Food and Nutrition Sciences
Vol.3 No.11(2012), Article ID:24466,10 pages DOI:10.4236/fns.2012.311194
In Vivo Antioxidant Activity of Fucoxanthin on Obese/Diabetes KK-Ay Mice
![]()
Laboratory of Bio-Functional Material Chemistry, Division of Marine Bioscience, Faculty of Fisheries Sciences, Hokkaido University, Hakodate, Japan.
Email: *kmiya@fish.hokudai.ac.jp
Received August 17th, 2012; revised September 18th, 2012; accepted September 25th, 2012
Keywords: Fucoxanthin; In Vivo Antioxidant Activity; Anti-Diabetic Effect; KK-Ay Mice
ABSTRACT
Dietary intake of 0.1% fucoxanthin significantly reduced lipid hydroperoxide levels of liver and abdominal white adipose tissue (WAT) of obese/diabetes KK-Ay mice. The fucoxanthin supplementation also significantly reduced blood glucose level and hepatic lipid contents of the mice. Oxidative stress is known to be induced in hyperglycemia and high fat conditions. Therefore, in vivo antioxidant activity of fucoxanthin found in the present study could be attributed to its anti-diabetic effect and its decreasing effect on hepatic lipids. On the other hand, little effect of fucoxanthin on lipid hydroperoxide levels was found in normal ICR mice. Although the content of fucoxanthin metabolites in the abdominal WAT of KK-Ay mice was about 50 times higher that in the liver, there was little difference in its in vivo antioxidant activity between in the liver and in the abdominal WAT. These results suggest that well-known ability of fucoxanthin to scavenge active oxygen species and/or free radicals would not be a main reason to explain its in vivo antioxidant activity.
1. Introduction
Polyphenols and carotenoids have been implicated as important dietary nutrients having antioxidant potential. The antioxidant property is the one of the possible mechanism by which they afford their beneficial health effects [1]. Recently, we have found that hepatic lipid hydroperoxide levels of mice fed brown seaweed lipids containing fucoxanthin, a characteristic carotenoid found in brown seaweeds, were significantly lower than that of control mice, even though total polyunsaturated fatty acids (PUFAs) content in the liver of mouse fed the brown seaweed lipids was higher than that of mouse fed control diet [2]. This suggested the involvement of fucoxanthin and/or polyphenols as major antioxidants in the brown seaweed lipids to the lower hydroperoxide levels in the liver.
Antioxidant activity of seaweed polyphenols have been reported by many researchers [3-6]. However, the evaluation of the effects exerted by algal polyphenols gives several problems when moving from in vitro experimental systems to the complexity of in vivo system. The major problem is their bioavailability and the difficulties in unraveling the complex mechanisms of absorption and metabolism. Up to date most of studies on the biological activities of algal polyphenols have been done in vitro systems using cultivated cell lines. In our previous study [2], no compound related to brown seaweed phenolic compounds or their metabolites was detected in high performance liquid chromatography (HPLC) analysis of the liver of mouse fed brown seaweed lipids, while fucoxanthin metabolites, mainly fucoxanthinol, were found in the liver. Furthermore, in vivo antioxidant activity level of different kinds of brown seaweed lipids was dependent on the fucoxanthin content, but not on the polyphenol content of each brown seaweed lipids. These results suggested that the in vivo antioxidant activity of the brown seaweed lipids found in the previous study [2] would be due to fucoxanthin metabolites, but not to polyphenols.
To make clear the in vivo antioxidant activity of fucoxanthin, the present study was aimed to examine the effect of purified fucoxanthin on lipid hyderoperoxide levels in the liver and in the abdominal WAT of obese/ diabetes model KK-Ay mouse.
2. Materials and Methods
2.1. Separation of Fucoxanthin
Crude lipids containing fucoxanthin were extracted from the commercial dried seaweed (Undaria pinnatifida) powder using acetone. Fucoxanthin was purified from the extracted crude lipids by silica gel column chromatography with n-hexane/acetone (8:2, v/v) as previously described [7]. The purity of fucoxanthin was >97% by HPLC analysis. The HPLC was carried out with a Hitachi L-2350 HPLC system (Hitachi Seisakusho Co., Tokyo, Japan) equipped with a pump (L-2130), an autosampler (L-2200) and a spectrophotometric detector (L-2400) [8]. The analyses were carried out at 25˚C using a ODS column (Develosil-ODS-UG-5, Nomura Chem. Co., Aichi, Japan) protected with a guard column having the same stationary phase. The mobile phase composition was methanol: hexane: dichloromethane: acetonitrile (10:2.5:2.5:85, v/v/v/v). The flow rate was set at 1.0 mL/min and the eluent was monitored at 450 nm.
2.2. Animals and Diets
All procedures for the use and care of animals were approved by the Ethical Committee of Experimental Animal Care at Hokkaido University, Japan. Animal experiments were carried out by using 6 weeks of age female ICR mice and 6 weeks of age female KK-Ay mice. Both mice were obtained from Japan CREA Co., Osaka, Japan. The mice were housed at 23˚C ± 1˚C and at 50% ± 10% humidity with a 12 h light/12 h dark cycle throughout the experiment. The mice had free access to drinking water and were fed a diet prepared according to the recommendations of American Institute of Nutrition (AIN-93G) [9].
After acclimation for 1 week, mice were randomly divided into each group of six and were fed the experimental diets for 3 weeks. The body weight, diet and water intake of each mouse was recorded every day. The composition of the diets is shown in Table 1. Soybean oil and lard were used for dietary lipids. Major fatty acids of soybean oil were 16:0 (10.5%), 18:1n−9 (23.3%), 18:2n−6 (51.8%), and 18:3n−3 (6.0%), while those of lard were 16:0 (24.8%), 18:1n−9 (42.5%) and 18:2n−6 (8.9%). Fucoxanthin (0.1%) was substituted with soybean oil (Table 1). All diets were vacuum-packed immediately after preparation and stored at −20˚C.
