Agricultural Sciences
Vol.3 No.1(2012), Article ID:16845,10 pages DOI:10.4236/as.2012.31004
Evaluating host plant resistance in cotton (Gossypium hirsutum L.) with varying gland densities to tobacco budworm (Heliothis virescens F.) and bollworm (Helicoverpa zea Boddie) in the field and laboratory
![]()
1Crop Genetics Research Unit, United States Department of Agriculture—Agricultural Research Service (USDA-ARS), Stoneville, USA; *Corresponding Author: Jodi.Scheffler@ars.usda.gov
2National Clonal Germplasm Repository, USDA-ARS, Stoneville, USA
3Southern Insect Management Research Unit, USDA-ARS, Stoneville, USA
4Biotechnology Regulatory Services, United States Department of Agriculture—Animal and Plant Health Inspection Service (USDAAPHIS), Riverdale, USA
Received 3 October 2011; revised 17 November 2011; accepted 21 December 2011
Keywords: Cotton; Gossypol; Helicoverpa; Heliothis; Terpenoid Aldehydes; Host Plant Resistance
ABSTRACT
Cotton (Gossypium hirsutum L.) produces a number of toxic terpenoid aldehyde (TA) compounds contained in epidermal glands that help protect the plant from pests and diseases. In the seed, one of these toxic compounds, gossypol, limits the use of the seed to ruminants such as dairy cows. There are breeding techniques and germplasm available to decrease gossypol in the seed, but the breeding process also needs to include methods to evaluate the plant’s ability to resist insect pests. Three approaches were used to assess resistance of cotton to herbivory from bollworm (Helicoverpa zea Boddie) and tobacco budworm (Heliothis virescens F.) including field counts, controlled field antibiosis assays and laboratory feeding tests of young field grown leaves. Results indicated that both field and laboratory evaluation could provide an assessment of the cotton host’s resistance. Measurements of terpenoid aldehydes (TAs) in the seed and the leaves, confirmed that the levels and types of TAs in the seed were not always good estimators of leaf TAs and that other TAs such as hemigossypolone and heliocides contribute to host plant resistance.
1. INTRODUCTION
Cotton (Gossypium hirsutum L.) produces not only an economically important natural fiber, but also oil and high quality protein from the seed [1]. For every kilogram of fiber produced, 1.6 kg of seed is available as a source of extra income for the grower. However, gossypol, a toxic terpenoid aldehyde (TA), decreases the value of cotton seeds and limits their use to feeding rations for ruminants such as dairy cows, goats or water buffalo [1]. Gossypol and other related terpenoid aldehyde (TA) compounds are contained in lysigenous glands normally found throughout the plant [2] and protect the plant from pests and pathogens [3-5]. While gossypol is the predominant TA in seeds and roots, other TAs predominate in “green” tissues such as leaves, bracts, calyces and boll hulls. The two most common are hemigossypolone (HGQ) and a group of related TAs often referred to as heliocides [4,6].
Earlier work [7-9] showed that glanding in cotton was controlled by two major genes, Gl2 and Gl3. A fully glanded plant is Gl2Gl2Gl3Gl3 and a completely glandless plant is gl2gl2gl3gl3. The number of dominant alleles present determines the density of the glands. While both genes are active in the vegetative and reproductive parts of the cotton plant, Gl2 is most strongly expressed in the seed and Gl3 in the non-reproductive plant parts. Regulatory mechanisms control TA production and in the above ground parts of the plant, when glands are not present TA compounds are not produced. Therefore, seed gossypol content is associated with the number glands present [10,11]. One method to decrease seed gossypol has been to completely eliminate all the glands on the entire plant. A glandless genetic mutant was reported by McMichael [12,13]; however, despite 20 years of development, totally glandless varieties have been unsuccessful because without TA containing glands on the plant, they suffer increased damage from a number of insect pests that can result in decreased yields [1,14,15].
Although glandless cotton has not been commercially viable, lines with decreased seed gossypol have been developed that retain sufficient glanding throughout the plant to provide adequate pest protection. Different combinations of dominant and recessive Gl2 and Gl3 alleles produce semi-glanded plants with gland densities 25% to 75% of a fully glanded (Gl2Gl2Gl3Gl3) plant. Sets of fully glanded and completely glandless near isogenic lines have also been produced [16,17]. The lower seed gossypol lines were tested to ensure that the gossypol levels remained consistent across years and environments. However, because of their potential vulnerability to herbivory, it is critical that the new lower seed gossypol lines are tested by actually exposing the plants to known pests and evaluating their response as hosts to those pests.
In parts of the southern United States, cotton is a host for a variety of insects, but the heliothines tobacco budworm (Heliothis virescens F.) and bollworm (Helicoverpa zea Boddie) are consistently present [18,19] and have a large economic impact on production [20]. They prefer young fruiting structures and terminal leaves, although they can attack developing bolls [5,21-23]. Studies have reported variation in cotton’s susceptibility to predation by these pests [24,25] and various methods have been used to study these differences [5,23,26,27]. With the advent of transgenic cotton expressing the bt gene, tobacco budworm (TBW) and bollworm (BW) have been well controlled and interest in selecting for host plant resistance (HPR) to heliothines declined. However, some research has supported the idea that other forms of HPR may compliment or enhance the action of the cry1AC protein [28-30]. With the growing concern over heliothines developing resistance to the bt toxin, there has been renewed interest in enhancing HPR by increasing levels of plant compounds toxic to heliothines.
The objective of this research was to find an effective and affordable way to assess host plant resistance (HPR) of new cotton breeding material. Newly developed semi-glanded lower seed gossypol germplasm lines, near isogenic lines fully glanded and glandless, as well as two control cultivars were used in the study. The study used three methods optimized to minimize time and infrastructure investments and easy to incorporate into a breeding program. The simplest method involved counting the heliothines present in the field at specified times during the growing season on the assumption that the number of heliothines present was an indication of a plant’s HPR. The second was a more labor intensive controlled evaluation of feeding antibiosis in the field and the third a laboratory assessment using field grown leaves that required the most equipment and labor.
2. MATERIALS AND METHODS
A glandless parent STV 7A gl was crossed to a normally-glanded parent, either upland cotton Stoneville 7A [31], JaJo 6078 (JaJo Genetics, Baton Rouge, LA), A1006 a high fiber quality elite line from Commonwealth Scientific and Industrial Research Organisation (CSIRO) or the Acala type MAXXA [31]. Selections from these crosses were advanced to the F11 generation by single plant selection followed by progeny evaluation for gland density and seed gossypol content in each generation [16,17]. Three semi-glanded progeny lines (GVS 5069, GVS 5070, GVS 741-2) from the cross STV 7A gl × A1006 were selected for evaluation in this study (Table 1). These lines were selected because A1006 and the three semi-glanded lines had the lowest levels of seed gossypol and were expected to show effects of herbivory. The glanded and glandless near isogenic lines were developed from two plants selected out of one segregating F7 progeny row. These two plants were phenotypically identical except that one had normal glanding and one was glandless. The four sets of near isogenic lines, evaluated in this study, were advanced by single plant selection and progeny evaluation from the F7 to F11 generation. Due to space limitations, JACO GL was not included in the 2008 field evaluation. Also included were the control (check) cultivars DP 432 (Delta & Pine Land, Scott MS) commonly grown in the area and H1220 (Paymaster 1220) [32]. Most upland cotton has glands on the calyx that extend half way up the calyx and are absent on the calyx lobes (crown). H1220 is a “highglanded” (HG) type with glands covering the entire calyx including the crown. H1220 has been identified in previous studies to exhibit enhanced host plant resistance [25].
