Open Journal of Yangtze Oil and Gas
Vol.02 No.02(2017), Article ID:75917,17 pages
10.4236/ojogas.2017.22008
Experimental Investigation of CO2-Water-Rock Interactions during CO2 Flooding in Carbonate Reservoir
Na Xiao1, Shi Li2, Meiqin Lin3
1Key Laboratory of Drilling and Production Engineering for Oil and Gas, College of Petroleum Engineering, Yangtze University, Wuhan, China
2Research Institute of Petroleum Exploration & Development, Beijing, China
3Enhanced Oil Recovery Research Center, China University of Petroleum, Beijing, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 20, 2017; Accepted: April 27, 2017; Published: April 30, 2017
ABSTRACT
Injecting CO2 into underground reservoir to displace oil is a viable means of reducing greenhouse gas emission to the atmosphere and enhancing oil recovery. To evaluate the effect of CO2-water-rock interactions on the characteristics of carbonate reservoir at high pressure, the mineralogy of calcite, the ion concentration in the reacted solution, the surface texture of calcite, the permeability of calcite after reacted with injected CO2 and deionized water was investigated by X-ray diffraction (XRD), inductive coupled plasma-ato- mic emission spectrometry (ICP-AES), scanning electronic microscope (SEM), and sand-packed model at pressure of 5.0 MPa. The results show that the mineral dissolution of calcite would occur when interacting with injected CO2 and water. The mineral dissolution of calcite caused the change of surface texture of calcite and increase in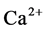 ,
, 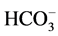 ion concentration in the solution. With the increase of CO2 pressure, the surface dissolution of calcite appeared more obvious. With the increase of reaction temperature, the surface dissolution of calcite also appeared more obvious and
ion concentration in the solution. With the increase of CO2 pressure, the surface dissolution of calcite appeared more obvious. With the increase of reaction temperature, the surface dissolution of calcite also appeared more obvious and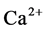 ,
, 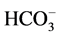 ion concentration in the solution increased first, then decreased. The mineral dissolution of calcite caused the improvement in water permeability of calcite/quartzsand-packed model.
ion concentration in the solution increased first, then decreased. The mineral dissolution of calcite caused the improvement in water permeability of calcite/quartzsand-packed model.
Keywords:
CO2 Flooding, CO2-Water-Rock Interaction, Calcite, Dissolution, Surfacetexture, Permeability

1. Introduction
The emission of carbon dioxide (CO2) is increasing natural greenhouse gas effects. It has been achieved a broad consensus to reduce CO2 emissions on a glo- bal scale. One of technological solutions to reduce CO2 emissions is to inject CO2 into underground reservoirs to displace oil, which can enhance oil recovery effectively and sequestrate CO2 simultaneously, mitigate global warming consequently [1] [2] . CO2 flooding will yield remarkable social and economic benefits [3] [4] .
The CO2-water-rock interactions caused by CO2 flooding of reservoirs are complex [5] [6] [7] . These interactions are highly reservoir specific and cannot easily be generalized [8] [9] . Injected CO2 dissolved in formation water to produce carbonic acid which will react with the reservoir rock [10] [11] [12] . The porosity and permeability of the reservoir [13] [14] [15] [16] [17] , the wettability of the rock surface and the characteristics of the crude oil will be changed by the reaction between carbonic acid and the rock [18] [19] , which will affect the efficiency of CO2 flooding and geological sequestration of CO2 [20] [21] [22] . And the carbonate minerals in the reservoir can especially easily react with CO2 and water to affect reservoir property. Carbonate reservoir is mainly composed of calcite, dolomite, ankerite and magnesite, and these minerals can easily dissolve in carbonate solution which leads to corrosion of the rock and increase of reservoir permeability. Ross [23] found that after the reaction of CO2, water and rock with carbonate mineral, carbonate minerals in the rock dissolved, and it formed a large number of secondary dissolution channels. Ross also analysed ion concentration of the displacement solution and found that the Ca2+ concentration in the solution increased significantly. Izgec [24] pointed out that a large number of loop hole formed in the process of the dissolution of carbonate mineral, it obviously improved the connectivity of pore, and so caused the permeability improved significantly. Raistrick [25] found that after the reaction of carbonate sample, CO2 and water, the concentration of Ca2+, Mg2+ and 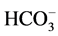 in effluent solution increased significant which indicating the carbonate mineral dissolution. In addition, some scholars also pointed out that the change of physical property of carbonate reservoir was related to formation water pressure, temperature, and rock mineral composition and CO2 partial pressure [26] .
in effluent solution increased significant which indicating the carbonate mineral dissolution. In addition, some scholars also pointed out that the change of physical property of carbonate reservoir was related to formation water pressure, temperature, and rock mineral composition and CO2 partial pressure [26] .