2.3. Sample Collections
After feeding with the control and experimental diets for 3 weeks, mice were starved for 12 h and dissected while anesthetized with diethyl ether. Liver, abdominal white adipose tissue (WAT), and other tissues were rapidly removed in their entirety and weighed. The protein content in the tissue was measured by Lowry method [10].
2.4. Blood Glucose
Blood glucose was determined using a blood glucose
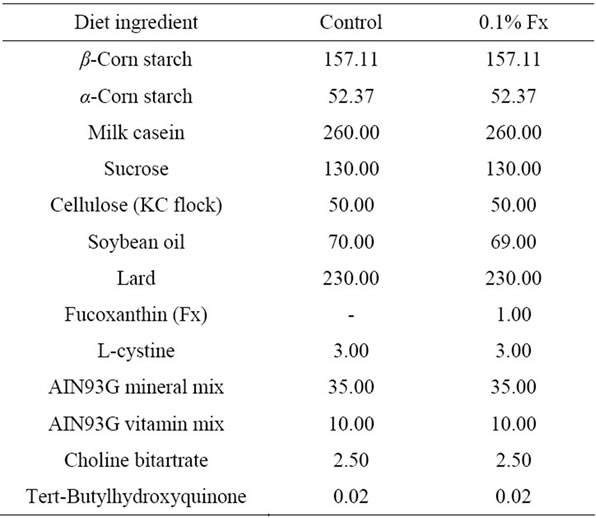
Table 1. Composition (g/kg) of experimental diets used in the present study.
monitor, the Glutest Neo Sensor (Sanwa Kagaku Kenkyusho Co. Ltd., Aichi, Japan), without fasting at 1 day before dissection (after 21 days of feeding). This sensor is an amperometric sensor with flavin adenine dinucleotide (FAD)-dependent glucose dehydrogenase and .
.
2.5. Hydroperoxide Analysis of Liver and Abdominal WAT Lipids
Each tissue was perfused with 0.9% NaCl and the lipids Each tissue was perfused with 0.9% NaCl and the lipids were extracted with chloroform/methanol (2:1, v/v) as described previously by Folch et al. [11]. Tissue samples from each mouse were analyzed separately. Lipid hydroperoxide is known to react stoichiometrically with non-fluorescent diphenyl-1-pyrenylphosphine (DPPP) to give fluorescent DPPP oxide [12]. The hydroperoxide content of tissue lipids was determined using this quantitative conversion of DPPP to DPPP oxide. Briefly, a 10 mg of lipid extract was weighed and dissolved in 5 ml chloroform (containing 10 mg/mL butylhydroxytoluene (BHT))/methanol (2:1, v/v). To a test tube with a screw cap, 100 mL of the sample solution and 50 mL of DPPP solution (1 mg/10 mL chloroform) were added and left for 60 min at 60˚C in a water bath. Then the solution was cooled in an ice bath and 3 ml of 2-propanol was added. The reaction mixture was diluted by 1:10 (v/v) before measurement by reversed phase HPLC. The HPLC was carried out with the same system as described above except that a fluoresence detector (Hitachi L-2485) was used for the analysis. All the HPLC analyses were done at 40˚C using a reversed-phase column (DevelosilODS-UG-5, Nomura Chem. Co.), protected with a guard column (10 × 4.0 mm i.d.) having the same stationary phase. The mobile phase was butanol-methanol (10:90, v/v) and the flow rate was 1.0 mL/min. The fluoresence detector (Hitachi L-2485) was set at Ex. 352 nm and Em. 380 nm. The hydroperoxide concentration in the sample solution was calculated from the DPPP oxide detected using a DPPP oxide standard curve. The hydroperoxides in the tissue lipids were expressed as μmol/g tissue.
2.6. Fucoxanthin Content of Extracted Lipids
An aliquot of the extracted lipids was dissolved in acetone and filtered with a 0.22 μm of membrane filter (PTFE Acrodisc; Wako, Osaka, Japan). The filtered sample was analyzed by HPLC. The HPLC was carried out with the same conditions as described in the previous section for fucoxanthin preparation. Fucoxanthinol and amarouciaxanthin A were identified as major metabolites of fucoxanthin by comparing with authentic purified fucoxanthinol and amarouciaxanthin A [2]. Unknown peak other than the peaks corresponding to fucoxanthinol and amarouciaxanthin A was also detected as shown in our previous paper [2]. Quantitative estimation of these peaks was done using standard curve. The detection wavelength was set at 450 nm. Purified fucoxanthinol was used as authentic standard for the quantification of fucoxanthin metabolites in extracted lipids from each tissue. The contents were expressed as μg/mg protein of tissue.
Fucoxanthinol was prepared from purified fucoxanthin by hydrolysis with porcine pancreas lipase, type II (Sigma, St. Louis, MO). Purified fucoxanthin (purity > 99%) was obtained by the HPLC separation of Undaria pinnatifida lipids as described previously [13]. One gram of fucoxanthin and 5 g of taurocholic acid sodium salt were once dissolved in methanol, and the solvent was dried under nitrogen. Then 20 g of lipase (Sigma) in 1l of potassium phosphate buffer (0.1 M, pH 7.0) was added to the mixture of fucoxanthin and taurocholic acid and dispersed by sonication. After incubation at 37˚C for 2 h, the reaction mixture was extracted with methanol/diethyl ether (1:1, v/v), and the diethyl ether phase containing fucoxanthinol was separated. Fucoxanthinol was further purified by silica gel column chromatography using acetone/n-hexane (1:1, v/v) and HPLC. The purity of fucoxanthinol was >99% by HPLC analysis.
