was done according to Christie [24], while gas chromatography/mass spectrometry was done following the method of Knapp [25].
2.5. Statistical Analysis
All experiments were carried out in triplicates. Data obtained were subjected to statistical analysis for tendency central and dispersion measured (p < 0.05).
3. RESULTS AND DISCUSSION
3.1. Chia Seed Proximal Characterization
Table 1 shows the proximal analysis of the chia flour. Chia is characterized by a high fat content. In the present study, chia showed a fat content of 35.13%. Its fat content was similar to the 33% and 32% reported by Ixtaina et al. [26] and Guiotto et al. [6], respectively. The protein (24.11%) and ash (4.58%) contents were near the 23% protein and 4.6% ash contents reported by Ayerza and Coates [9]. Guiotto et al. [6] reported a similar behavior with 29% and 5% of protein and ash contents, respectively. The nitrogen-free extract (NFE) in the raw chia flour (1.51%) was lower than the 7.42% reported by Salazar-Vega et al. [27], probably due to the 25.2% fat content observed in that study. Chia was a good source of crude fiber. The determined content was 34.46%, which is similar to that reported by Segura-Campos et al. [28] in raw chia flour (35.85%) but higher than that reported in defatted chia flour (21.43%). Proximate composition analysis showed that fat and fiber were the principal components in the raw chia flour.
3.2. Physicochemical Characterization of Chia Oil
Chia seed is mainly valued for its oil. Thus, many oil extraction methods had been utilized. Differences in the extraction methods resulted in variations in the oil yield, quality of fatty acids, fatty acid contents, total dietary fibers, and also the antioxidant content. In the present study, the oil yield was 27.3% and the percentage of oil recuperation was 83.44 with respect to the fat content

Table 1. Proximal composition of chia flour (% d.b).
registered in the proximal analysis. The yield was lower than reported by Ayerza and Coates [2] who reported oil yields of 28.5% - 32.7% in chia seeds from Colombia, Argentina, Peru and Bolivia.
With the physical characterization, chia showed a relative density value from 0.9241 (Table 2), which was similar to that reported in sunflower oils at 25˚C (0.909), as well as to safflower (0.92) and soy (0.919) oils at 20˚C [29]. According to Alvarado and Aguilera [30], the relative density is high to higher unsaturation’s content in the fatty acids. The results obtained here suggest that chia has a high number of unsaturated fatty acids, which is consistent with the results reported by Tosco [31].
Chia showed a refraction index value of 1.4761 at 25˚C. This value was similar to oil obtained from Argentinean and Guatemalan chia seeds with 1.4768 and 1.4763 at same temperature [32]. However, the index value was higher than reported by Codex Stan 210 [29] in sunflower (1.461), safflower (1.467) and soy (1.466) oils at 40˚C. According to Alvarado and Aguilera [30], the refraction index is dependent on the analysis temperature and unsaturation contents of the fatty acids. They establish that high analysis temperatures showed lower refraction index values, and high unsaturation content is related to high refraction index values.
Chia oil color showed more yellow (70) than red (9.1) units. This behavior was similar to that reported by Hosseinian et al. [33] for linseed oil, which showed 70 and 8.6 yellow and red units, respectively. However, the color of chia oil was different than palm (3.2, 27.4) and soy oil (4.6, 10.6), which registered more red than yellow units [34].