The entries were planted at the USDA-ARS Entomology Farm, Stoneville, Mississippi on 1-May 2008 and 5-May 2009 in four-row 10 m plots with 1 m row spacing using a randomized complete block design with four replications. Normal cultural practices were followed, however, to fully test the host plant resistance of the individual lines to tobacco budworms (TBW) and bollworms (BW), the plots were not sprayed with insecticides. Tolerance of the lines to the heliothines TBW and BW was estimated by counting heliothines present in the plot, or measuring weight gain of larvae placed on plants in the field, or feeding detached leaves to neonates under laboratory conditions. The number of heliothines (TBW
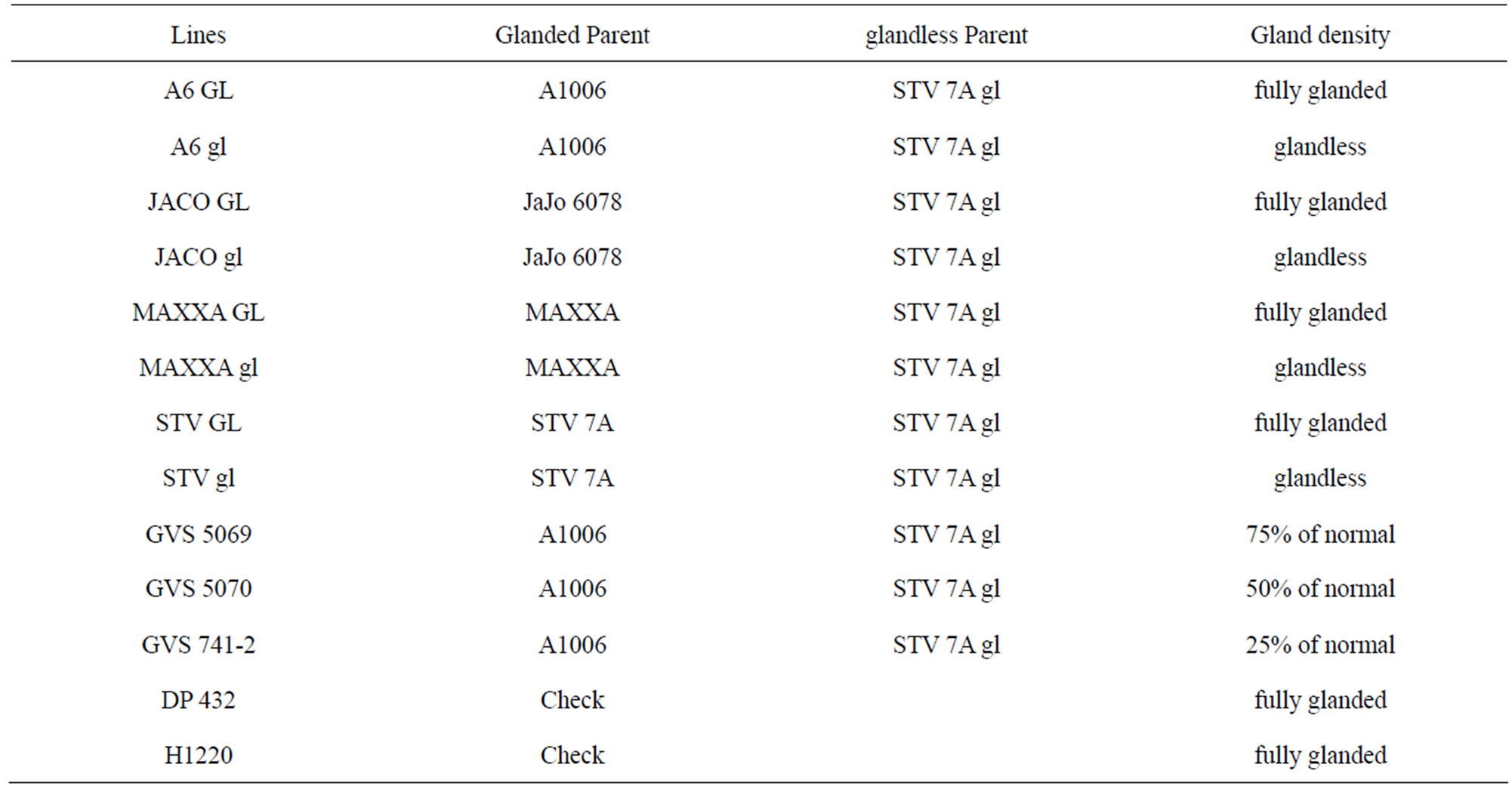
Table 1. Parentage and gland density of lines used in the study.
or BW) present was estimated by shaking insects off the plants onto a drop cloth [18]. Two samples, 5 row-meters each were taken per plot. To estimate the proportion of TBW and BW present in the field, the live larvae collected on the drop cloth were placed in insect artificial diet [33] under laboratory conditions and emerged adults were classified to assess the proportion of TBW:BW on a given sampling date.
Field infestation was assessed by placing a <24-h laboratory-reared tobacco budworm neonate of the USDA-Stoneville colony [27] or a bollworm from a commercial vendor (Benzon Research, Carlisle, PA) on the terminal meristem of a tagged plant and covering the terminal with a 10 cm × 12 cm organza bag (LeMelange, Wellington, FL). The terminal was examined to ensure it was not damaged and no insects were already on the terminal, before applying the neonate. There were four bags per plot. The bags were left for seven days, then the plant terminal was cut below the bag and taken to the laboratory. For each plot, larvae were removed from inside the bags, survivorship assessed and larval weights averaged. In 2008, a field test with TBW was started 29-Jul [89 days after planting (DAP)]. In 2009, a test with BW was added and started 14-Jul (70 DAP), and TBW was tested again 5-Aug (92 DAP). Each test had four bags per plot and four replications per line.
Laboratory evaluations were conducted in parallel with the field infestation tests. Newly expanded leaves, 2 cm wide, were collected in the field plots and transported to the laboratory. Five leaves per plot were placed in individual 37 ml plastic cups (T-125, Solo Cup Company, www.solocup.com). A newly emerged TBW or BW neonate, from laboratory reared colonies, was placed on each leaf and the cup covered with a lid. The larvae on the detached leaves were maintained in an incubator under 28˚C, 14:10 hour light: dark cycle and 60% - 85% relative humidity conditions for seven days. The cups were inspected daily and new leaves added as needed. After seven days, larval mortality and weight gain were recorded. Weights were recorded as the average weight of the surviving larvae from the five leaves per replication. The laboratory tests were started 30-Jul (90 DAP) in 2008 and 06-Aug (93 DAP) 2009.
In October of 2008 and 2009, a sample of seed was harvested from each plot and seed gossypol content was measured with HPLC using the method described by Scheffler and Romano [34]. Previous reports [5,35] and the authors’ unpublished data have indicated that the levels of TAs in leaves and other non-seed plant parts are not always correlated with seed gossypol content and TAs other than gossypol play a more important role in determining HPR. To investigate this possibility, in 2009 fifteen 2 cm wide leaves were harvested 17-Jul (73 DAP) and 07-Aug (94 DAP) and analyzed for total gossypol, hemigossypolone (HGQ) and total heliocides using a modified version of an HPLC method from Stipanovic, et al. [6] that allowed large numbers of samples to be analyzed. Before the leaves were processed, three of the leaves were sampled at random and scored for leaf gland density. The rating scale was 0 no glands to 6 fully glanded. Statistical analyses were carried out using SAS (SAS Institute, Cary, NC) for means and standard errors (Std. Err.) and EXCEL (Microsoft, Redmond WA) to calculate Pearson Product Moment Correlations (r value).
3. RESULTS
Seed gossypol and leaf gland densities were determined for the lines evaluated in the study and are presented in Table 2. Leaf gland density did not always reflect the seed gossypol content as highlighted by GVS 5070 with gland density rating of 3.0% and 0.17% seed gossypol in contrast to GVS 741-2 with a rating of 2.2 and average 0.32% seed gossypol.
