Journal of Minerals and Materials Characterization and Engineering
Vol.05 No.02(2017), Article ID:75040,17 pages
10.4236/jmmce.2017.52008
Adsorption and Post Adsorption Behavior of Schwertmannite with Various Oxyanions
Khandala Khamphila1, Ritsu Kodama1, Tsutomu Sato2, Tsubasa Otake2
1Graduate School of Engineering, Hokkaido University, Hokkaido, Japan
2Faculty of Engineering, Hokkaido University, Hokkaido, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 27, 2017; Accepted: March 28, 2017; Published: March 31, 2017
ABSTRACT
The adsorption and post adsorption behavior of schwertmannite with various oxyanions were investigated for clean-up contaminated water with hazardous oxyanions and safe disposal of spent schwertmannite. The result of adsorption experiments showed that the maximum capacities of oxyanions adsorption onto schwertmannite are 1.023, 0.934, 0.723 and 0.313 mmol/g for arsenate, phosphate, chromate and selenate, respectively. Based on the differences in the adsorption capacities, the selectivity of oxyanion adsorption on schwertmannite decreases as the order: arsenate ≥ phosphate > chromate >> selenate. Change in the Zeta potential after adsorption by arsenate, phosphate and chromate were very different from those after adsorption by selenate and of the original schwertmannite. This difference implies that the adsorption mechanism on schwertmannite with arsenate, phosphate and chromate is different from that with selenate and sulfate. Arsenate, phosphate, and chromate ions form inner-sphere complexes with the surface of schwertmannite, while selenate and sulfate ions form outer-sphere complexes with the surface of schwertmannite. Based on a comparison with anion adsorption, strong base anions form inner-sphere complexes, which induce a strong adsorption with schwertmannite as well as it is conducive to high adsorption capacity. From the results of aging experiments, schwertmannite with sulfate and selenate changed to a more stable phase, goethite, in a short time, whereas there is no change in the XRD patterns of schwertmannite with arsenate and phosphate after 30 days. The stability of schwertmannite after the adsorption increased in the following order: sulfate ≅ selenate  chromate < phosphate ≅ arsenate. The solubility of schwertmannite with different oxyanions was calculated according to solid solution theory. The solubility of schwertmannite decreased after adsorption of oxyanions with high selectivity. It is concluded that oxyanions with high selectivity can stabilize schwertmannite by decreasing the solubility of the schwertmannite after adsorption of the oxyanions.
chromate < phosphate ≅ arsenate. The solubility of schwertmannite with different oxyanions was calculated according to solid solution theory. The solubility of schwertmannite decreased after adsorption of oxyanions with high selectivity. It is concluded that oxyanions with high selectivity can stabilize schwertmannite by decreasing the solubility of the schwertmannite after adsorption of the oxyanions.
Keywords:
Adsorption, Oxyanions, Schwertmannite and Solid Solution