Chemical characterization showed that chia registered an acidity index of 2.053 mg KOH/g oil, which represents an average content of free fatty acids of 1.032% as
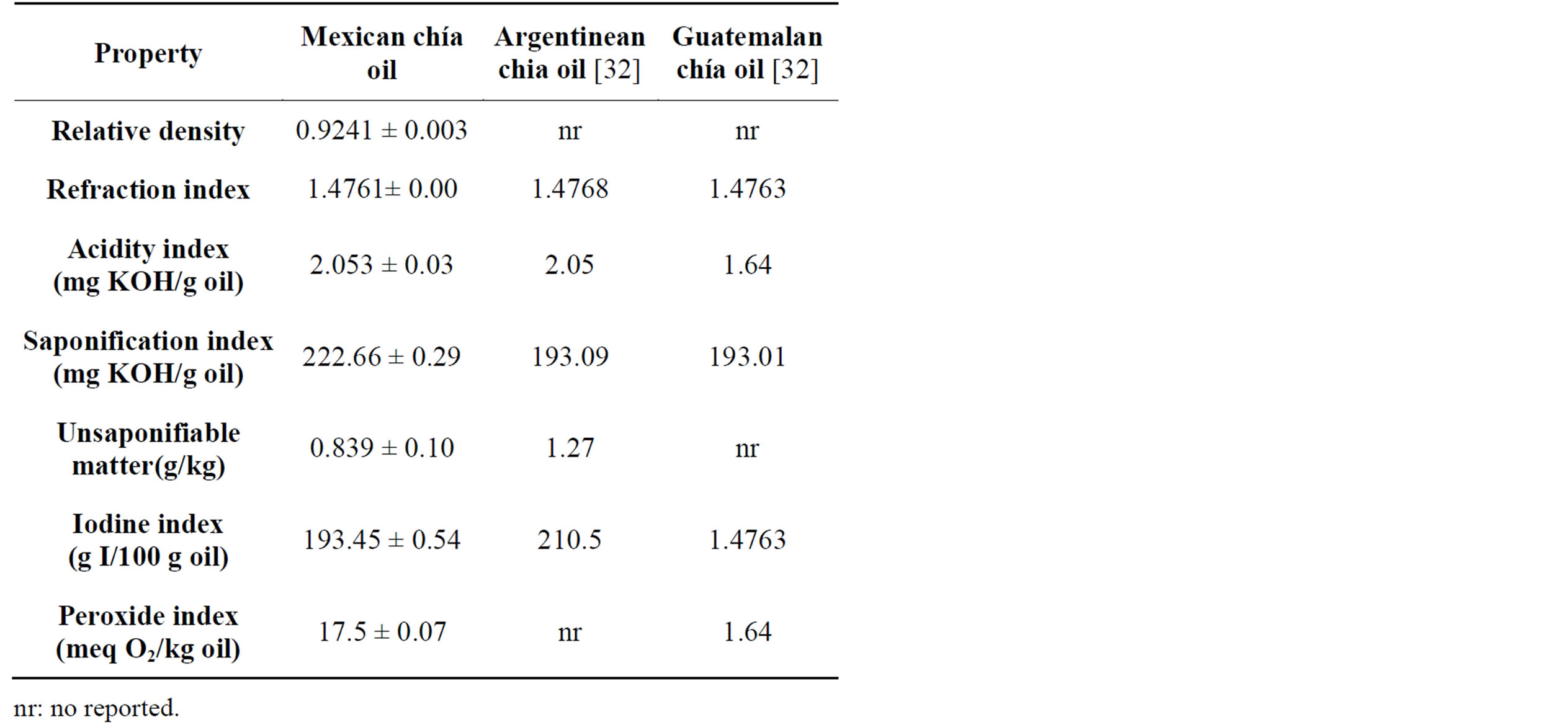
Table 2. Physical and chemical properties of chia oil.
oleic acid and 1.017% as linolenic acid. The results suggest that chia oil was not altered by chemical or enzymatic hydrolysis. This acidity index was similar to chia oil obtained by pressing from Argentinean seeds (2.05 mg KOH/g sample) but higher respect to oil from Guatemalan seeds with 1.64 mg KOH/g oil [32].
The saponification index registered here (222.66 mg KOH/g oil) was higher than reported in sunflower (188 - 194 mg KOH/g oil), safflower (186 - 198 mg KOH/g oil), soy (189 - 195 mg KOH/g oil) and virgin olive oil (184 - 196 mg KOH/g oil) [29,35]. Also, the index was higher respect to oils obtained from Argentinean and Guatemalan chía seeds (193 mg KOH/g oil) [32]. According to Adrian et al. [36], the saponification index is inversely proportional to the chain length of fatty acids. This suggests that chia oil is constituted by fatty acids of lower molecular weight than sunflower, safflower, soy and virgin olive oil.
Chia registered a low content of unsaponifiable matter (0.087%), which suggests a low content of organic matter (sterols, hydrocarbons, pigments, phospholipids, vitamins) and, hence, a low impurities quantity. The unsaponifiable matter registered here was lower than that reported by Adrian et al. [36] who reported values of 0.3 to 1.5% in natural fats or oils as registered in peanut oil (1%).
