Journal of Minerals and Materials Characterization and Engineering
Vol.04 No.01(2016), Article ID:62818,7 pages
10.4236/jmmce.2016.41004
Investigation of Aloe lateritia Gel as Corrosion Inhibitor for Mild Steel in 2 M HNO3 and 1 M H2SO4 Media
Lucas Paul1*, Revocatus L. Machunda2
1Department of Materials and Energy Science & Engineering, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
2Department of Water and Environmental Science and Engineering, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 December 2015; accepted 15 January 2016; published 18 January 2016
ABSTRACT
Corrsion inhibition of Aloe lateritia gel for Mild steel in 2 M HNO3 and 1 M H2SO4 solutions was investigated by potentiodynamic polarization, Scanning electron microscopy (SEM) and Foutier transform infrared (FT-IR). Inhibition efficiency increased with the increase of the concentration of the gel. The optimal concentration of the gel gives maximum inhibition efficiency of 77.4% and 70.3% in 1 M H2SO4 and 2 M HNO3 respectively. Polarization plots shows that, the gel works as a mixed type inhibitor altering both cathodic and anodic reaction. SEM proves the uniform and pitting corrosion at the surface of Mild steel in 1 M H2SO4 and 2 M HNO3 respectively. Using FT-IR potential function groups from pure gel and some stretch shift was observed from corrosion product and some stretch shift from corrosion products was observed.
Keywords:
Corrosion Inhibitor, Mild Steel, Potentiodynamic Polarization, Sulfuric Acid, Nitric Acid

1. Introduction
Mild steel is popular in the construction of different structures like pipelines, thermal chemical reactor and cooling system, since it is excellent in performance, highly recyclable, high life span, strength as well as more ductile [1] .
Corrosion is the catastrophic deterioration of material’s properties due to electrochemical reaction caused by potential differences between the oxidant (corrosive medium) and reductant (metals). The external energy absorbed by the metal during their extraction is the driving forces to return to their stable oxidation state (ores) by corrosion, since always exist in the temporary unstable state.
Mild steel suffers from corrosion when exposed acidic medium. For this study 2 M HNO3 and 1 M H2SO4 is going to be used as the corrosion medium. Nitric acid participates during corrosion by cathodic reactions:
 (1)
(1)
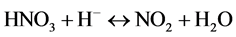 (2)
(2)
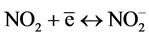 (3)
(3)
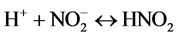 (4)
(4)
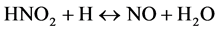 (5)
(5)
Interaction between nitrous acid (HNO2) from Equation (4) and nitric acid (HNO3) produces nitrogen dioxide (NO2).
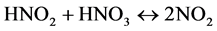 (6)
(6)
Finally the cathodic reaction becomes autocatalytic, where NO2 combine with ammonium (NH3) to produce ammonium salt (NH4NO2) and (NH4NO3) which finally decomposes to give nitrogen gas (N2 (g)) and nitrogen oxides. These products are accompanied by brown gas evolution.
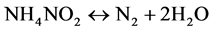 (7)
(7)
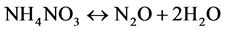 (8)
(8)
As reported by [2] .
Sulphuric acid is the most used chemicals in almost every industry like in petroleum production, fertilizer manufacture, paint production, metal pickling and extraction of non-metals [3] .
Major challenge with acid like H2SO4 and HNO3 they accelerate corrosion in several equipments, so there is a need to be inhibited. There are several methods has been used to control and prevent corrosion. However, use of corrosion inhibitor is more effective, especially in corrosion enhanced by acid. Inhibitor performs well due to ability to create a barrier for interaction between the materials and the medium. This is due to presence of electrons rich functional groups and atom like Oxygen (O), Sulpher (S), Nitrogen (N) and the multiple bonds in their conjugate system [4] .
Synthetic corrosion inhibitors used for several years have been reported to be unfriendly to the environment; therefore, searching for green ones is inevitable. Compounds from plants recently have been recognized to be the best eco-friendly inhibitors [5] - [8] .
This study intends to investigate the insight about corrosion of Mild steel in 1 M H2SO4 and 2 M HNO3 in presence of Aloe lateritia gel. The use of Aloe lateritia gels as corrosion inhibitor has been hardly reported. The inhibitory effect of these naturally occurring plant gels on corrosion of Mild steel in 1M H2SO4 and 2 M HNO3 was investigated by potentiodynamic polarization. Additionally, surface analysis was obtained from SEM. FT-IR was used to identify functional groups from the gels and that of corrosion products from metals which were inhibited.