1. Introduction
Water contamination is one of the serious problems worldwide. The source of the contamination is both from anthropogenic as well as from natural sources. For example, the source of arsenic in contaminated water may be from active or abandoned mines, and also mineral processing and sediments [1] [2] [3] [4] [5] . To treat toxic elements such as arsenic, there are numerous established processes such as coagulation/co-precipitation, adsorption, and ion-exchange etc. [6] [7] [8] . Adsorption is an example of simple easily applied techniques. Adsorption has been reported in removal of hazardous cations using activated carbon, zeolites, and clays [9] [10] . However, the removal of toxic oxyanions is more difficult than that of removing cations because natural waters contain anions with similar structures, including nitrate, sulfate, and phosphate often coexisting in high concentrations. However, iron oxides and hydroxides are excellent scavengers both for hazardous cations and also hazardous oxyanions. In iron oxides and hydroxides, there are several mineral species such as goethite, hematite, and ferrihydrite etc. Schwertmannite is a meta-stable iron oxy- and hydroxy-sulfate found in acidic iron- and sulfate-rich environments such as sulfide metal mines [11] [12] [13] [14] . Schwertmannite is well known to play an important role in the removal toxic elements from the acid mine drainage [15] [16] [17] and in natural attenuation processes of hazardous elements in acid mine water [18] .
As mentioned above, schwertmannite is a meta-stable phase and easily transforms to goethite in pure water, while it is also an excellent adsorbent. Both the adsorption capacity and the stability of adsorbents need to pay attention safety at the sites where adsorbents are deposited. However, it is also well known that the stability of schwertmannite is changed and stabilized after arsenate adsorption [19] , but the adsorption and post-adsorption behavior of schwertmannite with other kinds of oxyanions have not been fully or systematically investigated. With this background, the adsorption properties of oxyanions including arsenate, phosphate, chromate, and selenate of schwertmannite were investigated. The stability of schwertmannite after adsorption of the above oxyanions was compared to gain a better understanding of the post-adsorption behavior of schwert- mannite with the different oxyanions.
2. Material and Methods
2.1. Preparation of Schwertmannite
Schwertmannite was prepared by the method previously reported by Bigham et al. [13] . Mixing solution prepared by 0.04 M Na2SO4 solution and 0.04 M 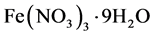 solution was held at 60˚C for 12 minutes, then cooled and dialyzed for 30 days, deionized water used for the dialyzing was changed every day. The produced suspension was filtered through a 0.2 mm cellulose membrane then immediately freeze-dried to prevent transformation to another phases. To evaluate the chemical composition of the schwertmannite, the product was dissolved by 6 M HCl solution, then the Fe contents in the solution were measured using inductively coupled plasma atomic emission spectroscopy (ICPE-9000, ICP-AES). In addition, the product was also dissolved by 0.01 NaOH solution, and the SO42-in the solution was measured using ion chromatography (Metrohm 861 Advanced Compact, IC). The procedures employed in this study are similar to those previously reported [19] . As a result, the chemical formula of the synthetic schwertmannite was determined as Fe8O8(OH)4.88(SO4)1.56. Further, X-ray diffraction (XRD) analyses were conducted to identify the synthesized phases by Rigaku X-Ray diffractometer with CuK
solution was held at 60˚C for 12 minutes, then cooled and dialyzed for 30 days, deionized water used for the dialyzing was changed every day. The produced suspension was filtered through a 0.2 mm cellulose membrane then immediately freeze-dried to prevent transformation to another phases. To evaluate the chemical composition of the schwertmannite, the product was dissolved by 6 M HCl solution, then the Fe contents in the solution were measured using inductively coupled plasma atomic emission spectroscopy (ICPE-9000, ICP-AES). In addition, the product was also dissolved by 0.01 NaOH solution, and the SO42-in the solution was measured using ion chromatography (Metrohm 861 Advanced Compact, IC). The procedures employed in this study are similar to those previously reported [19] . As a result, the chemical formula of the synthetic schwertmannite was determined as Fe8O8(OH)4.88(SO4)1.56. Further, X-ray diffraction (XRD) analyses were conducted to identify the synthesized phases by Rigaku X-Ray diffractometer with CuK  radiation (40 kV and 40 mA). From the XRD analyses, the synthesized products were identified as a schwertmannite because the XRD pattern was identical to that of the previously reported schwertmannite by Bigham et al. [11] .
radiation (40 kV and 40 mA). From the XRD analyses, the synthesized products were identified as a schwertmannite because the XRD pattern was identical to that of the previously reported schwertmannite by Bigham et al. [11] .
2.2. Adsorption Experiments
All adsorption experiments were conducted at a constant ionic strength (I = 0.01M, NaNO3) by using a 50 ml centrifuge tube adding 40 ml solution of Na2HPO4, Na2HAsO4, Na2CrO4, and Na2SeO4 with concentrations from 0 to 2 mM and 40 mg of the synthetic schwertmannite. The pH of the adsorption media was adjusted to 7.00 ± 0.28 by 0.1M NaOH solution. The samples were placed in a reciprocal shaker at 25˚C, 100 rpm for 24 hours. The filtered solids were freeze-dried for measurements of Zeta potential (Malvern Zetasizer Nano series Nano-ZS90 instrument) and liquid samples were used for the inductively coupled plasma atomic emission spectroscopy, (ICPE-9000, ICP-AES) and ion chromatography (Metrohm 861 Advanced Compact IC instrument) to determine the concentration of elements after the adsorption. The released 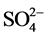 and Fe in the equilibrium solutions were also determined.
and Fe in the equilibrium solutions were also determined.