Counts to estimate density of heliothines in the plots (Figure 1) were not well correlated with the seed gossypol content in the lines in either year (r = −0.56, p > 0.05 in 2008, r = −0.15, p > 0.05 in 2009). The counts varied widely and few of the differences were statistically significant, suggesting that presence of heliothines may not indicate level of host plant tolerance in a line. The proportion of TBW (28%) to BW (72%) in the 2008 plots was slightly higher, but similar to that observed in 2009 (TBW 15%, BW 85%). As predicted based on gland density (Table 1), field testing of weight gain of TBW fed on different cotton lines did show differences (Figures 2 and 3). Correlations between % seed gossypol and TBW weight gain in field tests were r = −0.86, p <
0.01 in 2008, r = −0.79, p < 0.01 in 2009. The 2009 field test of BW showed similar trends (Figure 4). TBW laboratory test results were well correlated with field tests in 2008 (r = 0.79, p < 0.01 and Figure 2), but were less so in 2009 (r = 0.58, p < 0.05 and Figure 3), however, the rankings were similar in both years. Correlations between % seed gossypol and the TBW laboratory tests were r= −0.84, p < 0.01 in 2008 and r = −0.45, p > 0.05 in 2009 (Table 3). The 2009 BW laboratory results were consistent with predictions based on seed gossypol and gland density (Figure 4). There were several notable exceptions that did not perform as predicted based on seed gossypol content and leaf gland density (Table 2 and Figures 2-4). GVS 5069 which showed less larval weight gain than predicted with both TBW and BW and DP 432 which exhibited more than expected weight gain for TBW in 2009. Correlations were a good method to evaluate overall trends, but were not able to detect the exceptions to the trend that are the target of plant breeders.
The 2009 samples of young newly emerged leaves taken at mid-flower (73 DAP) and then at the same time the TBW laboratory feeding trial was initiated (94 DAP) were analyzed for gossypol, HGQ and total heliocides. Results showed that GVS 5069 (0.68% seed gossypol)
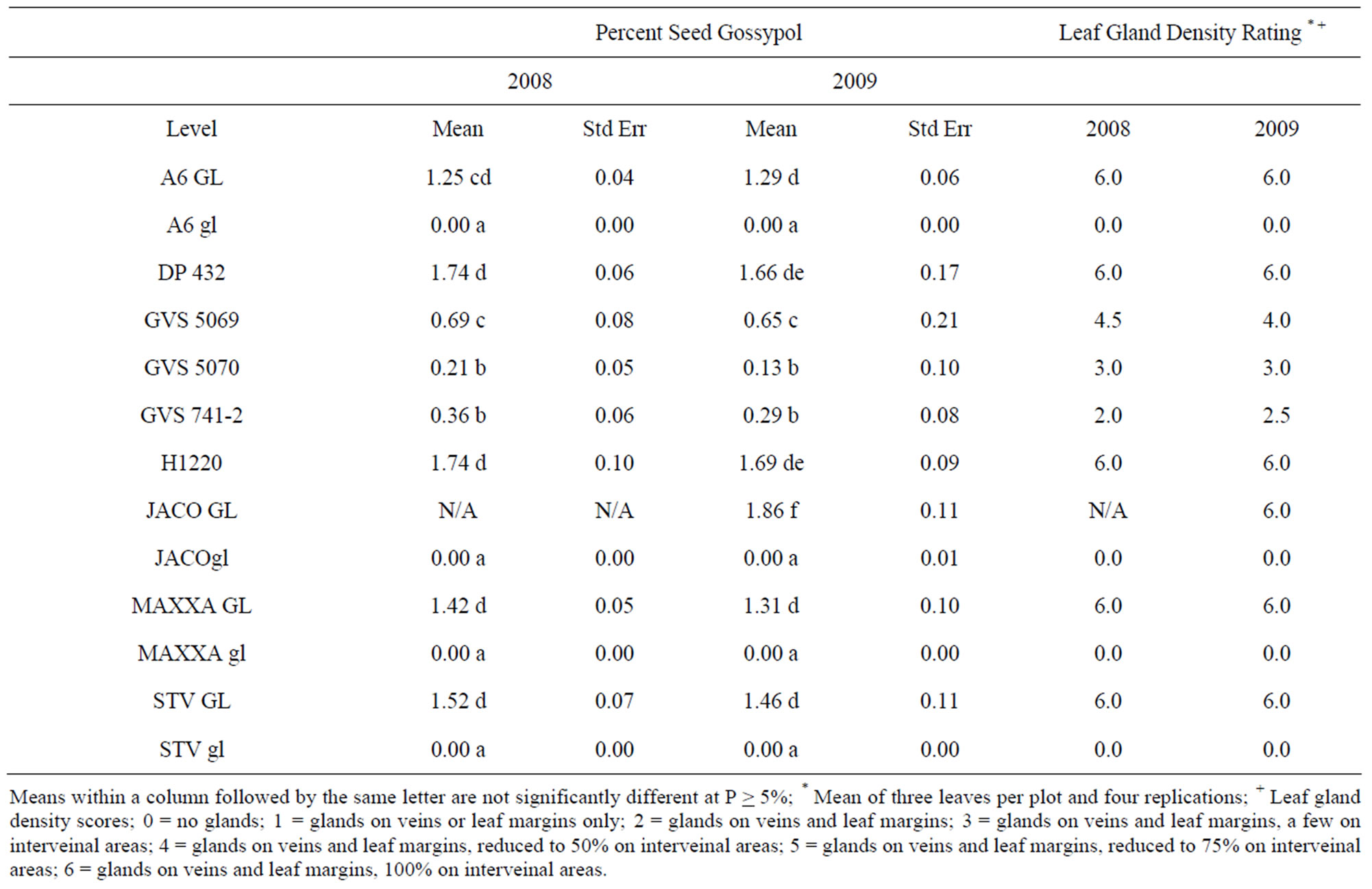
Table 2. Percent seed gossypol and leaf gland density rating.
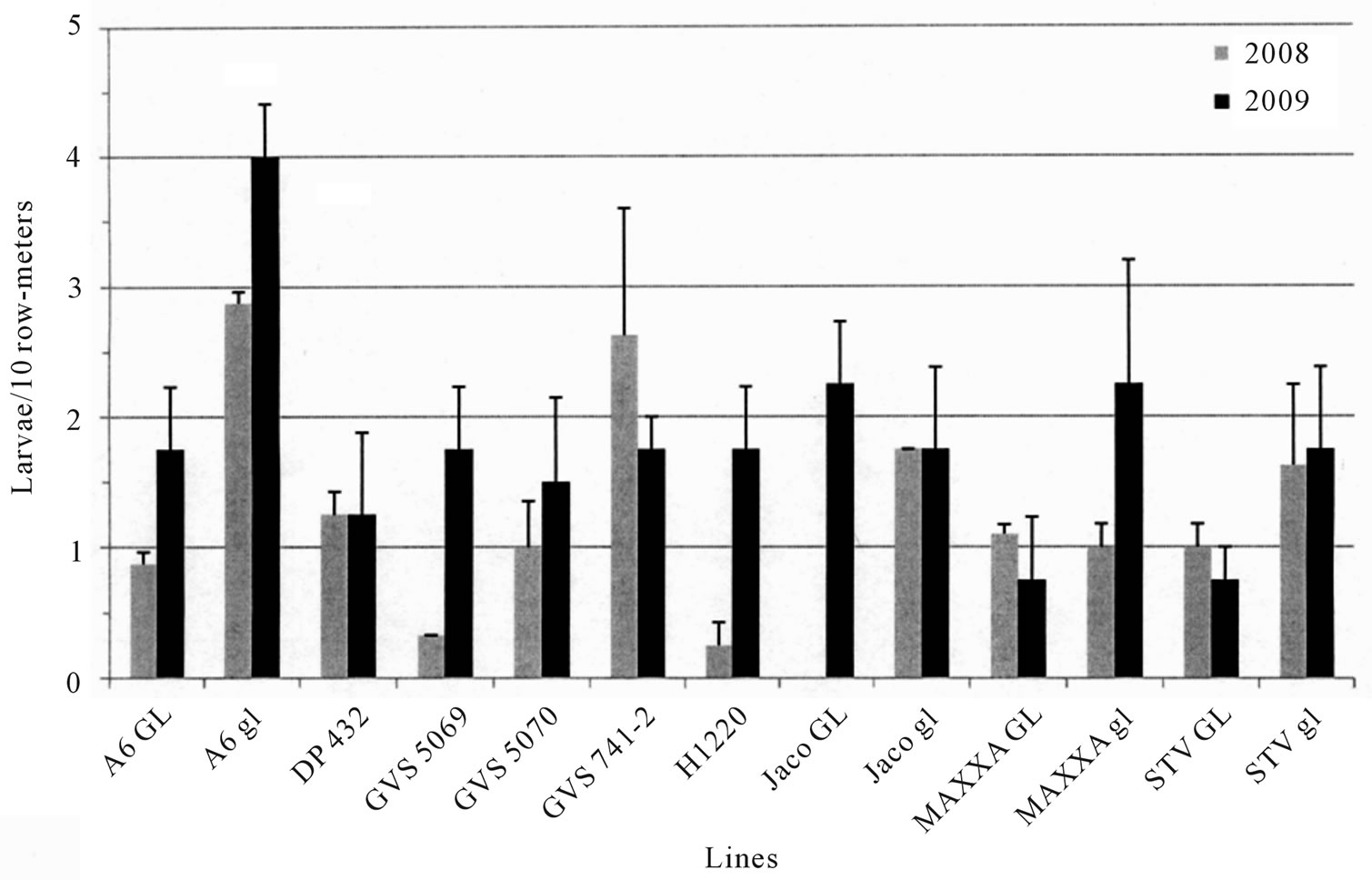
Figure 1. Natural heliothine infestation. Average number of Heliothines per 10 row-meters. The values are the mean of 2 samples per replication and four replications per line.