Potentiodynamic polarization was used to control the electrochemical reaction of corrosion by monitoring Anodic and Cathodic reactions:
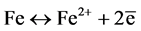 (9)
(9)
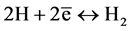 (10)
(10)
This is useful to determine the corrosion rate, passivation especially the region where metal is active and noble. It helps to determine pitting tendency of metal during corrosion reaction. The overall reaction produces the anodic current, for uninhibited system produces more current as compared to inhibited; due to high dissolution of metal. The expression below in Equation (11) uses the current to determine the inhibition efficiency when inhibitor is used [9] .
 (11)
(11)
where %IE is inhibition efficiency, 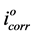 and icorr are corrosion current densities of unhibited and inhibited solutions respectively.
and icorr are corrosion current densities of unhibited and inhibited solutions respectively.
To determine extend of corrosion, SEM was used to monitor the level of deterioration of the metal surface. Inhibited metal expected to have a smooth surface comparatively with uninhibited; this is due to fact that inhibitor prevents interaction of the metal and the surrounding medium [10] . FT-IR was used to determine function groups of the inhibitor before and after being applied therefore, obtained corrosion product has comparable functional groups to that of inhibitor; this is along with that reported by [11] .
2. Materials and Methods
2.1. Sample Preparation
2 M HNO3 and 1 M H2SO4 was prepared by diluting 69% HNO3 and 98% H2SO4 with distilled water as corrosive medium. Plant inhibitor Aloe lateritia gel was obtained from the western part of Kahama district at Wendere village, Tanzania. The gel was obtained by cutting the leaves and squeezing, then filtering through the mesh of cloth to get pure gel. About 1 kg of fresh leaves was used to obtain 2 Litters of gel. It was kept cool in a refrigerator prior to testing this is, in similar to what reported by [12] . Concentration of inhibitors: (0%, 3.2%, 6.3%, 9.1%, 11.8%, 14.3% v/v) was used at room temperature.
Mild steel with following composition was used: C―0.15, Mn―1.26, V―0.017, Si―0.035, S―0.008, Cr―0.036, Ni―0.03, Al―0.083, Cu―0.038 and the remainder is Fe. The metal was cut into 2 cmx1 cm as working electrode for electrochemical measurements. The surface of metal was pre-cleaned using silicon carbide paper; it was made to silver mirror by cleaning using diamond paste. Finally, by using acetone and distilled water in an utrasonicator bath was washed and rinsed then stored for use.
2.2. Potentiodynamic Polarization
The experiment was performed in a conversational cell with three electrodes connected to a potentiostat (PGSTAT 302N). Mild steel was used as working electrode in 2 M HNO3 and 1 M H2SO4 corrosive medium. Platinum wire, saturated calomel electrode was used as a counter and reference electrodes respectively. The plots used to obtain the tafel curve were done by changing the potential from −250 to +250 mV at scan rate of 1mV∙s−1 against open circuit potential.
2.3. SEM for Surface Morphology Analysis
The surface of Mild steel exposed to uninhibited 2 M HNO3 and 1 M H2SO4 and to that containing optimal concentration of inhibitor, was examined by Field Scanning Electron Microscope (FE-SEM, Hilachi, S-4700). Before exposing to SEM the metal was cleaned thoroughly to remove the oxide layer and after 24 hours prior-o to measurement was washed using running distilled water and dried. Corrosion products were obtained by removing the oxide surface of the metal using the utrasonicator, and then were submitted for measurement.
2.4. FTIR for Functional Group Identification
The inhibitor gels and the corrosion products were subjected to Varian 660-IR for the determination of appropriate functional groups available. Few drops of Aloe lateritia gel and the corrosion products obtained after 24 hours of metal corrosion in optimal inhibitor were subjected to FTIR measurements.
3. Results and Discussion
3.1. Potentiodynamic Polarization
It was used to obtain polarization curves for the Mild steel in 2 M HNO3 and 1 M H2SO4 with and without the addition of Aloe lateritia gel.
The plots from Figure 1 and Figure 2 reveal that Tafel plot was obeyed by anodic and cathodic reactions. The linear part of the Tafel plots were extrapolated, at the point of intersection is where corrosion potential (Ecorr)
Figure 1. Polarization curves of mild steel in 2 M HNO3 in presence with different concentration of Aloe lateritia gel.
Figure 2. Polarization curves of mild steel in 1 M H2SO4 with different concentration of Aloe lateritia gels.
and corrosion current density (icorr) were obtained.