Therefore, in order to carry further study on the main controlling factors which result in the change of reservoir physical properties in the process of CO2 flooding in carbonate reservoir, calcite (main ingredient is CaCO3) was selected to react with CO2 and water in this paper. Then, the surface texture of calcite, the ion concentration in the reacted solution and the permeability of calcite/quartz packed model were investigated after their reaction. The mechanism of interaction of CO2-water-calcite was discussed, which provided the valuable reference for CO2 flooding and geological sequestration of CO2 in the carbonate reservoir.
2. Experimental
2.1. Materials and Preparation of Sample
Two types of calcite materials were used in the study, the plate and the grained. The plate samples were made by cutting calcite material into 8 mm sized blocks, washed with dionized water by ultrasonic vibration, dried at 70˚C for 12 h. The calcite material was crushed to grains and selected the grains with average diameter of 0.45 mm. The calcite material was provided by Shijiazhuang Cuanshi mining company, in China. The purity of CO2 source provided by Beijing Haipubeifen gas limited company was >99.95%. The water used in the experiments was deionized.
2.2. Experimental Methods
1) Calcite component analysis
The clay mineralogy of the calcite was identified and quantified by X-ray diffraction (XRD) (D/MAX 2500, Rigaku Industrial Corporation, Japan) which can determine the mineral composition and content in calcite.
2) Determination of the rocks’ apparent morphology
SEM measurements were carried out on a Leica Cambridge S-360 (Malvern Instruments Ltd., UK) at 25˚C. The acceleration voltage was 20 kV. The resolution of the instrument was 5 nm. The vacuum of the sample room was 1.33 × 10−3 - 1.33 × 10−4 Pa.
First, the calcite was cut and polished into 20 × 20 mm sheet, and the surface of it was cleaned with ultrasonic waves in deionized water. Then the calcite was dried and sticked on specimen holder by conductive adhesive, and it was coated by means of ion sputtering at last. The morphology of coating calcite slice was observed by scanning electron microscope, and then it was placed in the high pressure reactor. After the reaction, the calcite slice was took out and dried, the newly formed or altered surface texture in the reacted rock sample was investigated by SEM again.
3) The static evaluation method of CO2-water-rockchemical interaction
The chemical interaction of CO2-water-rock was identified by exposing the rock samples in a stainless steel reactor of 100 ml (Figure 1), equipped with a Teflon (PTFE) internal cup, manometer and automatic temperature controller, connected to an electric heating apparatus. The sheet calcite sample was put into the bottom of the high pressure reactor, and 100 mL deionized water was added into the high pressure reactor, then the CO2 was injected into it at specific pressure and temperature. After 20 days’ reaction, the calcite slice was took out and dried.
4) Aqueous sampling and ion concentration determination
The grained calcite was mixed with deionized water by ratio of 1:20, w:w, and then CO2 was injected into the reactor to 2.0 MPa. The reacted liquid was sampled every 2 days during reacting for 20 days. The major cation concentrations of the sampled solution were determined by inductive coupled plasma-atomic emission spectrometry, ICP-AES (Proflie, Leeman Labs, USA). The resolution of the instrument is 200 nm. The 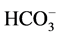 concentration of the sampled liquid was determined by double-tracer technique.
concentration of the sampled liquid was determined by double-tracer technique.
Figure 1. Scheme of equipment used for CO2-water-rock reactions (1. CO2 resource; 2. valve; 3. Reactor; 4. electric heating apparatus; P1, P2 is pressure gauge).
5) Permeability test
Steady state permeability variation caused by the presence of deionized water and CO2 was analyzed on a 30 cm sand-packed model which was a sand pipe filled with grained calcite and quartz with the same diameter mixed by a ratio of 1:1. The original permeability of the model was presented by water permeability. Having placed for 3 days, water permeability of the sand-packed model was measured again. Then CO2 was injected into the model to 5.0 MPa. After that the water permeability of the model was measured every 5 days. The test temperature is 65˚C.