2.7. Statistical Analysis
Results are shown as mean ± standard error of the mean (SEM) for seven mice. Differences between groups were examined for statistical significance using Scheffe’s, Tukey HSD or t-test for independent samples with Statistica (StatSoft software).
3. Results
3.1. Changes in Body Weight and Tissue Weights
Normal ICR and diabetic/obese KK-Ay mice were fed high-fat diet with or without fucoxanthin for 3 weeks (Table 1). Throughout the experimental period, no significant difference (P > 0.05) in total food intake and total water intake was observed among the two groups of ICR and KK-Ay mice. Intake of 0.1% fucoxanthin slightly reduced the body weight gain and total weight of mesentery, epididymal, perirenal and retroperitoneum WAT per body weight of both ICR and KK-Ay mice, however, the difference was not significant (Figure 1 and Table 2). On the contrary, brown adipose tissue
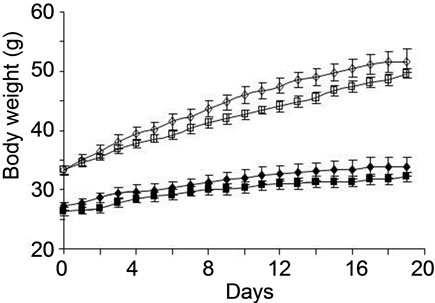
Figure 1. Changes in body weight of ICR and KK-Ay mice fed control and 0.1% Fx diet. Values represent means ± SD of six mice per group. ICR mice fed control (solid diamond), ICR mice fed Fx (solid square), KK-Ay mice fed control (open diamond), KK-Ay mice fed control (open square).
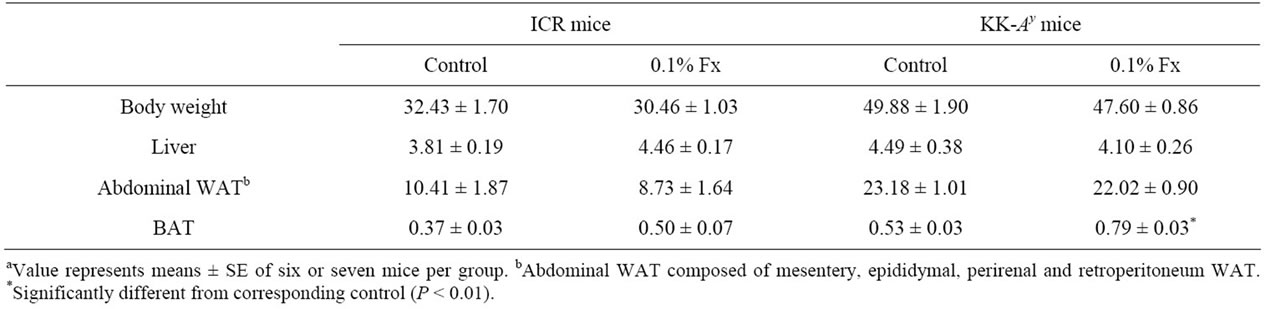
Table 2. Body weight (g), liver and adipose tissue (WAT: white adipose tissue; BAT: brown adipose tissue) weights (g/100 g body weight) of ICR and KK-Ay mice fed experimental dietsa.
(BAT) weight increased by feeding fucoxanthin in both kinds of mice. Especially, significant increase was observed in KK-Ay mice (Table 2).
3.2. Effect of Fucoxanthin Intake on Blood Glucose Level and Lipid Levels of Liver and WAT
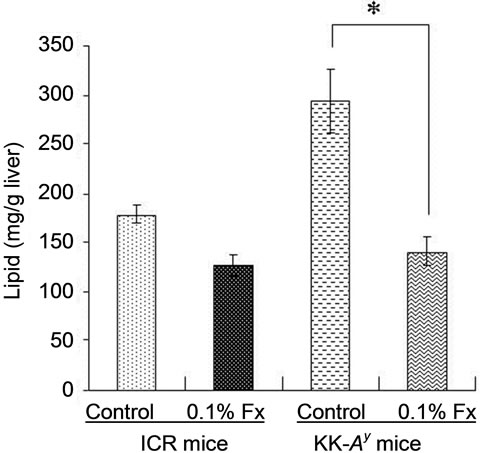
Since KK-Ay mouse is characterized by hyperglycemia, the plasma glucose level of the KK-Ay mice fed control diet was significantly higher than that of normal ICR mice (Figure 2). The blood glucose levels of KK-Ay mice significantly decreased by fucoxanthin intake, whereas fucoxanthin did not affect the blood glucose levels of ICR mice (Figure 2). The same effect of fucoxanthin was found in the liver lipid content (Figure 3(a)). Lipid content in the liver of KK-Ay mice fed control diet was higher than that of ICR mice, but the lipid level significantly reduced by fucoxanthin supplementation to the same level as that of control ICR mice. On the other hand, there was no significant difference in the lipid content of WAT between KK-Ay and ICR mice (Figure 3(b)). Little effect of fucoxanthin was also found in the lipid level of WAT of both KK-Ay and ICR mice (Figure 3(b)).