Related parameters like Cathodic Tafel slope (bc), anodic Tafel slope (ba), corrosion current density (icorr) and corrosion potential (Ecorr) were calculated and results are tablated in Table 1.
An increase in the concentration of inhibitor resulted in the following:
There is equal shift for the potential toward more negative and positive relating to cathodic and anodic respectively; this indicates that reaction mechanism was the same. From Table 1 it is observed that there is a decrease of corrosion current density (icorr) contrary to inhibitor concentration. This is attributed by the ability of inhibitor to prevent interaction between metal and the corrosive medium which leads to corrosion. Although, the corrosion current density (icorr) decrease as inhibitor’s concentration increases; no remarkable shift of corrosion potential (Ecorr) was observed. This is to say a mixed type inhibition for both anodic and cathodic reactions took place, the observation is associated with that reported by [13] .
Inhibition efficiency (%IE) was obtained by Equation (11) when the concentration of inhibitor increased %IE increases too. From Table 1 at 14.3% v/v of inhibitor %IE reached maximum 77.4% for 1M H2SO4 while for 2M HNO3%IE increased with concentration of inhibitor up to 11.8%v/v which attained 70.3%, then started to decrease as concentration increased. So the inhibitor performs better with H2SO4 than HNO3.
3.2. Surface Morphology
Mild steel was exposed to 1M H2SO4 and 2M HNO3 medium in the absence and presence of optimal amount
Aloe lateritia gel for 24 hours at room temperature. Their morphology was determined by SEM, the results from Figure 3(a) and Figure 3(c) verifies that the steel more corroded as the surface was more rough in blank medium. However, Figure 3(b) and Figure 3(d) is for the steel which was exposed in a corrosive medium with optimal concentrations of inhibitor.
The presence of inhibitor reduced the extent of corrosion, the surface become smoother compared to the uninhibited. This reveals the performance of inhibitor by protective layer formation; which separates the interaction between the metal and corrosive medium similar behavior observed by [14] . From Figure 3(a) and Figure 3(c) the metal exposed to blank 1 M H2SO4 and 2 M HNO3 displayed uniform and pitting corrosion respectively.
3.3. FT-IR Analysis
For determination of the functional groups few drops of Aloe lateritia gel and that of corrosion products was used. The results were displayed for FT-IR spectrum as shown in Figure 4.
Aloe lateritia gel and that of corrosion product was exposed to IR spectrum and the results are shown in Figure 4(a) and Figure 4(b). The stretch for O-H which was around 3339.60 cm−1 was shifted to 3360.29 cm−1, from 1635.49 cm−1 to 1641.08 cm−1 for C=C when the pure inhibitor gel and corrosion product respectively. The presence of stretch at 551.87 cm−1 indicates presence of g-Fe2O3 this verifies the interaction between the inhibitor and the metal surface was also observed as shown in Table 2 [15] .
Table 1. Potentiodynamic polarization parameters for Mild steel in 2 M HNO3 and 1 M H2SO4.
Table 2. Different frequencies of IR spectrum of corrosion product and pure Aloe lateritia gel.
Figure 3. SEM micrographs of mild steel surface exposed in (a) 1 M H2SO4 solution (b) 1 M H2SO4 with 14.3 (%v/v) Aloe lateritia gel; (c) 2 M HNO3 solution and (d) 2 M HNO3 with 11.8 (%v/v) Aloe lateritia gel at room temperature for 24 hours.


Figure 4. FT-IR spectrum for the (a) pure Aloe lateritia gel and (b) corrsion products respectively.
4. Conclusion
The inhibitor gel was observed to perform well on both H2SO4 and HNO3 medium. Inhibition efficiencies were observed to rise with a concentration of inhibitor. Maximum efficiencies of 77.4% at 14.3% v/v for 1 M H2SO4 and 70.3% at 11.8% v/v for 2 M HNO3 were noticed. Mixed type of inhibition was revealed since no remarkable shift of Ecorr. SEM images revealed effective performance of inhibitor since inhibited metal appeared to be smoother than uninhibited. FT-IR information’s proved the interaction between inhibitor and metal surfaces. Additionally the functional groups stretch shift and decrease of Icorr proves the film formation and lowering metal dissolution.
Acknowledgements
This work was supported by British Gas (BG) scholarship for my studies at Nelson Mandela African Institution of Science and Technology (NM-AIST). I appreciate Dr. Kiseok Jung for proofreading this work.