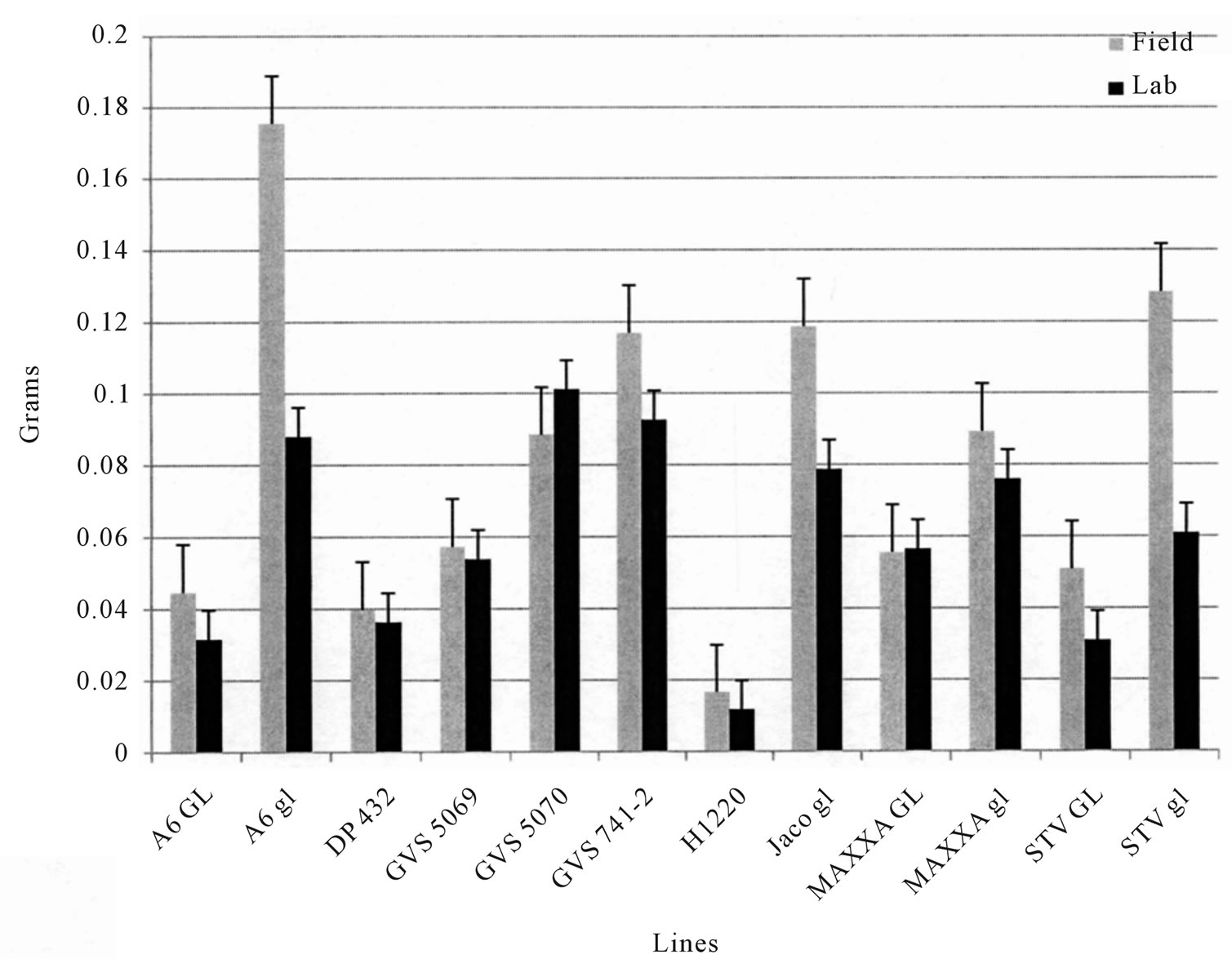
Figure 2. Mean (Std. Err.) weight gain of tobacco budworm (TBW, Heliothis virescens) larvae fed on fresh field grown leaves either in situ or in the laboratory in 2008. Values are the mean of 4 replications, 4 samples per replication.
had leaf gossypol and HGQ comparable to fully glanded lines and the highest average level of total heliocides (Tables 4 and 5) compared to all the lines evaluated. The check H1220 (1.7% seed gossypol) had the highest average level of HGQ and the second highest level of heliocides while DP 432 (1.7% seed gossypol) ranked as one of the lowest fully glanded lines for HGQ and heliocides. These differences were evident in both the
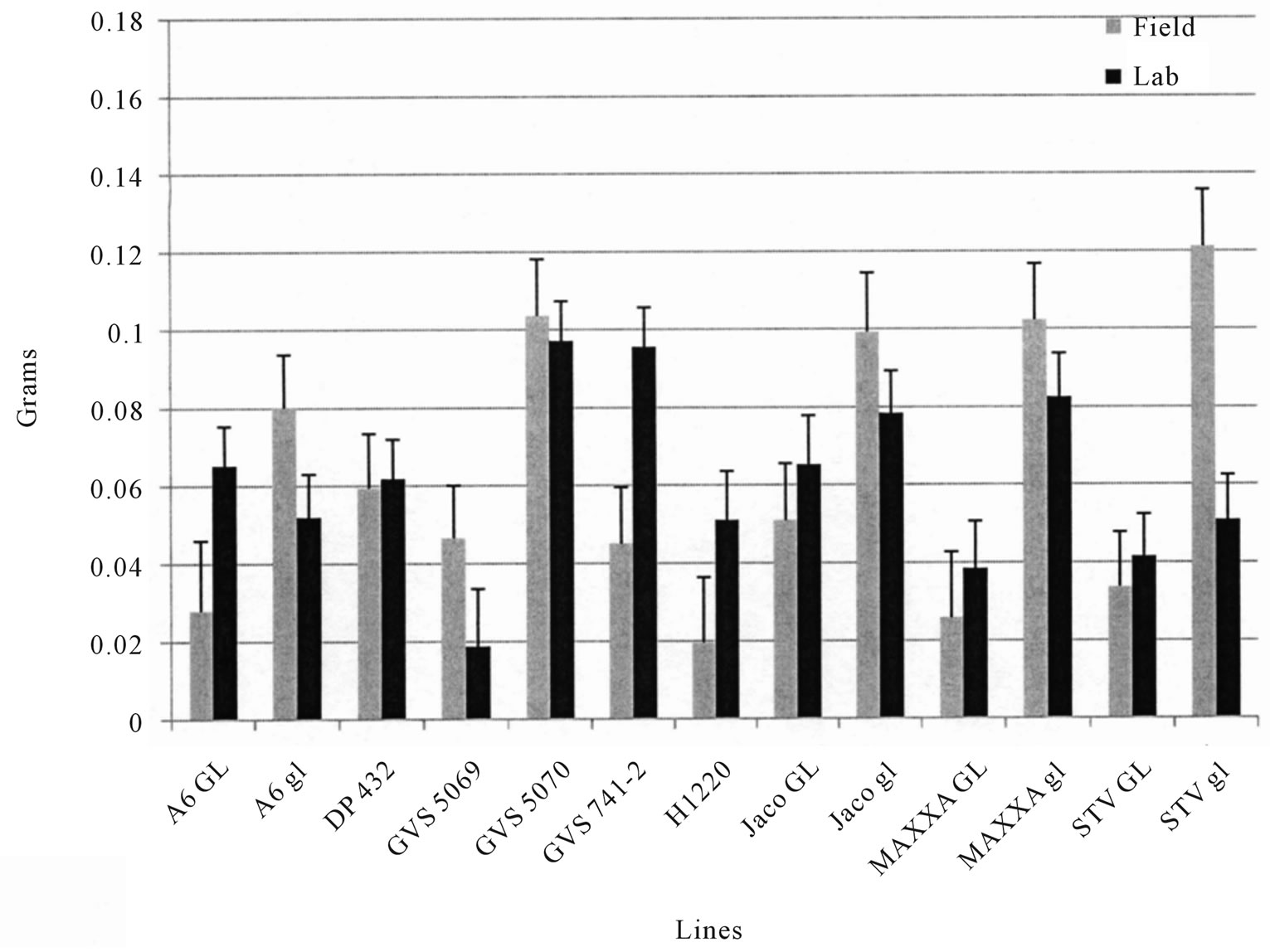
Figure 3. Mean (Std. Err.) weight gain of tobacco budworm (TBW, Heliothis virescens) larvae fed on fresh field grown leaves either in situ or in the laboratory in 2009. Values are the mean of 4 replications, 4 samples per replication.