3.3. Lipid Hydroperoxide Levels in Liver and WAT of KK-Ay and ICR Mice
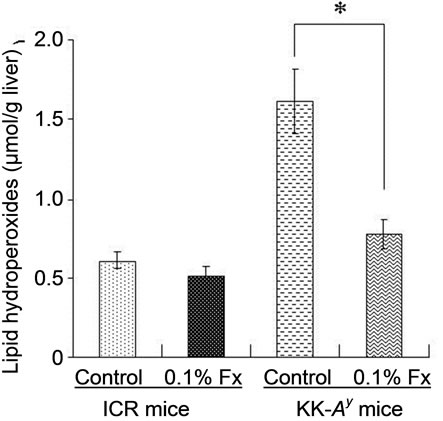
Lipid hydroperoxide levels in the liver and WAT of KK-Ay mice fed control diet were significantly higher than those of ICR mice (Figure 4). The hydroperoxide levels were significantly reduced by the addition of 0.1% fucoxanthin to the diet for KK-Ay mice. On the other hand, little change was found in lipid hydroperoxide levels of ICR mice with or without fucoxanthin.
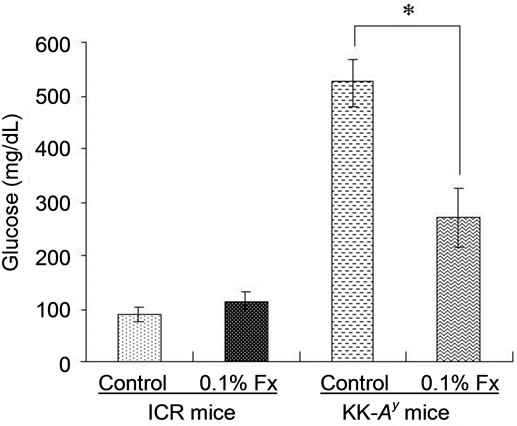
Figure 2. Blood glucose levels in ICR and KK-Ay mice. Blood glucose levels were measured at 21 days without fasting. Values represent the mean ± SEM of six mice per group. *Significantly different from corresponding control (P < 0.01). Fx: fucoxanthin.
3.4. Fucoxanthin Metabolite Contents
Contents (μg/mg protein) of fucoxanthin metabolites in liver and abdominal WAT are shown in Table 3. More fucoxanthin metabolites were detected in abdominal WAT of ICR and KK-Ay mice than in liver of both mice. The main fucoxanthin metabolite was amarouciaxanthin A for WAT. On the other hand, fucoxanthinol was most abundant fucoxanthin metabolite in the liver, being followed by amarouciaxanthin A and unknown metabolite,
 (a)
(a)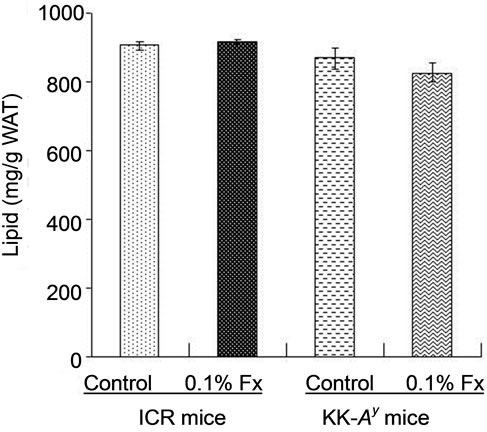 (b)
(b)
Figure 3. Lipid content of liver (a) and abdominal WAT (b) of ICR and KK-Ay mice. Values represent means ± SD of six mice per group. Fx: fucoxanthin.
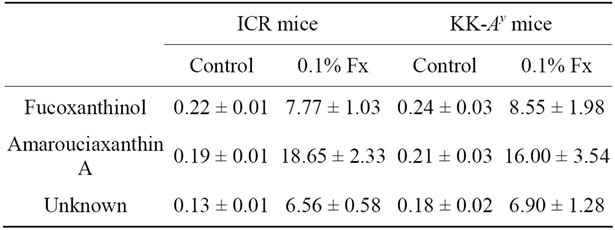
Table 3. Fucoxanthin metabolites (mg/mg protein) in liver and abdominal WAT of mice fed 0.1% fucoxanthin.
 (a)
(a)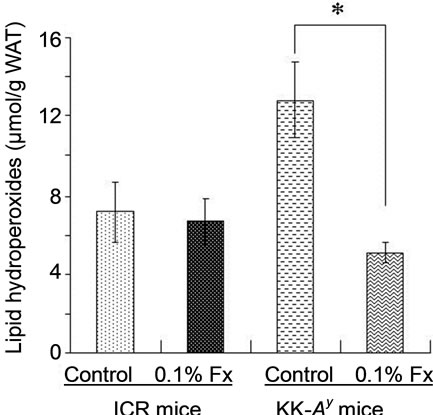 (b)
(b)
Figure 4. Lipid hydroperoxide levels in liver (a) and WAT (b) of ICR and KK-Ay mice. Values represent means ± SD of six mice per group. *Significantly different from corresponding control (P < 0.01). Fx: fucoxanthin.
respectively, whereas the difference in the content of three kinds of fucoxanthin metabolites in the liver was a little.
4. Discussion
In the present study, we used KK-Ay mice as an obese/diabetes animal model, while ICR mice were used as normal model mice. The inbred mouse strain KK, established in Japan as a diabetic strain, develops type 2 diabetes mellitus with mild obesity, mainly due to insensitivity of the peripheral tissue to insulin. Diabetes and obesity in KK mice is relatively moderate but introducetion of the Ay allele (KK-Ay) exacerbates the pathophysiological obese and diabetes conditions [14]. As expected, abdominal WAT weight (Table 2) and glucose level (Figure 2) of control KK-Ay mice were much higher than those of normal ICR control, although both animals were fed the same high-fat diet (Table 1). It is known that significant increase in oxidative stress is observed in obese subjects [15-17]. Hyperglycemia, fundamental abnormality found in diabetes, can also induce oxidative stress [17-20]. Therefore, the obese and diabetic conditions of KK-Ay mice resulted in oxidative stress to produce more lipid hydroperoxides than those found in normal ICR mice (Figure 4).
