Food and Nutrition Sciences
Vol.4 No.1(2013), Article ID:26622,4 pages DOI:10.4236/fns.2013.41004
Growth Performance of Broiler Chickens Fed Fossil Shell Growth Promoter
![]()
Department of Animal Science, Faculty of Agriculture and Forestry, University of Ibadan, Ibadan, Nigeri.
Email: gbemiadeyemo@yahoo.com
Received June 7th, 2012; revised October 22nd, 2012; accepted October 30th, 2012
Keywords: Fossil Shell; Performance; Calcium; Phosphorus; Broilers
ABSTRACT
A study was carried out to determine the influence of fossil shell (diatomaceous earth) supplemented diets on the performance and bone composition of broiler chickens. A total of 120 day old broiler chicks were used for the experiment and randomly allotted to 5 treatments (T1—0.9%, T2—1.2%, T3—1.5%, T4—1.8% and T5—0% inclusion levels respectively). Fossil shell inclusion had no significant influence on feed intake and feed conversion ratio, but had significant impact on weight gain. At the finisher phase no significant differences (p > 0.05) were observed for weight gain, feed intake and feed: gain ratio. Bone analysis showed that calcium content was not affected but fossil shell had significant influence on phosphorus content of the analyzed bones.
1. Introduction
Broiler birds have been developed with genetic potentials for a faster growth rate to attain market weight in the shortest time possible. These genetic potential cannot be fully utilized or expressed if the right or optimal environment is not provided, it therefore means that animals should be adequately provided with the right kind of nutrients for the maximum expression of their genetic endowment. However, a lot of factors militate against meeting these requirements for nutrient to ensure maximum productivity. Apart from the high cost of feed which accounts for about 70% or more of the total cost of production [1], arising from the stiff competition between man and livestock for the conventional feed ingredients; there is also the need to examine the level of utilization of the nutrients contained in the feed on the growth of the birds. The increasing genetic selection pressure, proper management and high density diets have promoted excellent performance.
However, there are some metabolic disorders currently observed derive from these improvements in genetics and management, problems like femoral head necrosis in broilers cause significant losses for producers. According to [2], it is estimated that about 3.2% of broiler production is lost due to skeletal malformation in the United States.
It has been found that bone tissue strength results from calcium and phosphorus deposition in the form of hydroxyapartite during bone mineralization. These two minerals make up 70% of the bone whereas the remaining 30% consist of organic matter mainly collagen. A deficiency of either calcium or phosphorus in the diet of young growing birds results in abnormal bone development even when the diet contains adequate Vitamin D3. Young broilers and pullets exhibit lameness usually around 10 - 14 days of age when their bones become rubbery and the rib cage is flattened and beaded at the attachment of the vertebrae. The use of fossil shell powder as feed additive has helped in tackling this problem, if the feed given does not supply the required amount of calcium and phosphorus in the right quality and quantity, either due to economic reasons or others. Fossil shells are largely made up of amorphous silica which dissolves easily in the animal body or system can be used to remedy the situation. It is also a natural diatomaceous earth which is mined, dried, ground, sieved and bagged. This gives a guarantee that it could indeed serve as a source of alternative and unconventional ingredient for the animal production industry. This study was designed to know the effect of fossil shell powder supplemented diets on the performance of broilers.
2. Materials and Methods
2.1. Experimental Procedure and Duration
The research was carried out at the pullet unit of the Teaching and Research farm of the University of Ibadan, Nigeria. A total of 120 mixed sex Abor Acre broiler chicks were purchased. The birds were raised in deep litter system with pens partitioned with wire-mesh. The floor of the pens was cushioned with saw dust prior to the arrival of the chicks with adequate heating provided. The experiment covered a period of eight (8) weeks. The birds were randomly assigned to five dietary treatments (T1—0% control, T2—0.9%, T3—1.2%, T4—1.5% and T5—1.8%) consisting of varying levels of fossil shell. Each treatment had 4 replicates. The growth promoter is a natural product composed of ground diatomaceous earth (fresh water type) used in animal feeding. Feed composition for the starter and finisher phases are presented in Tables 1 and 2 respectively. Feed and water were given to all chickens ad-libitum and the amount of feed consumed per week was calculate as the difference between initial feed given and final feed left. The birds were weighed weekly. Feed: gain ratio was also computed for the whole period.
2.2. Bone Analysis
At the end of the 8th week, six representative birds were selected per treatment out of a total of twenty-four birds in a treatment and killed. The tibia bones of the birds were removed carefully and all the flesh, cartilages and fat were removed after boiling. The bones were weighed, air dried, crushed, oven dried at 105˚C for 24 hours and charred at 600˚C for 6 hours. The calcium and phosphorus content of the bones was then determined using the UV-Vis Spectrophotometer (Model 753 W) manufactured by Easy Way Medical, England.
Table 1. Feed composition of dietary treatment (starter phase).