The Iodine index registered here (193.45 g I/100 g oil) showed the unsaturation grade of chia’s oil, and that this parameter is proportional to the number of double bonds in the fatty acid chains. Chia oil showed an Iodine index value that was higher than that reported by Codex stan 210 [29] in sunflower (118.141 g I/100 g oil), safflower (136 - 148 g I/100 g oil) and soy (124 - 139 g I/100 g oil) oils as well as that reported by Codex Stan 33 [35] in virgin olive oil (75 - 95 g I/100 g oil). The Iodine index in chia oil only could be compared with the Iodine index reported by Hosseinian et al. [33] in linseed oil (187 g I/100 g oil). The high values of density, refraction index and Iodine index revealed the high content of polyunsaturated fatty acids (PUFA), such as α linolenic (ω-3) and linoleic (ω-6) in chia oil.
Finally, the peroxide index registered in chia oil (17.5 meq O2/kg oil) was lower than reported by Codex Stan 210 [29] for any crude edible oil suggesting that this oil did not present signs of rancidity.
The phospholipid concentration of chia oil was 118 ppm. This value was lower than that reported in soy oil (349 - 975 ppm) [37], which is considered the principal source of these components as well as sunflower (342 - 657 ppm) [38] and rape (120.5 ppm) [39] oils. The importance of phospholipids in the food industry is their capacity to reduce the surface tension, thus, facilitating the production of stable emulsions.
The fatty acid profile (Table 3) from chia oil showed a higher content of α and γ linolenic and palmitic acids than reported by Ayerza and Coates [40] and Craig and Sons [41]. However, a lower content of oleic acid and similar contents of linoleic acid were registered in chia oil compared with that reported by Ayerza and Coates [40]. On the other hand, fatty acids such as pentadecanoic, arachidonic and docosahexaenoic were registered here and not in the aforementioned studies. The results confirm that chia oil is the vegetable source with the highest content of essential fatty acids, containing more than 60% as α-linoleic acid and more than 20% as linoleic acid. These results are only compared with linseed that registered 58.8% of α-linolenic acid and 14% - 16% of linoleic acid [33].
There are many factors that may cause variations in the concentration of the fatty acids in chia seed. One of them is the cultivation area of the plant itself. According to Mohd Ali et al. [42], differences in the environment, climate changes, availabilities of nutrient, year of cultivation, or soil conditions play crucial roles in the varia-