The amout of anions adsorbedwere calculated by the difference between the initial concentration and concentration in the solution after adsorption as shown in the equation reported previously [20] .
2.3. Aging Experiments
Dried solid schwertmannite was added to the solutions of the anions, for the adsorption process, the pH was adjusted by 0.1 M NaOH and 0.1 HNO3 to 7.00 ± 0.12 and 30 - 35 mg/g of the solid phase of anions of arsenate, phosphate, chromate or selenate were adsorbed on the schwertmannite, separately. The solids after the adsorption were mounted and dried on silica glass. The glasses with the mounted solid samples were kept in boxes with wet cotton at 60˚C to accelerate alterations in a moisture condition. At the different aging times, the samples were analyzed by XRD to determine the extent of the phase transformation.
3. Results
3.1. Oxyanion Adsorption Experiments
As shown in Figure 1, the amounts of adsorbed oxyanions increased with increasing initial concentration of anions. Arsenic ion adsorbed on schwertmannite with the highest adsorption capacity, while only small amount of selenate adsorbed on schwertmannite. The maximum adsorbed anion contents were 1.023, 0.934, 0.723 and 0.313 mmol/g for arsenate, phosphate, chromate, and selenate, respectively. From the differences in the capacity, the selectivity of oxyanion adsorption on schwertmannite decreases as the order: arsenate ≥ phosphate > chromate  selenate.
selenate.
The pHs of the initial solution adjusted to neutral then decreased to 3.5-4.5 after adsorption (Figure 2).
This decrease would be due to release of protons from schwertmannite during the adsorption. Further, the 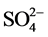 concentration in the reaction solutions was determined after the adsorption because
concentration in the reaction solutions was determined after the adsorption because  originally adsorbed on schwertmannite. The
originally adsorbed on schwertmannite. The 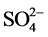 concentration in the reaction solution initially 0.123 ± 0.002 mmol/g without adsorption of any other oxyanions, which is a value similar to that in previously work [21] . During the adsorption process, the released
concentration in the reaction solution initially 0.123 ± 0.002 mmol/g without adsorption of any other oxyanions, which is a value similar to that in previously work [21] . During the adsorption process, the released 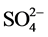 ion concentration in the solution increased with increasing initial concentration of anions in the solution (Figure 3(a)).
ion concentration in the solution increased with increasing initial concentration of anions in the solution (Figure 3(a)).
However, the increasing ratios were different among the anions in the following order: arsenate ≥ phosphate > chromate 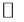 selenite. The released Fe concentration in the solution decreased with increasing initial concentration of
selenite. The released Fe concentration in the solution decreased with increasing initial concentration of
Figure 1. Adsorbed anion contents on schwertmannite as function of the initial concentration of anion in solution (n: Arsenate, p: Phosphate, : Chromate and ¿: Selenate).
Figure 2. Changes in pH of the solution at the different initial concentration of anions during adsorption processes; (a) Arsenate, (b) Phosphate, (c) Chromate and (d) Selenate.
Figure 3. Related SO4 (a) and Fe (b) concentrations from schwertmannite as function of the initial concentration of anions during adsorption process (n: Arsenate, p: Phosphate, : Chromate and ¿: Selenate).
anions in the solution (Figure 3(b)). The decreasing ratios were different among the anions in the following order, arsenate ≥ phosphate > chromate  selenite.
selenite.
The zeta potentials of schwertmannite before and after adsorption of the anions were measured at different pHs (Figure 4). Schwertmannite before ad-
Figure 4. Changes in zeta potential of schwertmannite before and after adsorption with different oxyanions (p: sulfate, £: Arsenate, r: Phosphate, : Chromate and ¯: Selenate.).
sorption, sulfate-schwertmannite, has a Point of Zero Charge (PZC) at around 7. The schwertmannite has positive and negative potentials at pH less and more than 7. Schwertmannite with selenate changes similar to that of schwertmannite with sulfate. PZC of selenite-schwertmannite is at around 6.2. Schwertmannite with arsenate, phosphate, and chromate showed different changes from that of schwertmannite with sulfate with a PZC at around 5. Among arsenate, phosphate, and chromate, schwertmannite with chromate changes is slightly different curve at pH range between 6 and 10 with less negative potentials.
3.2. Aging Experiments
From the results of the aging experiments, the XRD patterns of samples clearly indicated the difference in the stability of schwertmannite with different oxyanions and aging times as shown in Figure 5. In schwertmannite with sulfate ions (pure schwertmannite), the peaks from goethite appeared after 7 days, while the peaks from goethite appeared after 14 days in schwertmannite with selenate. In schwertmannite with chromate, the XRD pattern showed peaks form goethite after 30 days. There are no differences in the XRD patterns for schwertmannite with arsenate and phosphate even after 30 days. In comparison with pure schwertmannite, transformation to goethite was retarded for schwertmannite after adsorption with selenate, chromate, phosphate, and arsenate. The degree of retardation in transformation to goethite decreased as: arsenate = phosphate > chromate > selenate > sulfate.
4. Discussion
4.1. Difference of Adsorption with Different Anions
Among the oxyanions, there are differences in the adsorption capacity. As men-
Figure 5. X-ray diffractogram of the synthetic schwertmannite and schwertmannite after adsorption of each oxyanion with different aging times.
tioned above, the selectivity of oxyanion adsorption on schwertmannite decreases in the following order: arsenate ≥ phosphate > chromate  selenate. At the pH after the reaction, the dominant species of the oxyanions were
selenate. At the pH after the reaction, the dominant species of the oxyanions were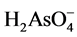 ,
, 