The higher hydroperoxide levels of KK-Ay mice markedly decreased by fucoxanthin supplementation to the same level as that found in control ICR mice (Figure 4). Blood glucose level of KK-Ay mice was significantly reduced by the supplementation of fucoxanthin (Figure 2), while no significant decrease in abdominal WAT was found by the fucoxanthin intake (Table 2). Therefore, the in vivo antioxidant activity of fucoxanthin on KK-Ay mice found in Figure 4 would be partly due to the significant reduction of blood glucose level. Anti-diabetic effect of fucoxanthin has been well studied at molecular level [21-24]. One of the molecular mechanisms is the regulatory effect of fucoxanthin metabolites accumulated in abdominal WAT on releasing biologically active mediators termed adipokines. With the exception of adiponectin and adipsin (complement factor D), most other adipokines has been implicated in obesity, type 2 diabetes, hypertension, inflammatory, and cardiovascular diseases [25]. Dietary fucoxanthin down-regulated the over expressions of these adipokines such as leptin, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and plasminogen activator inhibittor-1 (PAI-1) [21]. In addition, fucoxanthinol, one of the main metabolite of fucoxanthin, decreased nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) mRNA expression in RAW264.7 cells stimulated by palmitic acid [21]. This down-regulatory effect of fucoxanthin metabolite on iNOS and COX-2 expressions would be also related to its in vivo antioxidant activity [26-28], although the effect of fucoxanthin on the expression of iNOS and COX-2 was not analyzed in the present study.
Fucoxanthin shows an antiobesity effect through uncoupling protein 1 (UCP1) induction of WAT mitochondria [29]. This activity has been reported to appear at least more than 60 mg fucoxanthin intake/kg mouse/day [2], while the significant reduction of abdominal WAT of obese female volunteers was observed only by intake of fucoxanthin less than 0.024 mg/kg/day (2.4 mg intake/day for volunteers with 100 kg average weight) [30]. When fucoxanthin intake of mouse in the present study is roughly calculated from fucoxanthin content (0.1%) in the diet, average feed intake (>3 g diet per day), and average mouse body weight, it was around 50 mg fucoxanthin intake/kg mouse/day. This fucoxanthin level might not be enough to show an anti-obesity effect on the KK-Ay mice used in the present study (Table 2).
Dietary fucoxanthin also significantly reduced hepatic liver lipids of KK-Ay mice to the same level as that found in ICR control mice (Figure 3(a)). The decrease in the lipid substrates for oxidation could result in the lower lipid hydroperoxide levels of the liver (Figure 4(a)). Woo et al. [31] has reported the improvement effect of fucoxanthin on hepatic lipid profiles of C57BL/6N mice fed a high-fat diet containing 39% of calories as fat. Both 0.05% and 0.2% fucoxanthin supplementation signifycantly lowered triacylglycerol (TG) levels by 38% and 24%, respectively, and cholesterol levels by 26% and 24%, respectively, compared to the control group. From the analysis of enzymatic activities, they demonstrated that the effect of fucoxanthin on the lipid metabolism would be due to suppression of the hepatic lipogenesis and increase in hepatic lipolysis through regulation of nuclear receptors, peroxisome proliferators activated receptor α (PPARα) and PPARγ.
Because of the presence of long central chain of conjugated double bonds and its characteristic functional groups of the terminal rings, fucoxanthin shows ability to quench singlet molecular oxygen (1O2) and to trap free radicals [32]. Nomura et al. [33] demonstrated the 1,1-diphenyl-2-picrylhydrazyl (DPPH) quenching activeity of fucoxanthin isolated from the diatom Phaeodactylum tricornutum. Electron spin resonance (ERS) analysis showed the quenching ability of fucoxanthin against both organic radicals DPPH and 12-doxyl-steraic acid (12DS) [1]. Yan et al. [34] demonstrated the strong DPPH radical scavenging activity of organic extracts from different edible seaweeds and fucoxanthin was identified as the active compound. DPPH radical scavenging activity, ABTS radical scavenging activity, and hydroxyl radical scavenging activity of fucoxanthin were measured by chemiluminescence technique [35]. Therefore, in vivo antioxidant activity of fucoxanthin found in this study might have been related to its in vitro antioxidant capacity reported in above studies. However, as shown in Figure 4, dietary fucoxanthin had little effect on lipid hydroperoxide levels of both liver and abdominal WAT of ICR mice as compared with those of control ICR mice. The in vivo antioxidant activity of fucoxanthin was only found in diabetes and/or hepatic hyperlimidemia conditions found in KK-Ay mice. These results suggests that significant decrease in lipid hydroperoxides in both liver and abdominal WAT of obese/diabetes KK-Ay mice fed fucoxanthin (Figure 4) will be mainly based on the reduction of glucose level and decrease in hepatic lipids by the fucoxanthin intake, but not much on its ability to scavenge free radicals and active oxygen species.