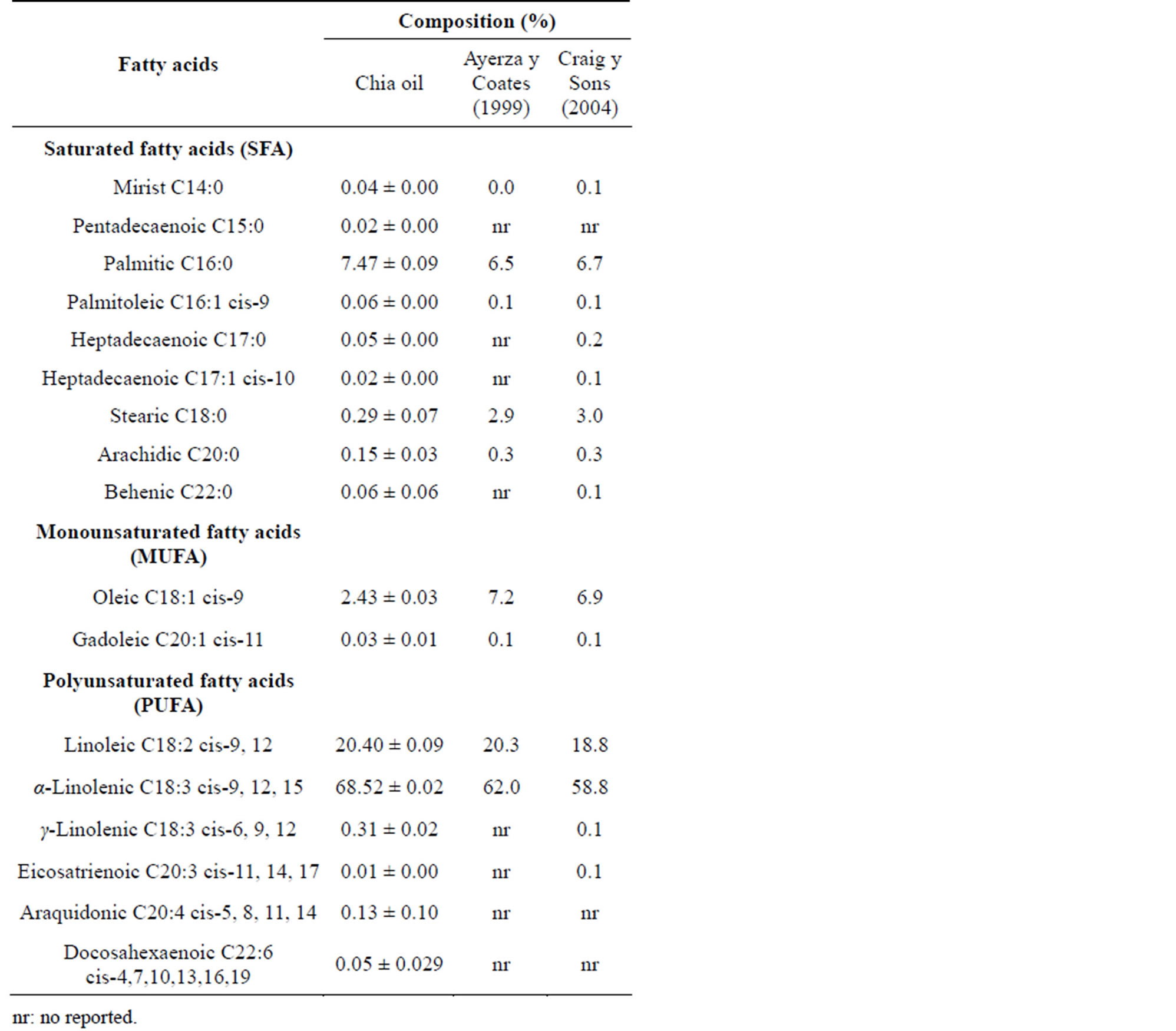
Table 3. Fatty acids profile of chia oil.
tions. In this respect, Ayerza [43] established the existence of an inverse relationship between altitude and the content of saturated fatty acids (SFA); at low elevation, an increase in fatty acid saturation was noted in areas where the temperature was high. In Argentina, Ayerza [7] demonstrated that temperature largely contributed to the type of fatty acid found in the oil. They found that during seed development from April to May, an increase in the temperature of the environment brought about a decrease in the PUFA content. Another factor that may contribute to differences in the chemical compositions of chia seed is the developmental stage of the plant. It was shown that the (α-linolenic acid) ALA content decreased by 23% from the early stage to the matured stage of the seed. This concurrently resulted in the increase of linolenic acid (LA) and lignin content [44].
The literature shows the high PUFA content in chia as well as its importance. Antruejo et al. [45] in a comparative study using flaxseed, rapeseed, and chia seed as chicken feed demonstrated that eggs from hens fed with chia had the highest ω-3 ALA content when compared to hens fed with flaxseed or rapeseed. Ayerza and Coates [46] and Fernandez et al. [47] conducted studies concerning the effects of chia seed feeding on rat plasma. Their findings indicated that serum triglycerides (TG) and low-density lipoprotein (LDL) were significantly reduced, whereas high-density lipoprotein (HDL) and ω-3 PUFA levels were elevated. They also noted that no adverse effects were observed on the rat’s thymus and IgE serum level. Furthermore, chia seed feeding was tested in pigs and rabbits, which resulted in an increase of PUFA in meat fats as well as aroma and flavor [48]. These are desirable characteristics of human food. In summary, the incorporation of chia seed into animal feed results in an increase of ALA and a decrease of cholesterol levels in meat and eggs. Hence, it is a good substitute source of PUFA for fish and other seed oils. Moreover, atypical organoleptic characteristics such as flavor and smell from marine sources were not found in chia [49]. This showed the superiority of chia seed against other nutritional sources.