The changes in the Zeta potential after adsorption by arsenate, phosphate and chromate were very different from the adsorption by selenate and that of original schwertmannite. This difference implies that the adsorption mechanism on schwertmannite for arsenate, phosphate and chromate is different from selenate and sulfate. The curves of the Zeta potential after adsorption for oxyanions shifted to negative. The degree of the shift increased in the following order, selenate 
4.2. Anion-Exchange with Different Anions
As described above, adsorption of oxyanions with schwertmannite such as with arsenate, phosphate and chromate is represented by the release of 
The quantity of released protons was estimated from the differences in proton concentrations which were calculated from the speciation analytical by REACT in GWB package [23] . From the slope of the regression line in Figure 6, schwertmannite released 0.61 mmol of SO42-after 1 mmol of As(V) adsorption. This value is similar to the value previously reported by Fukushi et al. [19] . Overall, the anion-exchange reaction can be expressed as below:

where Schindicates schwertmannite with sulfate and As-Sch is the schwertmannite with arsenate. Similarly, the anion-exchange reaction of phosphate with sulfate and chromate with sulfate become express as follows, respectively.

and

Figure 6. Relationship between the amount solid-phase (oxyanions) concentration with (a) released SO4 and (b) released of H+.
In this study, selenate adsorption on schwertmannite was not considered to be an ion-exchange with sulfate because the capacity of selenate adsorption is limited.
Bigham, et al. [13] reported the chemical formula of schwertmannite as follows:
where 

Table 1. Chemical composition of schwertmannite adsorbed by arsenate.
Table 2. Chemial composition of schwertmannite adsorbed by phosphate.
Table 3. Chemical composition of schwertmannite adsorbed by chromate
in the composition of schwertmannite and schwertmannite adsorbed oxyanions; the maximum adsorbed oxyanions are about half of SO4 which remains in schwertmannite. In the natural states based on field observations it was established that the 
From the chemical composition of schwertmannite and schwertmannite adsorbed by oxyanions, the mole numbers of SO4 which could be exchanged are 0.67, 0.61 and 0.47 which correspond to arsenate, phosphate and chromate adsorption, respectively. Therefore, schwertmannite with the maximum amount of oxyanions was as follows:
where St is the principal structural component and Ex is the exchangeable component. This shows that schwertmannite sorbing arsenate (Sch-As), phosphate (Sch-PO4) and chromate (Sch-Cr), which all behave as a solid-solution, are represented by the following chemical formulas, respectively
In the formula above, 
4.3. Estimating the Equilibrium Constant for Anion-Exchange Reaction
The exchange reaction is described by an equilibrium constant and the distribution coefficient ordinarily expressed separately for solid and aqueous solutions [25] [26] [27] . The equilibrium constant can be used to predict the amount of an element in the solid phase when the solution chemistry is known. The anion-exchange reaction from Equations (1)-(3) can then be formulated with end-number composition, similar to the previous work by Fukushi et al. [19] .