3. Results and Discussion
3.1. Composition Analysis of Calcite
The calcite used in the experiments is white. The crystal structure of calcite was shown in Figure 2, an obvious rhombus structure. According to the formation of the cubic packing arrangement form, 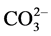 create a diamond surface grid. Most calcite crystal is colorless, transparent. It has smooth plane and straight edge, it aggregates in a variety of forms: flake, fibrous, dense block, membrane and drusy, etc. The result of X-ray diffraction of the calcite was shown in Figure 3. Compared with JCPDS (Joint Committee Powder Diffraction Standard), the major chemical component of the calcite was CaCO3. Based on quantitative calculation of intensity and half band width of the diffraction peak, CaCO3 accounted for 97.8%, so the calcite crystal had high quality.
create a diamond surface grid. Most calcite crystal is colorless, transparent. It has smooth plane and straight edge, it aggregates in a variety of forms: flake, fibrous, dense block, membrane and drusy, etc. The result of X-ray diffraction of the calcite was shown in Figure 3. Compared with JCPDS (Joint Committee Powder Diffraction Standard), the major chemical component of the calcite was CaCO3. Based on quantitative calculation of intensity and half band width of the diffraction peak, CaCO3 accounted for 97.8%, so the calcite crystal had high quality.
Figure 2. Crystal structure of calcite.
Figure 3. X-ray diffraction of calcite.
3.2. Surface Texture of Calcite after Reacted with CO2
In order to investigate the dissolution of rock caused by CO2-water-rock interactions, the surface of calcite slice dissolved by CO2 was observed by scanning electron microscope after the calcite slice reacted with CO2 under different pressure and temperature. The experiment results were shown in Figure 4 and Figure 5.
1) Surface texture of calcite before and after the interactions of CO2-water- calcite at different pressure
The change of the calcite morphology in different pressure and 30˚C was shown in Figure 4. As shown in Figure 4, the surface morphology of calcite




Figure 4. SEM micrographs of calcite samples that pre-and post-reacted with CO2 under different pressures.



Figure 5. SEM micrographs of calcite samples that pre- and post-reacted with CO2 in different temperature.
changed obviously after reaction with CO2 and water. At 30˚C, with the increase of CO2 pressure, honeycomb cave phenomenon of the calcite is more obvious caused by the reaction of CO2. At the pressure of 0.5 MPa, the surface of calcite appeared only a little corrosion phenomenon caused by the reaction of CO2. As the pressure rose to 10 MPa, the surface of calcite appeared obvious corrosion phenomenon, namely the morphology of calcite changed more obvious. This is mainly because the reaction of CO2 and calcite is greater with the increase of CO2 pressure, the surface corrosion of calcite appeared more obviously. This result is consistent with the study by Gilfillan and Tang et al. [27] [28] [29] .
2) Surface texture of calcite before and after the interactions of CO2-water- calcite at different temperature
The change of the calcite morphology in different temperature at 2 MPa was shown in Figure 5. As shown in Figure 5, at the temperature of 60˚C and 90˚C, the reaction of CO2 and calcite is greater than which was at the temperature of 30˚C, and the surface corrosion of calcite appeared more obviously. After the reaction of CO2 and calcite at 60˚C, many large pores appeared in the calcite surface, namely the calcite was dissolved more.
3.3. Ion Concentration in the Solution after Reacted with CO2
The main reason for the changes of the surface morphology of calcite is carbonic acid which is produced when CO2 dissolved in the water. Carbonic acid dissociated hydrogen ion which can react with calcium carbonate in calcite and produced calcium soluble bicarbonate. That is CO2-water-dolomite interactions lead to calcite corrosion. The chemical reaction formula of CO2-water-calcite interactions is shown in formula (1).

There was Ca2+ ion in the solution after calcite dissolution and the dissolution trace was shown in the surface of the calcite. For proving that, the major Ca2+ and 
1) Ion concentration in the reacted solution after the interactions of CO2-wa- ter-calcite at different pressure
Figure 6 and Figure 7 shows that with the increase in reaction time, Ca2+ and 


Figure 6. Ca2+ ion concentration vs. reaction time at different reaction pressure.