4. CONCLUSION
The results show that the chia oil presents interest- ing physicochemical properties for the food industry. Fatty acids such as pentadecanoic, arachidonic and docosahexaenoic were registered in chia oil and also contain more than 60% as α-linoleic acid and more than 20% as linoleic acid than other sources. The results confirm that chia oil is the vegetable source with the highest content of essential fatty acids. From a physiological point of view, chia oil is a potentially interesting food ingredient due to its health benefits from its high levels of PUFA.
ACKNOWLEDGEMENTS
This research forms part of Project financed by the Consejo Nacional de Ciencia y Tecnología (CONACYT, México).
REFERENCES
- Codex Mendoza 1542 (1925) Edition of francisco del paso and troncoso. Museo Nacional de Arqueologia, Historia y Etnografia, Mexico.
- Ayerza, R. and Coates, W. (2004) Composition of chia (Salvia hispánica) grown in six tropical and subtropical ecosystems of South America. Tropical Science, 44, 131- 135. http://dx.doi.org/10.1002/ts.154
- Reales, A., Rivera, D., Palazón, J.A. and Obón, C. (2004) Numerical taxonomy study of Salvia sect. Salvia (labiatae). Botanical Journal of the Linnean Society, 145, 353- 371. http://dx.doi.org/10.1111/j.1095-8339.2004.00295.x
- Ixtaina, Y., Nolasco, S.M. and Tomas, M.C. (2008) Physical properties of chia (Salvia hispanica L.) seeds. Industrial Crops and Products, 28, 286-293. http://dx.doi.org/10.1016/j.indcrop.2008.03.009
- Commission of the European Communities (2009) Commission regulation (EC) 827/2009. Official Journal of the European Union, 52, 12-13.
- Guiotto, E.N., Ixtaina, V.Y., Tomás, M.C.M. and Nolasco, S.M. (2013) Moisture-dependent engineering properties of chia (Salvia hispánica L.) seeds. In: Food Industry. INTECH, 381-397.
- Ayerza, R. (1995) Oil content and fatty acid composition of chia (Salvia hispanica L.) from five northwestern locations in Argentina. Journal of the American Oil Chemists’ Society, 72, 1079-1081.
- Sultana, C. (1996) Oleaginous flax. In: Karleskind, A. and Wolff, J.P., Eds., Oils and Fats Manual, 157-160.
- Ayerza, R. and Coates, W. (2001) The omega-3 enriched eggs: The influence of dietary linolenic fatty acid source combination on egg production and composition. Canadian Journal of Animal Science, 81, 355-362. http://dx.doi.org/10.4141/A00-094
- Martha, G.C., Armando, R.T. and Carlos, A.A. (2012) A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. Journal of Nutrition, 142, 64-69. http://dx.doi.org/10.3945/jn.111.147447
- Vedtofte, M.S., Jakobsen, M.U. and Lauritzen, L. (2011) Dietary alpha linoleic acid, linoleic acid and n-3 longchain PUFA and risk of ischemic heart disease. The American Journal of Clinical Nutrition, 94, 1097-1103. http://dx.doi.org/10.3945/ajcn.111.018762
- Nieman, D.C., Cayea, E.J., Austin, M.D., Henson, D.A., McAnulty, S.R. and Jin, F. (2009) Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutrition Research, 29, 414-418. http://dx.doi.org/10.1016/j.nutres.2009.05.011
- AOAC (1997) Association of Official Analytical Chemists. Official Methods of Analysis. 20th Edition, Washington DC.
- NMX-F-75-1987 (1987) Aceites y grasas vegetales. Determinación de la densidad relativa. Secretaría de Comercio y Fomento Industrial, México.
- Kirk, R., Sawyer, R. and Egan, H. (1996) Composición y análisis de alimentos de Pearson. Compañía Editorial Continental. 2nd Edition, México.
- NMX-F-116-1987 (1987) Aceites y grasas vegetales. Determinación de color. Secretaría de Comercio y Fomento Industrial, México.
- NMX-F-101-1987 (1987) Aceites y grasas vegetales. Determinación del índice de acidez. Secretaría de Comercio y Fomento Industrial, México.
- NMX-F-174-S-1981 (1981) Aceites y grasas vegetales. Determinación del índice de saponificación. Secretaría de Comercio y Fomento Industrial, México.