The equilibrium constant for Equations (4)-(6) are represented below:



where










where 
Table 4. Activities of dissolove solution speciation and mole fraction of schwertmannite adsorbed by arsenate.
Table 5. Activities of dissolve solution speciation and mole fraction of schwertmannite adsorbed by phosphate.
Table 6. Activities of dissolve solution speciation and mole fraction of schwertmannite adsorbed by chromate.
ficient, 
According to previous study by Fukushi et al. [19] , the 

4.4. Stability of Schwertmannite with Different Oxyanions
The transformation process of schwertmannite to goethite involves dissolution and re-precipitation processes. The results from the calculation of solubility show that a low solubility suggests a high stability of the mineral [28] . In the previous studies, the stability of the mineral was described from consideration of the activity of dissolved species in aqueous solution; this was used to estimate the solubility products to calculate the stability minerals [29] [30] . This corresponds to the experimental results where the association of the solubility between schwertmannite and schwertmannite adsorbed by oxyanions could be a result of the equilibrium constant of the exchange reaction from Equations (7)-(9). [31] [32] [33] [34] . The dissolution reaction equation of the synthesized schwertmannite can be expressed as:

From Equation (13), the equilibrium constant of schwertmannite could be expressed by:

where, the activity of H2O and the mineral is unity. The dissolution reaction equation for schwertmannite which has adsorbed different kinds of oxyanions could be written by:



From Equations (15)-(17), the equilibrium constants could be represented by:



As explained previously, Kd corresponds to Kex which is the equilibrium constant for anions-exchange reaction. Further, Kex could be expressed as the ratio between the dissolution reaction of schwertmannite and schwertmannite adsorbed by different kinds of oxyanions as:



The Kex for the oxyanions indicate the relative solubility of each of the schwertmannite with oxyanions, which are compared with schwertmannite with sulfate (Table 7). The schwertmannite with arsenate has the lowest solubility and is the moststable phase among the one with other oxyanions. From Table 7, the relative solubility of schwertmannite with chromateis is smaller than that of schwertmannite with arsenate and phosphate. This implies that schwertmannite with chromate would be less stable than with arsenate and phosphate. This is consistent with the results of the aging experiments as shown in Figure 5. This is also consistent with the finding of the released Fe concentrations after the oxyanion adsorption as shown in Figure 3(b). Consequently, adsorption by oxyanions with the higher selectivity may be expected to result in meta-stable schwertmannite becoming stable.
5. Conclusions
In this study, the adsorption and post adsorption behavior of schwertmannite with various kinds of oxyanions were investigated to determine the potential for cleaning-up of contaminated water with hazardous oxyanions and a safe disposal of spent schwertmannite. The result of the adsorption experiments shows that the selectivity of oxyanion adsorption on schwertmannite decreases as the following order: arsenate ³ phosphate > chromate 
Table 7. Details of the solubility of schwertmannite with different kinds of oxyanions adsorbed.
ferent from that with selenate and sulfate. Arsenate, phosphate and chromate ions form inner-sphere complexes with the surface of schwertmannite. The selenate and sulfate ions form outer-sphere complexes with the surface of schwert- mannite. Strong base anions such as arsenate and phosphate can form inner- sphere complexes, which induces a strong adsorption with schwertmannite as well as provides a high adsorption capacity.
The stability of schwertmannite after the adsorption increased in the following order: sulfate 