Figure 7. 
at the pressure of 0.5 MPa, 2 MPa and 5 MPa, but the increment is small. Ca2+ and 

The major chemical component of calcite is CaCO3 which reacted with carbonic acid to cause the dissolution of calcite. Consequently, Ca2+ and 

2) ion concentration in the reacted solution after the interactions of CO2-wa- ter-calcite at different temperature
Figure 8 and Figure 9 shows the changes of Ca2+ and 
Figure 8 shows that with the increase in reaction time, Ca2+ ion concentration
Figure 8. Ca2+ ion concentration vs. reaction time at different reaction temperature.
in the solution was improved at 30˚C and 60˚C. But the extent of the change of the concentration at different reaction temperature was different. Ca2+ ion concentration in the solution remained unchanged after reacted for 8 days at 30˚C. It took 6 days to reach a plateau of Ca2+ ion concentration in the solution at 60˚C. Ca2+ ion concentration in the reacted solution was higher at 60˚C than the ion concentration in the reacted solution at 30˚C at the same reaction time.
Figure 9 shows that the change trend of 



Figure 8 also shows that Ca2+ ion concentration in the solution reached a
Figure 9. 
maximum after reacted with CO2 for 2 days at 90˚C, and then decreased with the increase in the reaction time. 


3.4. Water Permeability of Sand-Packed Model after Reacted with CO2
The dissolution of calcite to Ca(HCO3)2 in the solution under the interaction of CO2-water-rock would affect the permeability of reservoir rock. Figure 10 shows that the change of water permeability of sand-packed model filled with mixed grained calcite and quartz under the presence of CO2 and water at 65˚C. Before injecting gas, the water permeability of the sand-packed model remained relatively stable during placing for 3 days. After injecting CO2, the water permeability of
Figure 10. Water permeability of sand-packed model vs. reaction time, 65˚C.
the model increased with the increasing of the reaction time. The reason caused the results above was that the grained calcite (CaCO3) in the sand pipe reacted with injected CO2 and water, and partial dissolution of the calcite occurred, which led to the appearance of the larger pores and the increase in the permeability of the model. Therefore, the porosity and permeability of reservoir containing the chemical component of CaCO3 will be likely increased due to the mineral dissolution of CaCO3 during CO2 flooding, which will affect the efficiency of CO2 flooding.
4. Conclusions
1) The mineral dissolution of calcite would occur when interacting with injected CO2 and water, and the dissolution caused the change of surface texture of calcite.
2) The surface dissolution of calcite appeared more obvious with the increase of pressure when reacting with injected CO2 and water.
3) The dissolution of calcite caused the increase in Ca2+, 
4) When the reaction temperature is lower, the dissolution of calcite increases with the increase of temperature. When the reaction temperature reaches up to a certain value, the dissociation constant of carbonic acid decreases which leads to less dissolution of calcite.
5) The dissolution of calcite caused improvement in water permeability of sand-packed model filled with mixed grained calcite and quartz.
Acknowledgements
This project was supported by National Natural Science Foundation of China (41302096) and CNPC Science & Technology Innovation Foundation Project (2015D-5006-0206).
Cite this paper
Xiao, N., Li, S. and Lin, M.Q. (2017) Experimental Investigation of CO2-Water-Rock Interactions during CO2 Flooding in Carbonate Reservoir. Open Journal of Yangtze Gas and Oil, 2, 108-124. https://doi.org/10.4236/ojogas.2017.22008
References
- 1. Jiang, H.Y., Shen, P.P., Li, Z.P., Qi, R.L. and Qi, X.J. (2007) Study on Worldwide Carbon Dioxide Storage and Unitization Methods. International Petroleum Economics, 7, 16-19.
- 2. Shen, P.P., Jiang, H.Y., Chen, Y.W., Li, Y.T. and Liu, J.S. (2007) EOR Study of CO2 Injection. Special Oil and Gas Reservoirs, 14, 1-4.
- 3. Qian, B.Z. and Zhu, J.F. (2008) Present Situation Together with Foreground That CO2 Sequestration and Drive Oil in the World. Energy Environmental Protection, 22, 1-4.
- 4. Xu, Z.G., Chen, D.Z. and Zeng, R.S. (2007) Geological Storage of CO2and Commercial Utilization. Advances in Earth Science, 22, 698-707.