- NMX-F-152-S-1981 (1981) Alimentos para humanos. Aceites y grasas vegetales o animales. Determinación del índice de yodo por el método de Wijs. Normas Mexicanas, México.
- NMX-F-154-1987 (1987) Alimentos. Aceites y grasas vegetales o animales. Determinación del índice de peró- xido. Normas Mexicanas. Dirección General de Normas, México.
- Hart, F. and Fischer, J. (1991) Análisis moderno de los alimentos. Ed. Acribia. Zaragoza, España.
- Bligh, E.G. and Dyer, W.J. (1959) A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911-917. http://dx.doi.org/10.1139/o59-099
- Rouser, G., Siakoto, A.N. and Fleischer, S. (1996) Quantitative analysis of phospholipids by thin-layer chromatography and phosphorous analysis of spots. Lipids, 1, 85- 86. http://dx.doi.org/10.1007/BF02668129
- Christie, W.W. (1959) Lipid analysis. 2nd Edition, Pergamon Press, USA, 51-61.
- Knapp, D. (1979) Handbook of analytical derivatization reactions. Wiley-Interscience.
- Ixtaina, V.Y., Vega, A., Nolasco, S.M., Tomás, M.C., Gimeno, M. and Bárzana, E. (2010) Supercritical carbon dioxide extraction of oil from mexican chia seed (Salvia hispanica L.); characterization and process optimization. Journal of Supercritical Fluids, 55, 192-199. http://dx.doi.org/10.1016/j.supflu.2010.06.003
- Salazar-Vega, I.M., Rosado-Rubio, G., Chel-Guerrero, L.A., Betancur-Ancona, D.A. and Castellanos-Ruelas, A.F. (2009) Composición en acido graso alfa linolénico en el huevo y carne de aves empleando chia (Salvia hispanica) en el alimento. Interciencia, 34, 209-213.
- Segura-Campos, M.R., Salazar-Vega, I.M., Chel-Guerrero, L.A. and Betancur-Ancona, D. (2013) Biological potential of chia (Salvia hispanica) protein hydrolysates and their incorporation into functional foods. LWT-Food Science and Technology, 50, 723-731.
- Codex Alimentarius Stan 210 (2003) Norma del CODEX para Aceites Vegetales Especificados.
- Alvarado, J. and Aguilera, J. (2001) Métodos para medir propiedades físicas en industrias de alimentos. Ed. Acribia. Zaragoza, España, 15, 347-348.
- Tosco, G. (2004) Los beneficios de la Chía en humanos y animales. Actualidades Ornitológicas, 119, 7.
- Ixtaina, V.Y., Martínez, M.L., Sportono, V., Mateo, C.M., Maestri, D.M., Diehl, B.W.K., Nolasco, S.M. and Tomas, M.C. (2011) Characterization of chia seed oils obtained by pressing and solvent extraction. Journal of Food Composition and Analysis, 24, 166-174. http://dx.doi.org/10.1016/j.jfca.2010.08.006
- Hosseinian, F.S., Rowland, G.G., Bhirud, P.R., Dyck, J.H. and Tyler, R.T. (2004) Chemical composition and physicochemical and hydrogenation characteristics of high-palmitic acid solin (low-linolenic acid flaxseed) oil. Journal of the American Oil Chemists’ Society, 81, 185-188. http://dx.doi.org/10.1007/s11746-004-0879-6
- Patil, R.T. and Ali, N. (2006) Effect of pre-treatments on mechanical oil expression of soybean using a commercial oil expeller. International Journal of Food Properties, 9, 227-236. http://dx.doi.org/10.1080/10942910600592315
- Codex Alimentarius Stan 33 (1989) Norma del Codex para los aceites de oliva vírgenes y refinados, y los aceites refinados de orujo de aceituna.
- Adrian, J., Potus, J., Poiffait, A. and Douvillier, P. (2000) Análisis nutricional de los alimentos. Acribia. Zaragoza, 56-58.