Cite this paper
LucasPaul,Revocatus L.Machunda, (2016) Investigation of Aloe lateritia Gel as Corrosion Inhibitor for Mild Steel in 2 M HNO3 and 1 M H2SO4 Media. Journal of Minerals and Materials Characterization and Engineering,04,33-39. doi: 10.4236/jmmce.2016.41004
References
- 1. Hmimou, J., Rochdi, A., Touir, R., Ebn Touhami, M., Rifi, E., El Hallaouti, A., Anouar, A and Chebab, D. (2012) Study of Corrosion Inhibition of Mild Steel in Acidic Medium by 2-Propargyl-5-p-Chlorophenyltetrazole: Part I. Journal of Materials and Environmental Science, 3, 543-550.
- 2. Wiersma, B.J. (2002) Corrosion Testing of Carbon Steel in Acid Cleaning. Google Search [Online]. https://www.google.co.tz/?gws_rd=ssl#q=corrosion+Testing+of+carbon+steel+in+acid+cleaning
- 3. Shankar Rao, V. and Singhal, L.K. (2008) Corrosion Behavior and Passive Film Chemistry of 216 L Stainless Steel in Sulphuric Acid. Journal of Materials Science, 44, 2327-2333. http://dx.doi.org/10.1007/s10853-008-2976-4
- 4. Umoren, S.A., Gasem, Z.M. and Obot, I.B. (2013) Natural Products for Material Protection: Inhibition of Mild Steel Corrosion by Date Palm Seed Extracts in Acidic Media. Industrial & Engineering Chemistry Research, 52, 14855- 14865. http://dx.doi.org/10.1021/ie401737u
- 5. Bouyanzer, A., Hammouti, B. and Majidi, L. (2006) Pennyroyal Oil from Mentha pulegium as Corrosion Inhibitor for Steel in 1 M HCl. Materials Letters, 60, 2840-2843. http://dx.doi.org/10.1016/j.matlet.2006.01.103
- 6. Rahim, A.A., Rocca, E., Steinmetz, J. and Jain Kassim, M. (2008) Inhibitive Action of Mangrove Tannins and Phosphoric Acid on Pre-Rusted Steel via Electrochemical Methods. Corrosion Science, 50, 1546-1550. http://dx.doi.org/10.1016/j.corsci.2008.02.013
- 7. Chauhan, L.R. and Gunasekaran, G. (2007) Corrosion Inhibition of Mild Steel by Plant Extract in Dilute HCl Medium. Corrosion Science, 49, 1143-1161. http://dx.doi.org/10.1016/j.corsci.2006.08.012
- 8. Abdel-Gaber, M., Abd-El-Nabey, B.A., Sidahmed, I.M., El-Zayady, A.M. and Saadawy, M. (2006) Inhibitive Action of Some Plant Extracts on the Corrosion of Steel in Acidic Media. Corrosion Science, 48, 2765-2779. http://dx.doi.org/10.1016/j.corsci.2005.09.017
- 9. Principles, E. and Polarization, P. (1980) Application Note CORR-1 Basics of Corrosion Measurements. Princeton Applied Research.
- 10. Zohdy, K.M. (2015) Surface Protection of Carbon Steel in Acidic Solution Using Ethylenediaminetetraacetic Disodium Salt. International Journal of Electrochemical Science, 10, 414-431.
- 11. Mohamed, W.A., Rateb, N.M. andShakour, A.A. (2004) Performance of Copper Corrosion Inhibitors in a Museum Environment—A Comparative Study Using FTIR Spectroscopy. Metal 04: Proceedings of the International Conference on Metals Conservation, Canberra, 4-8 October 2004, 369-378.
- 12. Hart, K. and James, A.O. (2014) Sabinet—The Inhibitive Effect of Aloe Vera Barbadensis Gel on Copper in Hydrochloric Acid Medium. Journal of Emerging Trends in Engineering and Applied Sciences, 5, 24-29.
- 13. Fouda, A.S., Attia, A.A. and Negm, A.A. (2014) Some Thiophene Derivatives as Corrosion Inhibitors for Carbon Steel in Hydrochloric Acid. Journal of Metallurgy, 2014, Article ID: 472040. http://dx.doi.org/10.1155/2014/472040
- 14. Kadhum, A.A.H., Mohamad, A.B., Hammed, L.A., Al-Amiery, A.A., San, N.H. and Musa, A.Y. (2014) Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Materials, 7, 4335-4348. http://dx.doi.org/10.3390/ma7064335
- 15. Leelavathi, S. and Rajalakshmi, R. (2013) Dodonaea viscosa (L.) Leaves Extract as Acid Corrosion Inhibitor for Mild Steel—A Green Approach. Journal of Materials and Environmental Science, 4, 625-638.
NOTES
*Corresponding author.