Acknowledgements
The authors wish to express gratitude to assisting technical staff and for the financial support from Laboratory of Environmental Geology, Faculty Engineering, Hokkaido University. The Japan International Cooperation Agency (JICA) provided financial support to KK during the PhD program.
Cite this paper
Khamphila, K., Kodama, R., Sato, T. and Otake, T. (2017) Adsorption and Post Adsorption Behavior of Schwertmannite with Various Oxyanions. Journal of Minerals and Materials Characterization and Engineering, 5, 90-106. https://doi.org/10.4236/jmmce.2017.52008
References
- 1. Kumar, A.R. and Riyazuddin, P. (2011) Speciation of Selenium in Groundwater: Seasonal Variations and Redox Transformations. Journal of Hazardous Materials, 192, 263-269.
- 2. Kotas, J. and Stasicka, Z. (2000) Chromium Occurrence in the Environment and Methods of Its Speciation. Environmental Pollution, 107, 263-283.
- 3. Richard, F.C. and Bourg, A.C.M. (1991) Aqueous Geochemistry of Chromium: A Review. Water Research, 25, 807-816.
- 4. Smedley, P.L. and Kinniburgh, D.G. (2002) A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Applied geochemistry, 17, 517-568.
- 5. Saxena, V.K., Kumar, S. and Singh, V.S. (2004) Occurrence, Behaviour and Speciation of Arsenic in Groundwater. Current Science, 86, 281-284.
- 6. Rubio, J., Souza, M.L. and Smith, R.W. (2002) Overview of Flotation as a Wastewater Treatment Technique. Mineral Engineering, 15,139-155.
- 7. Mohan, D. and Pittman, C.U. (2007) Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. Journal of Hazardous Materials, 142, 1-53.
- 8. Gonzalez, C.M., Hernandez, J., Parsons, J.G. and Gardea-Torresdey, J.L. (2010) A Study of the Removal of Selenite and Selenate from Aqueous Solutions Using a Magnetic Iron/Manganese Oxide Nanomaterial and ICP-MS. Microchemical Journal, 96, 324-329.
- 9. Sawhney, B.L. (1972) Selective Sorption and Fixation of Cations by Clay Minerals: A Review. Clays and Clay Mineral, 20, 93-100.
https://doi.org/10.1346/CCMN.1972.0200208 - 10. Erdem, E., Karapinar, N. and Donat, R. (2004) The Removal of Heavy Metal Cations by Natural Zeolites. Journal Colloid and Interface Science, 280, 309-314.
- 11. Bigham, J.M., Schwertmann, U., Carlson, L. and Murad, E. (1990) A Poorly Crystallized Oxyhydroxysulfate of Iron Formed by Bacterial Oxidation of Fe(II) in Acid Mine Waters. Geochimica et Cosmochimica Acta, 54, 2743-2758.
- 12. Bigham, J.M., Carlson, L. and Murad, E. (1994) Schwertmannite, a New Iron Oyhydroxy-Sulfate from Pyhasalmi, Filand and Other Localitites. Mineralogical Magazine, 58, 641-648.
https://doi.org/10.1180/minmag.1994.058.393.14 - 13. Bigham, J.M., Schwertmann, U., Traina, S.J., Winland, R.L. and Wolf, M. (1996) Schwertmannite and the Chemical Modeling of Iron in Acid Sulfate Waters. Geochimica et Cosmochimica Acta, 60, 2111-2121.
- 14. Waychunas, G.A., Myneni, S.C.B., Traina, S.J., Bigham, J.M., Fuller, C.C. and Davis, J.A. (2001) Reanalysis of the Schwertmannite Structure and the Incorporation of SO42-Groups: An IR, XAS, WAXS and Simulation Study. Lawrence Berkeley National Laboratory.
- 15. Webster, J.G., Swedlund, P.J. and Webster, K.S. (1998) Trace Metal Adsorption onto an Acid Mine Drainage Iron(III) Oxy Hydroxy Sulfate. Environmental Science and Technology, 32, 1361-1368.
https://doi.org/10.1021/es9704390 - 16. Liao, Y., Liang, J. and Zhou, L. (2011) Adsorptive Removal of As(III) by Biogenic Schwertmannite from Simulated As-Contaminated Groundwater. Chemosphere, 83, 295-301.