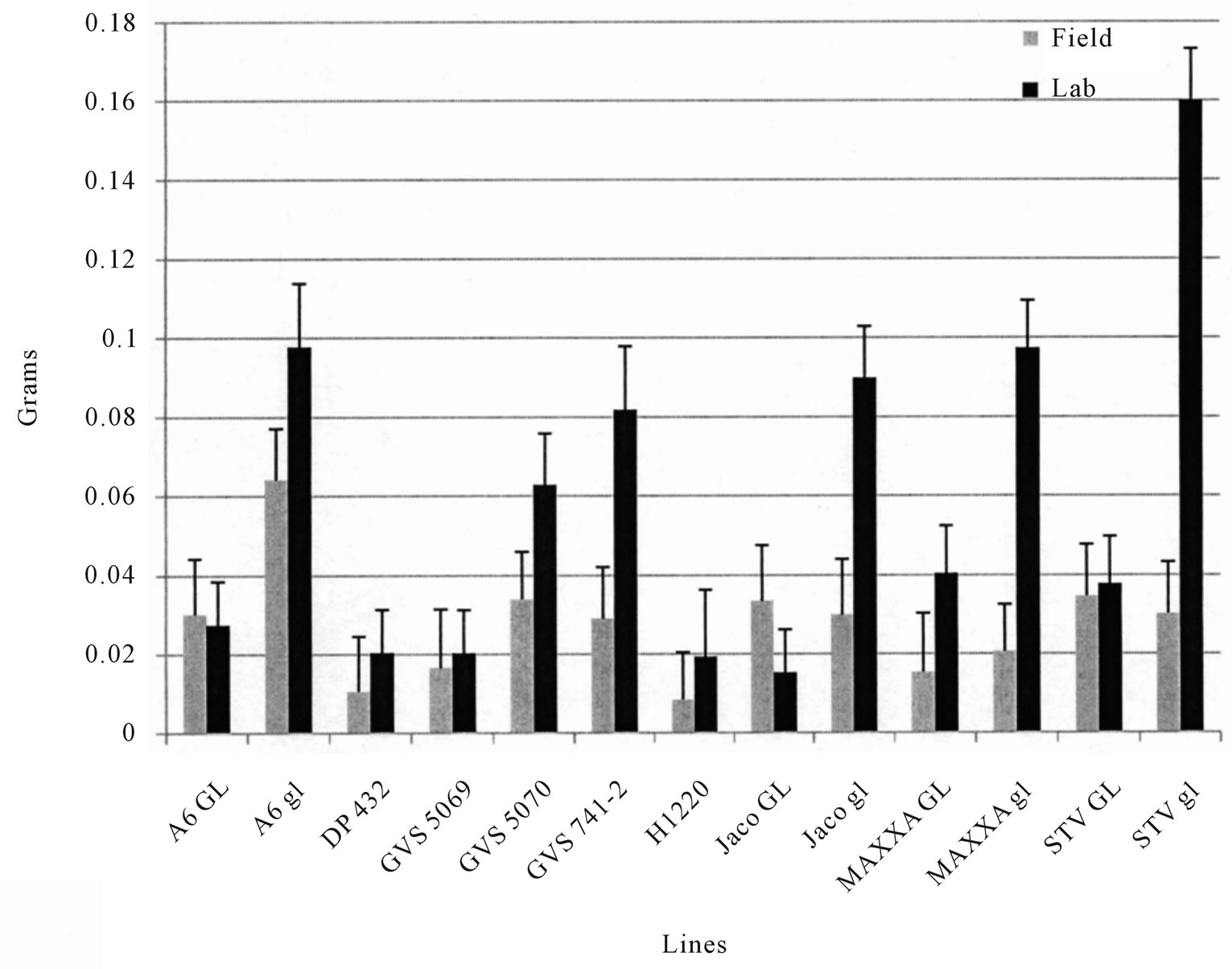
Figure 4. Mean (Std. Err.) weight gain of bollworm (BW, Helicoverpa zea) larvae fed on fresh field grown leaves either in situ or in the laboratory in 2009. Values are the mean of 4 replications, 4 samples per replication.
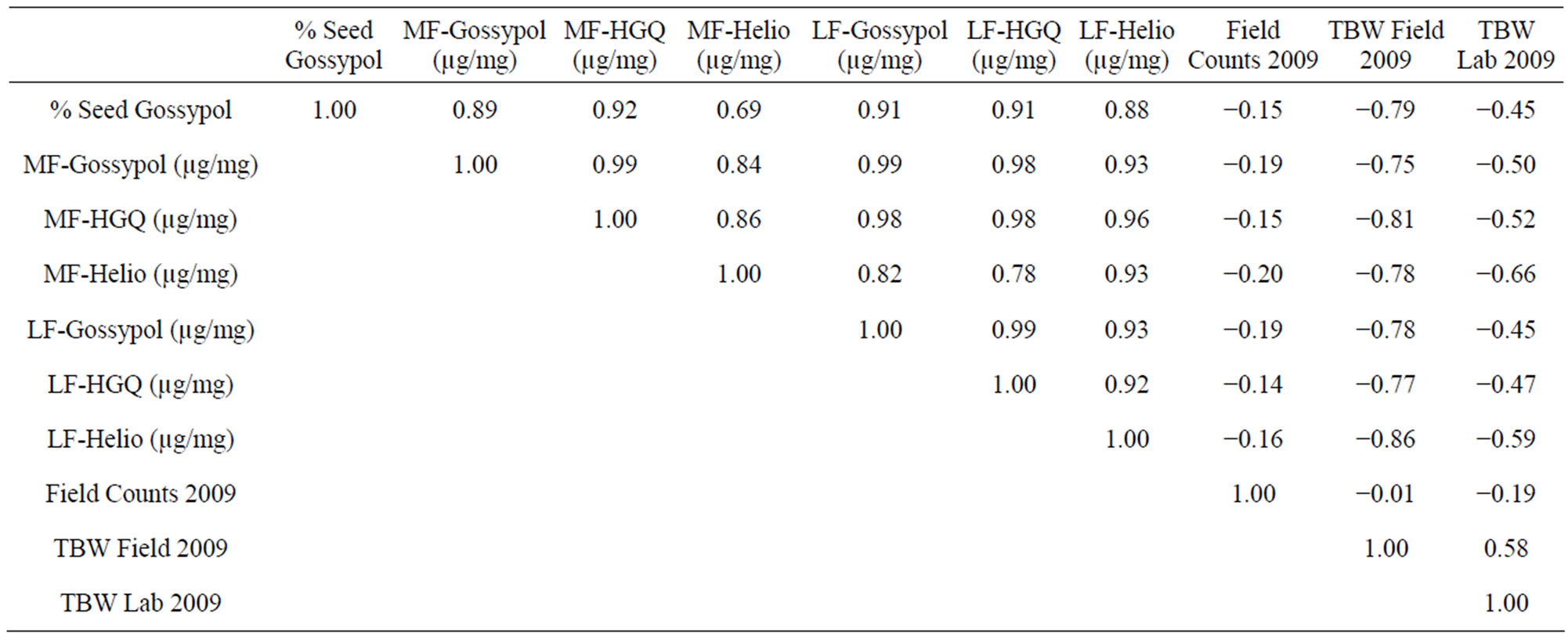
Table 3. Pearson Product Moment Correlations (r value) for mean of the 2009 TA data collected at mid flower (MF, 73 DAP) and late flower (LF, 94 DAP), % seed gossypol, field heliothine counts, larval weight gain in field and laboratory tests.