- 5. Yu, Z.C., Yang, S.Y., Liu, L., Li, S. and Yang, Y.Z. (2012) An Experimental Study on Water-Rock Interaction during Water Flooding in Formations Saturated with CO2. Acta Petrolei Sinica, 33, 1032-1042.
- 6. Wang, G.H., Zhao, J., Zhang, F.J., Tao, Y., Yang, X.Y. and Wang, H.Y. (2013) Interactions of CO2 Brine Rock in Sandstone Reservoir. Journal of Central South University: Nature Science Edition, 44, 1167-1173.
- 7. Peng, X.J., Liu, M.Q. and Xia, X.B. (2013) Interaction of Rock-Brine-Supercritical CO2 in Sandstone Reservoir. Journal of China University of Mining & Technology, 42, 302-307.
- 8. Knauss, K.G., Johnson, J.W. and Steefel, C.I. (2005) Evaluation of the Impact of CO2, Co-Contaminant Gas, Aqueous Fluid and Reservoir Rock Interactions on the Geologic Sequestration of CO2. Chemical Geology, 217, 339-350.
- 9. Pauwels, H., Gaus, I., Nindre, Y.M.L., Pearce, J. and Czernichowski, L.I. (2007) Chemistry of Fluids from a Natural Analogue for a Geological CO2 Storage Site (Montmiral, France): Lessons for CO2-Water-Rock Interaction Assessment and Monitoring. Applied Geochemistry, 22, 2817-2833.
- 10. Assayag, N., Matter, J., Goldberg, D., Ader, M. and Agrinier, P. (2009) Water-Rock Interactions during a CO2 Injection Field-Test: Implications on Host Rock Dissolution and Alteration Effects. Chemical Geology, 265, 227-235.
- 11. Emberley, S., Hutcheon, I., Shevalier, M., Durocher, K., Mayer, B., Gunter, W.D. and Perkins, E.H. (2005) Monitoring of Fluid-Rock Interaction and CO2 Storage through Produced Fluid Sampling at the Weyburn CO2 Injection Enhanced Oil Recovery Site. Applied Geochemistry, 20, 1131-1157.
- 12. Kharaka, Y.K., Cole, D.R., Thordsen, J.J., Kakouros, E. and Nance, H.S. (2006) Gas-Water-Rock Interactions in Sedimentary Basins: CO2 Sequestration in the Frio Formation. Journal of Geochemical Exploration, 89, 183-186.
- 13. Bertier, P., Swennen, R., Laenen, B., Lagrou, D. and Dreesen, R. (2006) Experimental Identification of CO2-Water-Rock Interactions Caused by Sequestration of CO2 in Westphalian and Buntsandstein Sandstones of the Campine Basin (NE-Belgium). Journal of Geochemical Exploration, 89, 10-14.
- 14. Gu, L.B., Li, Z.P. and Hou, X.L. (2007) Experimental Research of Reservoir Physical Changes Induced by CO2 Flooding. Journal of Oil and Gas Technology, 29, 258-260.
- 15. Ketzer, J.M., Iglesias, R., Einloft, S., Dullius, J., Ligabue, R. and Lima, V.D. (2009) Water-Rock-CO2 Interactions in Saline Aquifers Aimed for Carbon Dioxide Storage: Experimental and Numerical Modeling Studies of the Rio Bonito Formation (Permian), Southern Brazil. Applied Geochemistry, 24, 760-767.
- 16. Liu, L.H., Suto, Y., Bignall, G., Yamasaki, N. and Hashida, T. (2003) CO2 Injection to Granite and Sandstone in Experimental Rock/hot Water Systems. Energy Conversion and Management, 44, 1399-1410.
- 17. Rosenbauer, R.J., Koksalam, T. and Palandri, J.L. (2005) Experimental Investigation of CO2-Brine-Rock Interactions at Elevated Temperature and Pressure: Implications for CO2 Sequestration in Deep-Saline Aquifers. Fuel Processing Technology, 86, 1581-1597.
- 18. Chalbaud, C., Robin, M., Lombard, J.M., Martin, F., Egermann, P. and Bertin, H. (2009) Interfacial Tension Measurements and Wettability Evaluation for Geological CO2 Storage. Advances in Water Resources, 32, 98-109.