- 17. Paikaray, S., Göttlicher, J. and Peiffer, S. (2011) Removal of As(III) from Acidic Waters Using Schwertmannite: Surface Speciation and Effect of Synthesis Pathway. Chemical Geology, 283, 134-142.
- 18. Fukushi, K., Sasaki, M., Sato, T., Yanase, N., Amano, H. and Ikeda, H. (2003) A Natural Attenuation of Arsenic in Drainage from an Abandoned Arsenic Mine Dump. Applied Geochemistry, 18,1267-1278.
- 19. Fukushi, K., Sato, T. and Yanase, N. (2003) Solid-Solution Reactions in As(V) Sorption by Schwertmannite. Environmental Science & Technology, 37, 3581-3586.
https://doi.org/10.1021/es026427i - 20. Vanderborght, B.M. and Van Grieken, R.E (1977) Enrichment of Trace Metals in Water by Adsorption on Acitivated Carbon. Analytical Chemistry, 49, 311-316.
https://doi.org/10.1021/ac50010a032 - 21. Fukushi, K., Sato, T., Yanase, N., Minato, J. and Yamada, H. (2004) Arsenate Sorption on Schwertmannite. American Mineralogist, 89, 1728-1734.
https://doi.org/10.2138/am-2004-11-1219 - 22. Burton, E.D., Bush, R.T., Johnston, S.G., Watling, K.M., Hocking, R.K., Sullivan, L.A. and Parker, G.K. (2009) Sorption of Arsenic(V) and Arsenic(III) to Schwertmannite. Environmental Science & Technology, 43, 9202-9207.
https://doi.org/10.1021/es902461x - 23. Bethke, C.M. (2007) Geochemical and Biogeochemical Reaction Modeling. 2nd Edition, Cambridge University Press, Cambridge.
https://doi.org/10.1017/CBO9780511619670 - 24. Carlson, L., Bigham, J.M., Schwertmann, U., Kyek, A. and Wagner, F. (2002) Scavenging of As from Acid Mine Drainage by Schwertmannite and Ferrihydrite: A Comparison with Synthetic Analogues. Environmental Science & Technology, 36, 1712-1719.
https://doi.org/10.1021/es0110271 - 25. Sverjensky, D.A. (1984) Prediction of Gibbs Free Energies of Calcite-Type Carbonates and the Equilibrium Distribution of Trace Elements between Carbonates and Aqueous Solutions. Geochimica et Cosmochimica Acta, 48, 1127-1134.
- 26. Tesoriero, A.J. and Pankow, J.F. (1996) Solid Solution Partitioning of Sr2+, Ba2+, and Cd2+ to Calcite. Geochimica et Cosmochimica Acta, 60, 1053-1063.
- 27. Rimstidt, J.D., Balog, A. and Webb, J. (1998) Distribution of Trace Elements between Carbonate Minerals and Aqueous Solutions. Geochimica et Cosmochimica Acta, 62, 1851-1863.
- 28. Knorr, K.H. and Blodau, C. (2007) Controls on Schwertmannite Transformation Rates and Products. Applied Geochemistry, 22, 2006-2015.
- 29. Dove, P.M. and Rimstidt, J.D. (1985) The Solubility and Stability of Scorodite, FeAsO4•2H2O. American Mineralogist, 70, 838-844.
- 30. Yu, J.Y., Heo, B., Choi, I.K., Cho, J.P. and Chang, H.W. (1999) Apparent Solubilities of Schwertmannite and Ferrihydrite in Natural Stream Waters Polluted by Mine Drainage. Geochimica et Cosmochimica Acta, 63, 3407-3416.
- 31. Busenberg, E. and Plummer, L.N. (1989) Thermodynamics of Magnesian Calcite Solid-Solutions at 25℃ and 1 atm Total Pressure. Geochimica et Cosmochimica Acta, 53, 1189-1208.
- 32. Glynn, P.D. and Reardon, E.J. (1990) Solid-Solution Aqueous-Solution Equilibria: Thermodynamic Theory and Representation. American Journal of Science, 290, 164-201.
https://doi.org/10.2475/ajs.290.2.164 - 33. Plummer, N.L., Busenberg, E., Glynn, P.D. and Blum, A.E. (1992) Dissolution of Aragonite-Strontianite Soild Solutions in Nonstoichiometric Sr(HCO3)2-Ca(HCO3)2-CO2-H2O Solutions. Geochimica et Cosmochimica Acta, 56, 3045-3072.
- 34. Barham, R.J. (1997) Schwertmannite: A Unique Mineral, Contains a Replaceable Ligand, Transforms to Jarosites, Hematites, and/or Basic Iron Sulfate. Journal of Materials Research, 12, 2751-2758.
https://doi.org/10.1557/JMR.1997.0366