Carotenoids have been implicated as important dietary nutrients having antioxidant potential, being involved in the scavenging 1O2 and peroxy radicals generated in the process of peroxidation [36]. There is little doubt that, under the right conditions, carotenoids can protect cells, tissues and other structures such as lipoproteins against oxidative attack. To be effective antioxidants, carotenoids must be present in sufficient concentrations and at the specific locations where the reactive oxygen species and/or free radicals are generated [37]. Several carotenoids have been reported to decrease the risk of diseases and disorders by reduction of oxidative stress in the target tissues, since they can be present in sufficient amounts and right location to be a meaningful antioxidant in the tissues. For example, lutein and zeaxanthin has been studied widely and proven to show diverse beneficial effects on human health, particularly on optimising eye health [38]. The biological mechanisms for the protective effects of both carotenoids may include powerful blue-light filtering activities and antioxidant properties. There have been several studies on the relationship between the antioxidant activity of astaxanthin and the prevention of cardiovascular disease [39-41]. Prevention of atherosclerosis by astaxanthin intake can be explained by their protective effects on low density lipoprotein (LDL) and vein endothelial cells against oxidative injury and dysfunction. In case of fucoxanthin, the localization has been reported in abdominal WAT [29]. The high accumulation of fucoxanthin metabolites in abdominal WAT was also observed in the present study (Table 3). In spite of higher distribution of fucoxanthin metabolites to the abdominal WAT, however, little difference in the in vivo antioxidant activity of dietary fucoxanthin was found between in the liver and in the abdominal WAT (Figure 4). This result suggests a little involvement of direct action of fucoxanthin on active oxygen species and/or free radicals to its in vivo antioxidant activity found in the present study.
It is difficult to explain all the physiological effects of carotenoids only by their antioxidant activity [42]. Modulation effect of carotenoids on specific gene and protein expression in biological systems may be more important to explain biological effect of carotenoids. Fucoxanthin can quench 1O2; however the ability is lower than those of β-carotene, lycopene, and ataxanthin because of the lower number of conjugated double bonds present in the molecule. On the other hand, our previous studies shows that fucoxanthin improves insulin resistance and decreases blood glucose levels through down-regulation of several adipokines, up-regulation of glucose transporter 4 (GLUT4) expression in skeletal muscle, and promotion of GLUT4 translocation to the cell membrane [21-24]. This regulatory effect of fucoxanthin on target molecules would be main mechanism to explain its in vivo antioxidant activity.
5. Conclusion
The present study demonstrated that dietary brown seaweed carotenoid, fucoxanthin, reduced in vivo oxidative stress through the reduction of blood glucose level and hepatic lipid content of obese/diabetes model KK-Ay mice. The in vivo antioxidant activity of fucoxanthin was only found in diabetes condition, but not in a normal condition, suggesting that ability of fucoxanthin to scavenge active oxygen species and/or free radicals would not be a main reason to explain its in vivo antioxidant activity.
6. Acknowledgements
This work was supported by Grants-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (23380068), and partially supported by a National Project “Knowledge Cluster Initiative” (“Hakodate Marine Biocluster”), MEXT (Ministry of Education, Culture, Sports, Science and Tecnology, Japan).
REFERENCES
- H. Nishino, “Cancer Prevention by Carotenoids,” Macroeconomic Reports, Vol. 402, No. 1-2, 1998, pp. 159- 163. doi:10.1016/S0027-5107(97)00293-5
- M. K. W. A. Airanthi, N. Sasaki, S. Iwasaki, N. Baba, M. Abe, M. Hosokawa and K. Miyashita, “Effect of Brown Seaweed Lipids on Fatty Acid Composition and Lipid Hydroperoxide Levels of Mouse Liver,” Journal of Agricultural and Food Chemistry, Vol. 59, No. 8, 2011, pp. 4156-4163. doi:10.1021/jf104643b
- H. S. Kang, H. Y. Chung, J. Y. Kim, B. W. Son, H. A. Jung and J. S. Choi, “Inhibitory Phlorotannins from the Edible Brown Alga Ecklonia stolonifera on Total Reactive Oxygen Species (ROS) Generation,” Archives of Pharmacal Research, Vol. 27, No. 2, 2004, pp. 194-198. doi:10.1007/BF02980106
- M. Nakai, N. Kageyama, K. Nakahara and W. Miki, “Phlorotannins as Radical Scavengers from the Extract of Sargassum Ringgoldianum,” Marine Biotechnology, Vol. 8, No. 4, 2009, pp. 409-414. doi:10.1007/s10126-005-6168-9
- T. Shibata, K. Ishimaru, S. Kawaguchi, H. Yoshikawa and Y. Hama, “Antioxidant Activities of Phlorotannins Isolated from Japanese Laminariaceae,” Journal of Applied Phycology, Vo. 20, No. 5, 2008, pp. 705-711. doi:10.1007/s10811-007-9254-8
- Y. Zou, Z. J. Qian, Y. Li, M. M. Kim, S. H. Lee and S. K. Kim, “Antioxidant Effects of Phlorotannins Isolated from Ishige Okamurae in Free Radical Mediated Oxidative Systems,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 16, 2008, pp. 7001-7009. doi:10.1021/jf801133h
- T. Tsukui, K. Konno, M. Hosokawa, H. Maeda, T. Sashima and K. Miyashita, “Fucoxanthin and Fucoxanthinol Enhance the Amount of Docosahexaenoic Acid in the Liver of KKAy Obese/Diabetic Mice,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 13, 2007, pp. 5025-5029. doi:10.1021/jf070110q
- M. Terasaki, A. Hirose, B. Narayan, Y. Baba, C. Kawagoe, H. Yasui, N. Saga, M. Hosokawa and K. Miyashita, “Evaluation of Recoverable Functional Lipid Components with Special Reference to Fucoxanthin and Fucosterol Contents of Several Brown Seaweeds of Japan,” Journal of Phycology, Vol. 45, No. 4, 2009, pp. 974-980. doi:10.1111/j.1529-8817.2009.00706.x
- P. G. Reeves, F. H. Nielsen and G. C. Fahey, “AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet,” Journal of Phycology, Vol. 123, No. 11, 1993, pp. 1939-1951.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275.