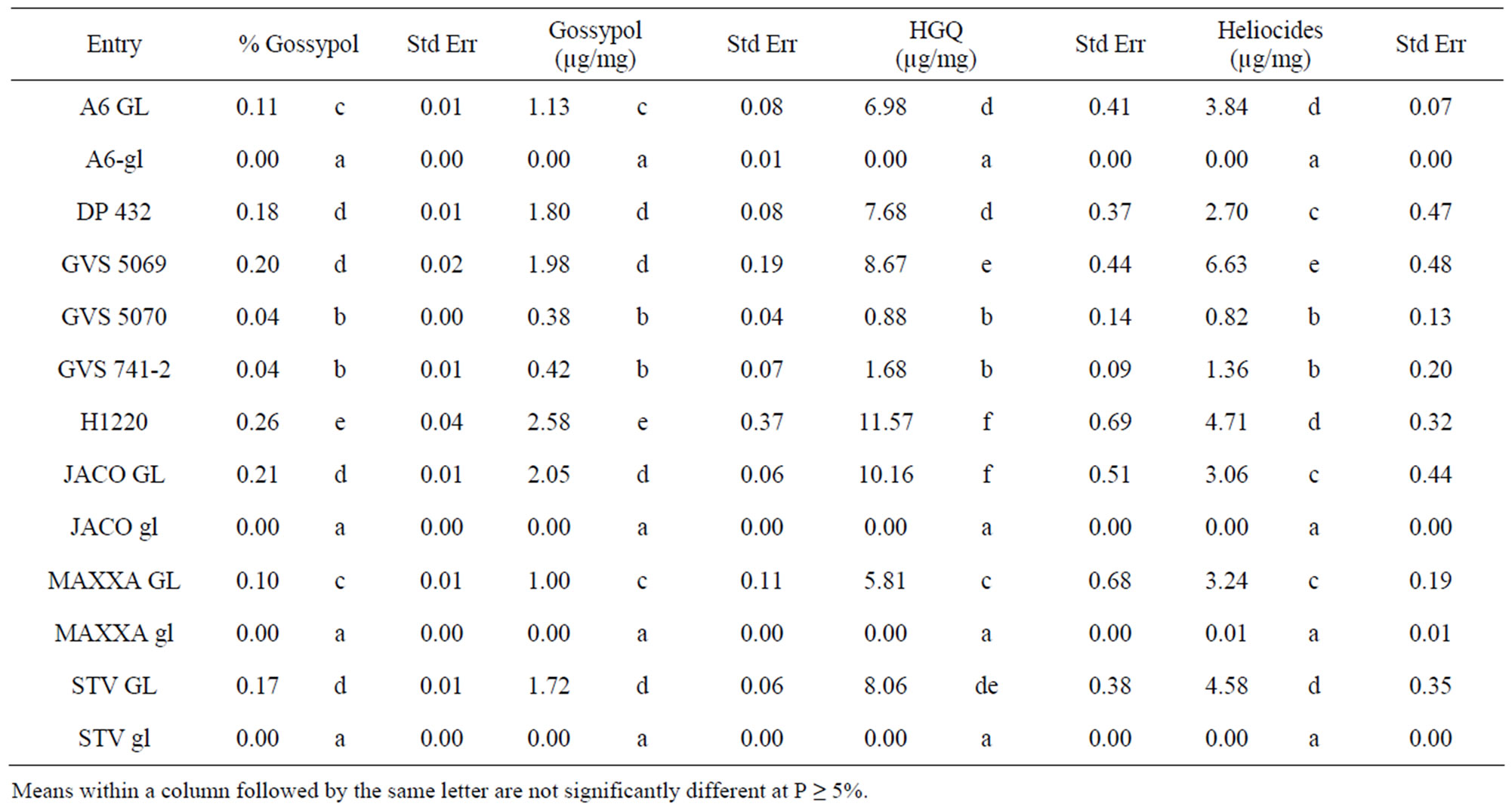
Table 4. Gossypol, hemigossypolone (HGQ) and total heliocide content in terminal leaves collected at mid flower (73 days after flowering) in 2009.
TBW field and laboratory tests.
4. DISCUSSION
Although it is important to document the presence of the insect pests under investigation, natural infestations are often not uniform and it appears that field insect counts alone will not provide a useful selection criterion to evaluate a plant’s ability to resist/tolerate TBW or BW. Field antibiosis evaluations using a known number of larvae feeding on a plant terminal for a specified number of days, provided a better alternative to random counts as reflected in Table 3. Previous studies showed TBW females preferentially laid their eggs on the cotton plant terminal and the newly hatched larvae fed first on the young terminal leaves before migrating to the young squares and young bolls [5,36], so the field test should model normal behavior and provide exposure to existing climatic conditions. It was sometimes difficult to locate the larva, as the terminal had grown markedly during the
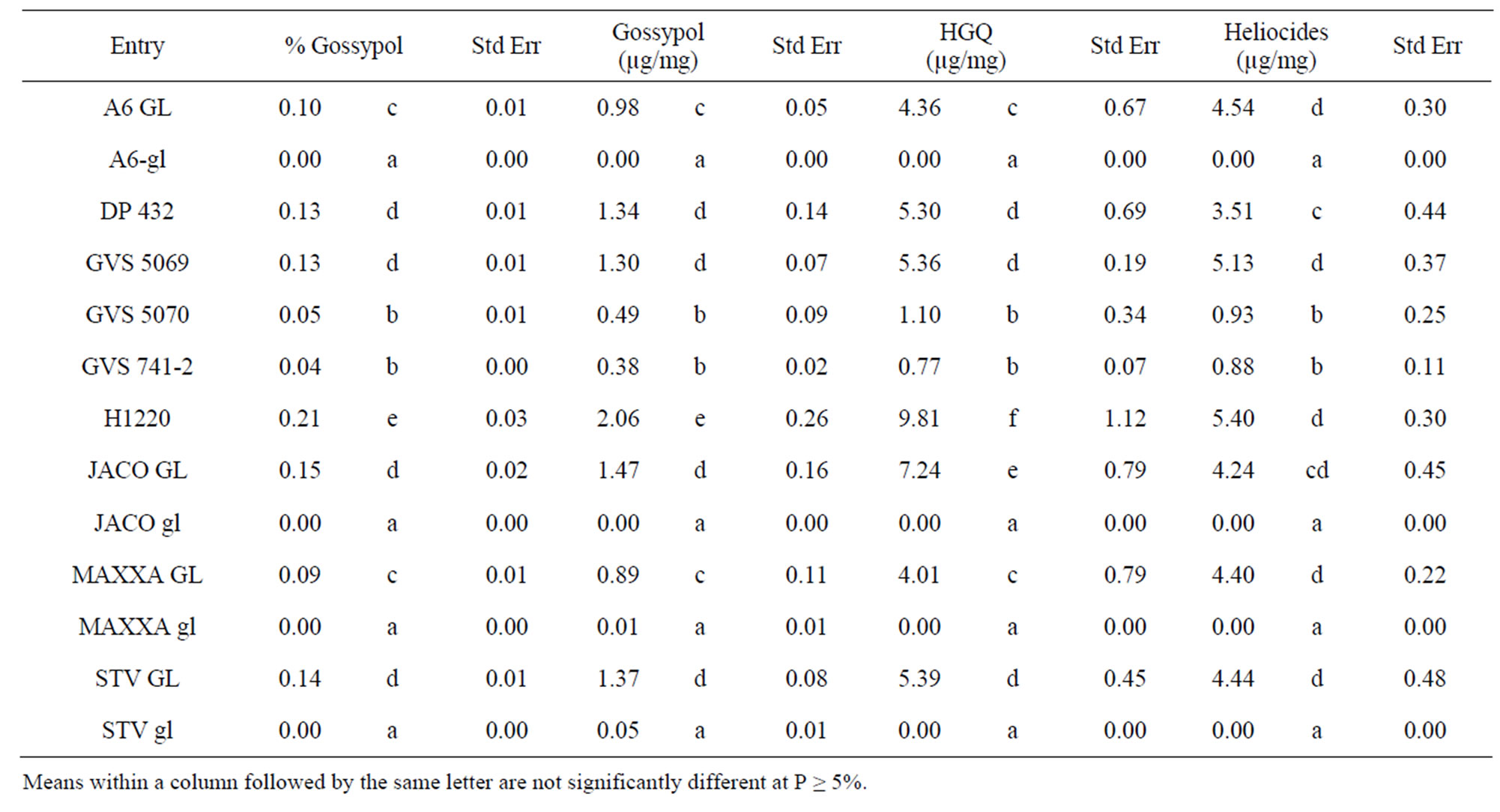
Table 5. Gossypol, hemigossypolone (HGQ) and total heliocide content in terminal leaves collected at mid flower (94 days after flowering) in 2009.