- Tosi, E.A., Cazzoli, A.F. and Tapiz, L.M. (1998) Phosphorus in oil. Production of molybdenum blue derivative at ambient temperature using noncarcinogenic reagents. Journal of the American Oil Chemists’ Society, 75, 41-44. http://dx.doi.org/10.1007/s11746-998-0007-x
- Carelli, A.A., Ceci, L. and Crapiste, G.H. (1998) Phosphorus-to-phospholipid conversion factors for crude and degummed sunflower oils. Journal of the American Oil Chemists’ Society, 79, 1177-1180. http://dx.doi.org/10.1007/s11746-002-0623-2
- Yang, B., Wang, Y. and Yang, J. (2006) Optimization of enzymatic degumming process for rapeseed oil. Journal of the American Oil Chemists’ Society, 83, 653-658. http://dx.doi.org/10.1007/s11746-006-1253-4
- Ayerza, R. and Coates, W. (1999) An ω-3 fatty acid enriched diet: Its influence on egg fatty acid composition, cholesterol and oil content. Canadian Journal of Animal Science, 79, 53-58. http://dx.doi.org/10.4141/A98-048
- Craig, R. and Sons, M. (2004) Application for approval of whole chia (Salvia Hispania. L) seed and ground whole chia as novel food ingredients. Advisory Committee for Novel Foods and Process. Company David Armstrong, Ireland, 1-29.
- Ali, N.M., Yeap, S.K., Ho, W.Y., Beh, B.K., Tan, S.W. and Tan, S.G. (2012) The promising future of chia, Salvia hispanica L. Journal of Biomedicine and Biotechnology, 2012, Article ID: 171956. http://dx.doi.org/10.1155/2012/171956
- Ayerza, R. (2010) Effects of seed colour and growing locations on fatty acid content and composition of two chia (Salvia hispanica L.) genotypes. Journal of the American Oil Chemists’ Society, 87, 1161-1165. http://dx.doi.org/10.1007/s11746-010-1597-7
- Peiretti, P.G. and Gai, F. (2009) Fatty acid and nutritive quality of chia (Salvia hispanica L.) seeds and plant during growth. Animal Feed Science and Technology, 148, 267- 275. http://dx.doi.org/10.1016/j.anifeedsci.2008.04.006
- Antruejo, A., Azcona, J.O., Garcia, P.T., Gallingerd, C., Rosminie, M., Ayerzaf, R., Coatesf, W. and Perez, C.D. (2011) Omega-3 enriched egg production: The effect of α-linolenic ω-3 fatty acid sources on laying hen performance and yolk lipid content and fatty acid composition. British Poultry Science, 52, 750-760. http://dx.doi.org/10.1080/00071668.2011.638621
- Ayerza, R. and Coates, W. (2007) Effect of dietary α-linolenic fatty acid derived from chia when feed as ground seed, whole seed and oil on lipid content and fatty acid composition of rat plasma. Annals of Nutrition and Metabolism, 51, 27-34. http://dx.doi.org/10.1159/000100818
- Fernandez, I., Vidueiros, S.M., Ayerza, R., Coates, W. and Pallaro, A. (2008) Impact of chia (Salvia hispanica L.) on the immune system: Preliminary study. Proceedings of the Nutrition Society, 67, E12.
- Coates, W. and Ayerza, R. (2009) Chia (Salvia hispanica L.) seed as an n-3 fatty acid source for finishing pigs: Effects on fatty acid composition and fat stability of the meat and internal fat, growth performance, and meat sensory characteristics. Journal of Animal Science, 87, 3798-3804. http://dx.doi.org/10.2527/jas.2009-1987
- Ayerza, R., Coates, W. and Lauria, M. (2002) Chia as an omega-3 fatty acid source for broilers: Influence on fatty acid composition, cholesterol and fat content of white and dark meat, on growth performance and on meat flavor. Poultry Science, 81, 826-837.
ABBREVIATIONS
ALA: α-Linolenic Acid AOAC: Association of Official Analytical Chemists HDL: High-Density Lipoprotein KOH: Potassium Hydroxide LA: Linolenic Acid LDL: Low-Density Lipoprotein MUFA: Monounsaturated Fatty Acid NFE: Nitrogen-Free Extract NMX: Norma Oficial Mexicana PUFA: Polyunsaturated Fatty Acid SFA: Saturated Fatty Acids TG: Serum Triglycerides