- J. Folch, M. Lees and G. H. S. Stanley, “A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues,” The Journal of Biological Chemistry, Vol. 226, No. 1, 1957, pp. 497-509.
- K. Akasaka, I. Sasaki, H. Ohrui and H. Meguro, “Simple Fluorometry of Hydroperoxides in Oils and Foods,” Bioscience Biotechnology & Biochemistry, Vol. 56, No. 4, 1992, pp. 605-607. doi:10.1271/bbb.56.605
- H. Maeda, M. Hosokawa, T. Sashima, K. Funayama and K. Myashita, “Fucoxanthin from Edible Seaweed, Undaria pinnatifida, Shows Antiobesity Effect through UCP1 Expression in White Adipose Tissues,” Biochemical and Biophysical Research Communications, Vol. 332, No. 2, 2005, pp. 392-397. doi:10.1016/j.bbrc.2005.05.002
- J. Suto, S. Matsuura, K. Imamura, H. Yamanaka and K. Sekikawa, “Genetic Analysis of Non-Insulin-Dependent Diabetes Mellitus in KK and KK-Ay Mice,” European Journal of Endocrinology, Vol. 139, No. 6, 1998, pp. 654-661. doi:10.1530/eje.0.1390654
- H. K. Vincent and A. G. Taylor, “Biomarkers and Potential Mechanisms of Obesity-Induced Oxidant Stress in Humans,” International Journal of Obesity, Vol. 30, No. 3, 2006, pp. 400-418. doi:10.1038/sj.ijo.0803177
- H. K. Vincent, K. E. Innes and K. R. Vincent, “Oxidative Stress and Potential Interventions to Reduce Oxidative Stress in Overweight and Obesity,” Diabetes, Obesity and Metabolism, Vol. 9, No. 6, 2007, pp. 813-839. doi:10.1111/j.1463-1326.2007.00692.x
- A. Andrikopoulos, “Obesity and Type 2 Diabetes: Slow Down!—Can Metabolic Deceleration Protect the Islet Beta Cell from Excess Nutrient-Induced Damage?” Molecular and Cellular Endocrinology, Vol. 316, No. 2, 2010, pp. 140-146. doi:10.1016/j.mce.2009.09.031
- I. Grattagliano, V. O. Palmieri, P. Portincasa, A. Moschetta and G. Palasciano, “Oxidative Stress-Induced Risk Factors Associated with the Metabolic Syndrome: A Unifying Hypothesis,” The Journal of Nutritional Biochemistry, Vol. 19, No. 8, 2008, pp. 491-504. doi:10.1016/j.jnutbio.2007.06.011
- H. Yang, X. Jin, C. W. K. Lam and S.-K. Yan, “Oxidative Stress and Diabetes Mellitus,” Clinical Chemistry and Laboratory Medicine, Vol. 49, No. 11, 2011, pp. 1773- 1782. doi:10.1515/cclm.2011.250
- Y.-C. Shi and T.-M. Pan, “Red Mold, Diabetes, and Oxidative Stress: A Review,” Applied Microbiology and Biotechnology, Vol. 94, No. 1, 2012, pp. 47-55. doi:10.1007/s00253-012-3957-8
- M. Hosokawa, T. Miyashita, S. Nishikawa, S. Emi, T. Tsukui, F. Beppu, T. Okada and K. Miyashita, “Fucoxanthin Regulates Adipocytokine mRNA Expression in White Adipose Tissue of Diabetic/Obese KK-Ay Mice,” Archives of Biochemistry and Biophysics, Vol. 504, No. 1, 2010, pp. 17-25. doi:10.1016/j.abb.2010.05.031
- H. Maeda, M. Hosokawa, T. Sashima and K. Miyashita, “Ditary Combination of Fucoxanthin and Fish Oil Attenuates the Weight Gain of White Adipose Tissue and Decrease Blood Glucose in Obese/Diabetic KK-Ay Mice,” Agricultural and Biological Chemistry, Vol. 55, No. 19, 2007, pp. 7701-7706. doi:10.1021/jf071569n
- H. Maeda, M. Hosokawa, T. Sashima, K. MurakamiFunayama and K. Miyashita, “Anti-Obesity and AntiDiabetic Effects of Fucoxanthin on Diet-Induced Obesity Conditions in a Murine Model,” Molecular Medicine Reports, Vol. 2, No. 6, 2009, pp. 897-902. doi:10.3892/mmr_00000189
- S. Nishikawa, M. Hosokawa and K. Miyashita, “Fucoxanthin Promotes Translocation and Induction of Glucose Transporter 4 in Skeletal Muscles of Diabetic/Obese KK-Ay Mice,” Phytomedicine, Vol. 19, No. 6, 2012, pp. 389-394. doi:10.1016/j.phymed.2011.11.001
- Y. Deng and P. E. Scherer, “Adipokines as Novel Biomarkers and Regulators of the Metabolic Syndrome,” Annals of the New York Academy of Sciences, Vol. 1212, 2010, pp. E1-E19. doi:10.1111/j.1749-6632.2010.05875.x
- F. Farinati, M. Piciocchi, E. Lavezzo, M. Bortolami and R. Cardin, “Oxidative Stress and Inducible Nitric Oxide Synthase Induction in Carcinogenesis,” Digestive Diseases, Vol. 28, No. 4-5, 2010, pp. 579-584. doi:10.1159/000320052
- S. K. Pathak, R. A. Sharma, W. P. Steward, J. K. Mellon, T. R. L. Griffiths and A. J. Gescher, “Oxidative Stress and Cyclooxygenase Activity in Prostate Carcinogenesis: Targets for Chemopreventive Strategies,” European Journal of Cancer, Vol. 41, No. 1, 2005, pp. 61-70. doi:10.1016/j.ejca.2004.09.028
- K. Uchida, “A Lipid-Derived Endogenous Inducer of COX-2: A Bridge between Inflammation and Oxidative Stress,” Molecular Cell, Vol. 25, No. 3, 2008, pp. 347- 351.