seven day infestation period and larvae were still quite small. However, it was easy to simply cut off the bag enclosing the larva infested plant terminal in the field and process it in the lab. The field test was simple and required minimal inputs and equipment. Although under field conditions, it was not possible to control light, moisture, temperature or other environmental factors that might influence the study, the results in both years indicated it provided reliable results.
In an attempt to control more of the environmental conditions, simplify the evaluation and more accurately measure antibiosis effects, newly emerged leaves were harvested from the plant terminal and brought into the lab. An individual leaf along with one neonate was placed into a 30 ml condiment cup with a lid and allowed to feed for seven days in a climate controlled incubator. Leaves were added as needed to ensure the larva had a fresh and adequate food supply. As each larva was contained individually in a small cup with one leaf, it was easy to recover larvae at the end of the test period.
Despite inability to control environmental conditions in the field, field and laboratory test results were in broad agreement and often as predicted based on the seed and leaf gland density values (Table 3, Figures 2-4); however, there were exceptions. The checks DP 432 (1.7%, 6.0) and H1220 (1.7%, 6.0) had the same values for seed gossypol and leaf gland density (Table 2), but had very different ratings for both the field and laboratory feeding tests. The line GVS 5069, with 0.68% total gossypol and 4.2 gland density score, performed as well or better than the fully glanded lines except H1220. A6 GL had the lowest seed gossypol (1.25%) of all the fully glanded lines, but had comparable HGQ and heliocide values.
In addition to observations that the levels of TAs in leaves and other “green” plant parts were not always correlated with seed gossypol content [6] (Scheffler unpublished data), earlier investigations suggested that other compounds might be effective chemical agents against TBW and BW [5,35,37]. The results presented here indicate that heliocides and HGQ may well play as important a role or, in some cases more important role in protecting the plant from heliothines than gossypol. The levels decreased only slightly during the two weeks from mid-flower to late flower, but it would be useful to know how the levels of TAs change throughout the season and if there were differences between the leaves and other plant parts such as bracts, calyces, petals, and bolls. A previous study by Dilday and Shaver [38], collected the entire flower buds (calyx, petal, ovary and stigma) from four cotton lines at 74 DAP to 116 DAP and measured “gossypol plus other related terpenoid aldehydes” by eliminating the chlorophyll and measuring the absorbance with a spectrophotometer. They found that “gossypol” in the flower buds changed during the season, but they did not measure individual plant parts or separate out gossypol from the other terpenoid aldehydes, which requires some form of chromatography separation such as HPLC.
As demonstrated by GVS 5069 which has low seed gossypol, but high leaf heliocides and HGQ, the levels in different plant organs can be modified independently, providing opportunities for modification through selection. The squares and young bolls are important targets of growing larvae [5,36,37] and the levels in these tissues may be as important as leaf TA levels. To gain a more complete picture of the possible interactions between TBW or BW and cotton plant TA levels, TAs are currently being evaluated in a number of tissues from a range of cotton lines throughout the growing season.
It would be easier to simply select cotton lines based on TA levels in plant tissues than to conduct field and/or laboratory assays. However, a number of researchers have reported other factors including leaf shape, trichome density or nectaries may contribute to a cotton plant’s level of “host plant resistance” [24,25,40] and the total effect of these factors can only be evaluated at the whole plant level. While it may well be possible to do preliminary screening based on TA levels, the advanced material should still be evaluated as whole plants either directly in the field using the method described here to measure weight gain of larvae enclosed on plant terminals or with the laboratory assay that correlated with field results. The plant material used here to evaluate these methods was a well characterized group of lines and the field and laboratory tests demonstrated that a line with reduced seed gossypol and gland density could exhibit antibiosis at the same level or better than fully glanded lines. The next step will be to see if a preliminary TA HPLC based screening, followed by field and laboratory tests would allow the breeder to select for improved HPR in lines with normal glanding and lower seed gossypol. HPR resistance alone will not make a line high yielding, but selecting for higher levels of TAs in addition to yield and fiber quality may give the plant breeder another trait to assure stable yield without added inputs.
5. ACKNOWLEDGEMENTS
The authors are indebted to Robert Stipanovic and Lorraine Puckhaber for sharing their HPLC protocols and Jennifer Tonos for successfully adapting the HPLC assay methods for our lab and Chad Roberts and Pameka Johnson for valuable technical assistance. This work was supported by Cotton Inc. Grants No. 08-399 and No. 09-540.
REFERENCES
- Lusas, E.W. and Jividen, G.M. (1987) Glandless cottonseed: A review of the first 25 years of processing and utilization research. Journal of the American Oil Chemists’ Society (JAOCS), 64, 839-854. doi:10.1007/BF02641491
- Adams, R., Geissman, T.A. and Edwards, J.D. (1960) Gossypol, a pigment of cottonseed. Chemical Reviews, 60, 555-574. doi:10.1021/cr60208a002
- Bottger, G.T., Shehan, E.T. and Lukefahr, M.J. (1964) Relation of gossypol content of cotton plants to insect resistance. Journal of Economic Entomology, 57, 183-185.
- Bell, A.A. and Stipanovic, R.D. (1977) The chemical composition, biological activity, and genetics of pigment glands in cotton. Proceedings of the Beltwide Cotton Conferences, 10-12 January 1977, Atlanta, GA. Cotton Foundation Publisher, Memphis, TN, 244-258.
- Hedin, P.A., Parrott, W.L. and Jenkins, J.N. (1992) Relationships of glands, cotton square terpenoid aldehydes, and other allelochemicals to larval growth of Heliothis virescens (Lepidoptera: Noctuidae). Journal of Economic Entomology, 85, 359-364.
- Stipanovic, R.D., Puckhaber, L.S. and Bell, A.A. (2006) Ratios of (+)- and (–)-gossypol in leaves, stems, and roots of selected accessions of Gossypium hirsutum Var. marie galante (Watt) Hutchinson. Journal of Agricultural and Food Chemistry, 54, 1633-1637. doi:10.1021/jf052319e
- Lee, J.A. (1962) Genetical studies concerning the distribution of pigment glands in the cotyledons and leaves of upland cotton. Genetics, 47, 131-142.
- Lee, J.A. (1965) The genomic allocation of the principal foliar-gland loci in Gossypium hirsutum and Gossypium barbadense. Evolution, 19, 182-188. doi:10.2307/2406373
- Lee, J.A. (1978) Inheritance of gossypol level in Gossypium IV. Results from the reciprocal exchange of the major gossypol-gland alleles between G. hirsutum L. and G. barbadense L. Crop Science, 18, 482-484. doi:10.2135/cropsci1978.0011183X001800030032x
- Lee, J.A., Cockerham, C.C. and Smith, F.H. (1968) The inheritance of gossypol level in Gossypium I. Additive, dominance, epistatic and maternal effects associated with seed gossypol in two varieties of Gossypium hirsutum L. Genetics, 59, 285-298.