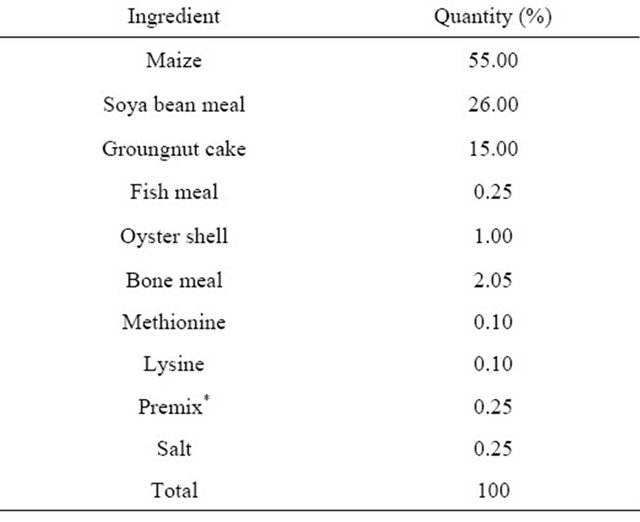
*Premix supplied per kg of diet: Vit A, 10,000 IU; Vit D, 2800 IU; Vit E, 35,000 IU; Vit K, 1900 mg; Vit B12 19 mg; Riboflavin, 7000 mg; Pyridoxine, 3800 mg; Thiamine, 2200 mg; D-Pantothenic acid, 11,000 mg; Nicotinic acid, 45,000 mg; Folic acid, 1400 mg; Biotin, 113 mg; Cu, 8000 mg; Mn, 64,000 mg; Zn, 40,000 mg Fe, 32,000 mg; Se, 160 mg; Iodine, 800 mg; Cobalt, 400 mg; Choline, 475,000 mg; Methionine, 50,000 mg; BHT, 5000 mg; Spiramycin, 5000 mg.
Table 2. Feed composition of dietary treatment (finisher phase).
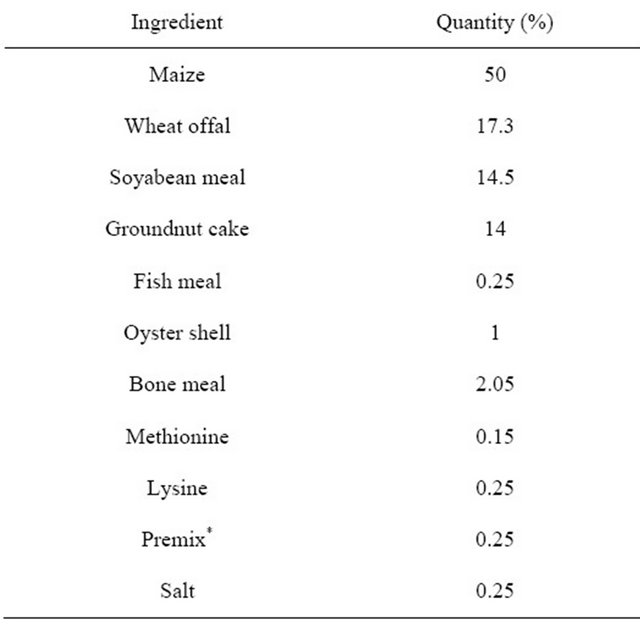
*Premix supplied per kg of diet: Vit A, 10,000 IU; Vit D, 2800 IU; Vit E, 35,000 IU; Vit K, 1900 mg; Vit B12 19 mg; Riboflavin, 7000 mg; Pyridoxine, 3800 mg; Thiamine, 2200 mg; D-Pantothenic acid, 11,000 mg; Nicotinic acid, 45,000 mg; Folic acid, 1400 mg; Biotin, 113 mg; Cu, 8000 mg; Mn, 64,000 mg; Zn, 40,000 mg Fe, 32,000 mg; Se, 160 mg; Iodine, 800 mg; Cobalt, 400 mg; Choline, 475,000 mg; Methionine, 50,000 mg; BHT, 5000 mg; Spiramycin, 5000 mg.
2.3. Calcium Determination
The bone ash was mixed together and 0.5 g was weighed into 100 ml volumetric flask, 5 ml of concentrated Hydrochloric acid was added to dissolve the bone ash. The flask was marked up using distilled water, after which 2 ml was diluted into 100 ml volumetric flask with distilled water, out of this 2 ml was diluted to 10 ml with 0.5% lanthanum solution. The amount of calcium was determined using the UV-Vis Spectrophotometer (Model 753 W) manufactured by Easy Way Medical, England.
2.4. Phosphorus Determination
The bone ash solution (2 mls/10 mls) was used. 3.0 mls of the solution was measured and poured into a test tube and 4 mls of distilled water was added. 7 mls of water was measured and poured into another test tube to serve as blank. Then 1 ml of ammonium molybdate was added to both test tubes and mixed properly and allowed to stand for 30 minutes. After which 1ml of hydroquinone and sodium sulphite solution was added and mixed and allowed to rest for 30 minutes before reading [3].