- K. Miyashita, S. Nishikawa, F. Beppu, T. Tsukui, M. Abe and M. Hosokawa, “Allenic Carotenoid, Fucoxanthin, as a Novel Marine Nutraceutical from Brown Seaweed,” Journal of Agricultural and Food Chemistry, Vol. 91, No. 7, 2011, pp. 1166-1174. doi:10.1002/jsfa.4353
- M. Abidov, Z. Ramazanov, R. Seifulla and S. Grachev, “The Effects of Xanthigen™ in the Weight Management of Obese Premenopausal Women with Non-Alcoholic Fatty Liver Disease and Normal Liver Fat,” Diabetes, Obesity and Metabolism, Vol. 12, No. 1, 2010, pp. 72-81. doi:10.1111/j.1463-1326.2009.01132.x
- M.-N. Woo, S.-M. Jeon, H.-J. Kim, M.-K. Lee, S.-K. Shin, Y. C. Shin, Y.-B. Park and M.-S. Choi, “Fucoxanthin Supplementation Improves Plasma and Hepatic Lipid Metabolism and Blood Glucose Concentration in HighFat Fed C57BL/6N Mice,” Chemico-Biological Interactions, Vol. 186, No. 3, 2010, pp. 316-322. doi:10.1016/j.cbi.2010.05.006
- K. Miyashita, “Function of Marine Carotenoids,” Forum of Nutrition, Vol. 61, 2009, pp. 136-146. doi:10.1159/000212746
- T. Nomura, M. Kikuchi, A. Kubodera and Y. Kawakami, “Proton-Donative Antioxidant Activity of Fucoxanthin with 1,1-Diphenyl-2-Picrylhydrazyl (DPPH),” Biochemistry & Molecular Biology International, Vol. 42, No. 2, 1997, pp. 361-370.
- X. Yan, Y. Chuda, M. Suzuki and T. Nagata, “Fucoxanthin as the Major Antioxidant in Hijikia fusiformis, a Common Edible Seaweed,” Bioscience Biotechnology & Biochemistry, Vol. 63, No. 3, 1999, pp. 605-607. doi:10.1271/bbb.63.605
- N. M. Sachindra, E. Sato, H. Maeda, M. Hosokawa, Y. Niwano, M. Kohno and K. Miyashita, “Radical Scavenging and Singlet Oxygen Quenching Activity of Marine Carotenoid Fucoxanthin and Its Metabolites,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 21, 2007, pp. 8516-8522. doi:10.1021/jf071848a
- R. Edge, D. J. McGarvey and T. G. Truscott, “The Carotenoids as Anti-Oxidants: A Review,” Journal of Photochemistry and Photobiology B, Vol. 41, No. 3, 1997, pp. 189-200. doi:10.1016/S1011-1344(97)00092-4
- H. Sies, “Total Antioxidant Capacity: Appraisal of a Concept,” Journal of Nutrition, Vol. 137, No. 6, 2007, pp. 1493-1495.
- L. Ma and X.-M. Lin, “Effects of Lutein and Zeaxanthin on Aspects of Eye Health,” Journal of the Science of Food and Agriculture, Vol. 90, No. 1, 2010, pp. 2-12. doi:10.1002/jsfa.3785
- J. P. Fredric, G. W. David and L. C. Charles, “Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease,” American Journal of Cardiology, Vol. 101, No. 10, 2008, pp. 58D- 68D.
- R. G. Fassett and J. S. Coombes, “Astaxanthin, Oxidative Stress, Inflammation and Cardiovascular Disease,” Future Cardiology, Vol. 5, No. 4, 2009, pp. 333-342. doi:10.2217/fca.09.19
- J.-P. Yuan, J. Peng, K. Yin and J.-H. Wang, “Potential Health-Promoting Effects of Astaxanthin: A High-Value Carotenoid Mostly from Microalgae,” Molecular Nutrition & Food Research, Vol. 55, No. 1, 2011, pp. 150-165. doi:10.1002/mnfr.201000414
- J. W. Erdman Jr., N. A. Ford and B. L. Lindshield, “Are the Health Attributes of Lycopene Related to Its Antioxidant Function?” Archives of Biochemistry and Biophysics, Vol. 483, No. 2, 2009, pp. 229-235. doi:10.1016/j.abb.2008.10.022
Abbreviations
BAT: brown adipose tissue;
BHT: butylhydroxytoluene;
COX-2: cyclooxygenase-2;
DPPP: diphenyl-1-pyrenyl-phosphine;
FAD: flavin adenine dinucleotide;
GLUT4: glucose transporter 4;
HPLC: high performance liquid chromatography;
iNOS: nitric oxide synthase;
LDL: low density lipoprotein;
MCP-1: monocyte chemoattractant protein-1;
PAI-1: plasminogen activator inhibitor-1;
PPAR: proliferators activated receptor;
PUFA: polyunsaturated fatty acid;
SEM: standard error of the mean;
TAG: triacylglycerol;
TNF-α: tumor necrosis factor-α;
UCP1: uncoupling protein 1;
WAT: white adipose tissue.
NOTES
*Corresponding author.