- Lee, J.A. (1977) Inheritance of gossypol level in Gossypium III: Genetic potentials of two strains of Gossypium hirsutum L. differing widely in seed gossypol level. Crop Science, 17, 827-930. doi:10.2135/cropsci1977.0011183X001700060002x
- McMichael, S.C. (1959) Hopi cotton, a source of cottonseed free of gossypol pigments. Agronomy Journal, 51, 630. doi:10.2134/agronj1959.00021962005100100025x
- McMichael, S.C. (1960) Combined effects of glandless genes gl2 and gl3 on pigments in the cotton plant. Agronomy Journal, 52, 385-396. doi:10.2134/agronj1960.00021962005200070005x
- Jenkins, J.N., Maxwell, F.G. and Lafever, H.N. (1966) The comparative preference of insects for glanded and glandless cottons. Journal of Economic Entomology, 59, 352-355.
- Hess, D.C. (1977) Genetic improvement of gossypol-free cotton varieties. Cereal World, 22, 98-103.
- Romano, G.B. and Scheffler, J.A. (2008) Lowering seed gossypol content in glanded cotton lines. Plant Breeding, 127, 619-624. doi:10.1111/j.1439-0523.2008.01545.x
- Scheffler, J.A. and Romano, G.B. (2012) Registration of GVS1, GVS2 and GVS3 upland cotton lines with varying gland densities and two near isogenic lines, GVS4 and GVS5. Journal of Plant Registrations, Article in Press.
- Blanco, C.A., Teran-Vargas, A.P., Lopez Jr., J.D., Kauffman, J.V. and Wei, X. (2007) Densities of Heliothis virescens and Helicoverpa zea (Lepidoptera: Noctuidae) in three plant hosts. Florida Entomologist, 90, 742-750. doi:10.1653/0015-4040(2007)90[742:DOHVAH]2.0.CO;2
- Hernandez, G. and Blanco, C.A. (2010) Abundance of Heliothis virescens (Lepidoptera: Noctuidae) during spring in Northwestern Mississippi. Southwestern Entomologists, 35, 361-365. doi:10.3958/059.035.0316
- Luttrell, R.G. (1994) Cotton pest management: Part 2. A U.S. perspective. Annual Review of Entomology, 39, 527- 542. doi:10.1146/annurev.en.39.010194.002523
- Kincaid, R.T., Laster, M.L. and Brazzel, J.R. (1967) Damage to cotton by the tobacco budworm. Journal of Economic Entomology, 60, 1163-1164.
- Pack, T.M. and Tugwell, P. (1976) Clouded and tarnished plant bug injury symptoms and damage on fruit parts. Report Series 226, Arkansas Agricultural Experiment Station, Fayetteville, AR.
- Allen, K.C., Luttrell, R.G. and Parker, C.D. (2009) Influence of within-season densities of heliothines and tarnished plant bugs on variability in end-of-season cotton yield mapping. Journal of Cotton Science, 13, 11-22.
- Jenkins, J.N. and Wilson, F.D. (1996) Host plant resistance. In: King, E.G., Phillips, J.R. and Coleman R.J., Eds., Cotton Insects and Mites: Characterization and Management, Cotton Foundation Publisher, Memphis, TN, 563-597.
- Jones, J.E. (1998) Public breeding efforts in the midsouth—Host plant resistance. Proceedings of the Beltwide Cotton Conferences, 5-9 January 1998, San Diego, CA. Cotton Foundation Publisher, Memphis, TN, 526-535.
- White, C.A., Leonard, B.R., Burris, E. and Graves, J.B. (1999) Laboratory and field evaluations of Bacillus thuringiensis Berliner insecticides against tobacco budworm (Lepidoptera: Noctuidae). Journal of Cotton Science, 3, 92-101.
- Blanco, C.A., Teran-Vargas, A.P., Abel, C.A., Portilla, M., Rojas, M.G., Morales-Ramos, J.A. and Snodgrass, G.L. (2008) Plant host effect of the development of Heliothis virescens F. (Lepidoptera: Noctuidae). Environmental Entomology, 37, 1538-1547. doi:10.1603/0046-225X-37.6.1538
- Sachs, E.S., Benedict, J.H. and Altman, D.W. (1993) Gene pyramiding to improve insect resistance of Bt cotton Proceedings of the Beltwide Cotton Conferences, 10-14 January 1993, New Orleans, LA, Cotton Foundation Publisher, Memphis, TN, 808-813.
- Tabashnik, B.E., Carriere, Y., Dennehy, T.J., Morin, S., Sisterson, M.S., Roush, R.T., Shelton, A.M. and Zhao, J.-Z. (2003) Insect resistance to transgenic Bt crops: Lessons from the laboratory and field. Journal of Economic Entomology, 96, 1031-1038. doi:10.1603/0022-0493-96.4.1031
- Carriere, Y., Ellers-Kirk, C., Biggs, R., Higginson, D.M., Dennehy, T. J. and Tabashnik, B.E. (2004) Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. Journal of Economic Entomology, 97, 1710-1718. doi:10.1603/0022-0493-97.5.1710
- D.T., Bowman, Gutierrez, O.A., Percy, R.G., Calhoun, D.S. and May, O.L. (2006) Pedigrees of upland and pima cotton cultivars released between 1970 and 2005. Bulletin 1155, Mississippi Agricultural and Forestry Experiment Station, Stoneville, MS.
- Calhoun, D.S., Jones, J.E., Dickson, J.I., Caldwell, W.D., Burris, E., Leonard, B.R., Moore, S.H. and Aguillard, W. (1997) Registration of “H1220” cotton. Crop Science, 37, 1013-1014. doi:10.2135/cropsci1997.0011183X003700030068x
- Blanco, C.A., Portilla, M., Abel, C.A., Winters, H., Ford, R. and Street, D. (2009) Soybean flour and wheat germ proportions in insect artificial diet and their effect on the growth rates of Heliothis virescens (F.) (Lepidoptera: Noctuidae). Journal of Insect Science, 5, Article 59. http://www.insectscience.org/9.59/i1536-2442-9-59.pdf
- Scheffler, J.A. and Romano, G.B. (2008) Modifying gossypol in cotton (Gossypium hirsutum L.): A cost effective method for small seed samples. Journal of Cotton Science, 12, 202-209.
- Stipanovic, R.D., Bell, A.A. and Benedict, C.R. (1999) Cotton plant resistance: The role of pigment gland constituents. In: Cutler, H.G. and Cutler, S., Eds., Biologically Active Natural Products, CRC Press, Boca Raton, FL, 211-220. doi:10.1201/9781420048629.ch18
- Wilson, L.T. and Waite, G.K. (1982) Feeding pattern of Australian Heliothis on cotton. Environmental Entomology, 11, 297-300.
- Benson, C.G., Fitt, G.P., Leach, D.N., Mares, C.L., Naiker, M.N. and Wyllie, S.G. (1994) Volatile terpenes and terpenoid aldehydes in Australian-grown Gossypium hirsutum L. cultivars and lines. In: Constable, G.A. and Forrester, N.W., Eds., Challenging the Future: Proceedings of the World Cotton Congress 1, 14-17 February, Brisbane, Australia, 351-355.
- Dilday, R.H. and Shaver, T.N. (1981) Seasonal variation in flowerbud gossypol content in cotton. Crop Science, 21, 956-960. doi:10.2135/cropsci1981.0011183X002100060037x
- Ramalho, F.S., McCarty Jr., J.C., Jenkins, J.N. and Parrott, W.L. (1984) Distribution of tobacco budworm (Lepidoptera: Noctuidae) larvae within cotton plants. Journal of Economic Entomology, 77, 591-594.
- Hedin, P.A., Parrott, W.L. and Jenkins, J.N. (1991) Effects of cotton plant allelochemicals and nutrients on behavior and development of tobacco budworm. Journal of Chemical Ecology, 17, 1107-1121. doi:10.1007/BF01402937