- 19. Nobakht, M., Moghadam, S. and Gu, Y.G. (2008) Mutual Interactions between Crude Oil and CO2 under Different Pressures. Fluid Phase Equilibria, 265, 94-103.
- 20. Sayegh, S.G., Krause, F.F., Girard, M. and Debree, C. (1990) Rock/Fluid Interactions of Carbonated Brines in a Sandstone Reservoir: Pembina Cardium, Alberta, Canada. SPE Formation Evaluation, 54, 399-405.
https://doi.org/10.2118/19392-PA - 21. Omole, O. and Osoba, J.S. (1983) Carbon Dioxide-Dolomite Rock Interaction during CO2 Flooding Process. 34th Annual Technical Meeting of the Petroleum Society of CIM, Banff, 10-13 May 1983, 1-13.
https://doi.org/10.2118/83-34-17 - 22. Marini, L. (2007) Geological Sequestration of Carbon Dioxide Thermodynamics, Kinetics and Reaction Path Modelling. Developments in Geochemistry, 11, 453.
- 23. Ross, G.D., Todd, A.C. and Tweedie, J.A. (1981) The Effect of Simulated CO2 Flooding on the Permeability of Reservoir Rocks. Proceedings of the 3rd European Symposium on Enhanced Oil Recovery, Bournemouth, 21-23 September 1981, 351-366.
- 24. Lzgec, O., Demiral, B., Bertin, H.J., et al. (2006) Experimental and Numerical Modeling of Direct Injection of CO2 into Carbonate Formations. SPE 100809.
- 25. Raistrick, M., Hutcheon, I., Shevalier, M., et al. (2009) Carbon Dioxide-Water-Silicate Mineral Reactions Enhance CO2 Storage; Evidence from Produced Fluid Measurements and Geochemical Modeling at the IEA Wey Burn-Midale Project. Energy Procedia, 1, 3149-3155.
- 26. Zhou, L.S. (2014) Study on the Effect of Water-CO2-Rock Interactions on Physical Properties of Unconsolidated Sandstone Reservoir in the Process of CO2 Flooding. Southwest Petroleum University, Chengdu.
- 27. Ye, J.P., Feng, S.L., Fan, Z.Q., Wang, G.Q., William, D.G., Sam, W. and John, R.R. (2007) Micro-Pilot Test for Enhanced Coalbed Methane Recovery by Injecting Carbon Dioxide in South Part of Qinshui Basin. Acta Petrolei Sinica, 28, 77-80.
- 28. Gilfillan, S.M.V., Ballentine, C.J., Holland, G., Blagburn, D., Lollar, B.S., Stevens, S., Schoell, M. and Gassidy, M. (2008) The Noble Gas Geochemistry of Natural CO2 Gas Reservoirs from the Colorado Plateau and Rocky Mountain Provinces, USA. Geochimica et Cosmochimica Acta, 72, 1174-1198.
- 29. Tang, Y., Du, Z.M., Sun, L., Liu, W. and Chen, Z.H. (2011) Influence of CO2 Dissolving in Formation Water on CO2 Flooding Process. Acta Petrolei Sinica, 32, 311-314.
- 30. Gong, Q.J., Deng, J., Wang, Q.F., Yang, L.Q. and She, M. (2008) Calcite Dissolution in Deionized Water from 50°C to 250°C at 10 MPa: Rate Equation and Reaction Order. Acta Gelologica Sinica, 82, 994-1001.
- 31. Yan, Z.W., Liu, H.L. and Zhang, Z.W. (2009) Influences of Temperature and CO2 on the Solubility of Calcite and Dolomite. Garsologica Sinica, 28, 7-10.
- 32. Cai, X.J. (1993) Temperature and Pressure in Equilibrium State while Calcite, Dolomite and Siderite Reacting on Liquid Water Containing CO2 Respectively. Journal of Mineralogy and Petrology, 13, 37-41.
- 33. Johnson, J.W., Oelkers, E. and Helgeson, H.C. (1992) Software Package for Calculating the Standard Molal Thermodynamic Properties of Minerals, Gases, Aqueous Species and Relations among Them as Functions of Temperature and Pressure. Computers & Geosciences, 18, 899-947.
