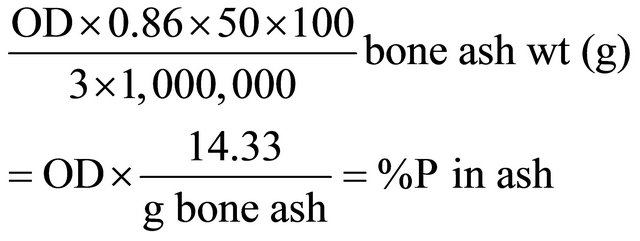
3. Results
3.1. Performance Characteristics
Results of the performance variables for the starter phase are presented in Table 3. Values for feed intake and feed conversion ratio showed no significant differences (p > 0.05) among the treatment means. Results showed that for feed intake, there were no significant differences (p > 0.05) observed, all treatments had the same mean value. For feed: gain ratio, T4 had the highest value (2.91) while T1 had the lowest value (2.31). Weight gain however showed significant differences (p < 0.05) between T1 and T4 (1.30 and 1.03 respectively). Results for the finisher phase presented in Table 4 showed no significant differences (p > 0.05) and were observed for weight gain, feed intake and feed: gain ratio. Values for feed: gain ratio showed that T4 had the highest value (3.10) while the control (T5) had the lowest value (2.06). However T2 (4.39) and T5 (3.62) had the highest and lowest values respectively for feed intake. Weight gain values showed that T1 (1.78) and T4 (1.36) had the highest and lowest values respectively.
3.2. Bone Analysis
No significant differences (p > 0.05) were observed for calcium content, results however showed that T4 had the highest value (0.138), while T1 and T2 had the lowest values (0.125 and 0.125) respectively as shown in Table 5. Phosphorus content however displayed significant differences (p < 0.05), between the treatments (T3; 0.295 vs T1; 0.280, T4; 0.280). Also both T2 and T5 had the same values of 0.288 each this is shown in Table 5.
Table 3. Performance of broilers fed fossil shell supplemented diets at the starter phase.
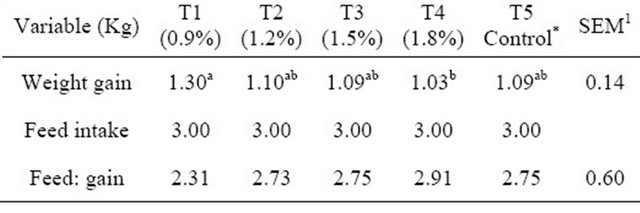
*Control = 0% fossil shell inclusion; 1SEM—Standard error of mean; a,bmeans with different superscripts in the same row are significantly different.
Table 4. Performance of broilers fed fossil shell supplemented diets at the finisher phase.
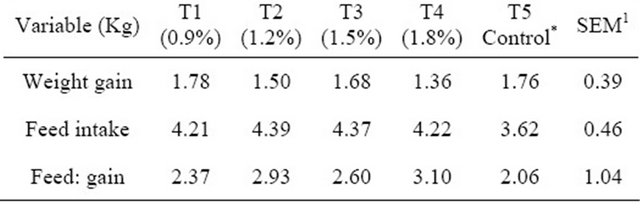
*Control = 0% fossil shell inclusion; 1SEM—Standard error of mean.
Table 5. Bone composition of broilers fed fossil shell supplemented diets.
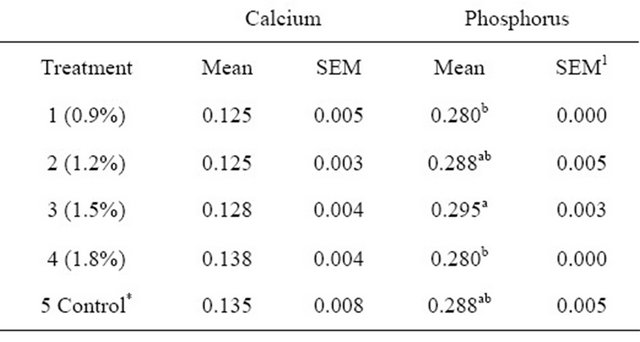
*Control = 0% fossil shell inclusion; 1SEM—Standard error of mean; a,bmeans with different superscripts in the same column are significantly different.
4. Discussion
The efficacy, with which broilers convert nutrients from feed into body tissue, is arguably the most important determinant of profitability in broiler production [4]. It has been reported that, animals fed with fossil shell powder have no problems of worms or internal parasites [5]. Fossil shell powder inclusion in diets daily tends to keep the animals free of parasites and toxic chemicals so it can get maximum benefits from the feed and water it consumes. The differences observed which were not significant might be due to factors, such as adequate amount of nutrients in diets particularly calcium and phosphorus, the rate of utilization of available energy by animals, reduce competition amongst the birds for feed because of adequate amount of feed and water spaces.
There was an increase in weight gain generally throughout the eight weeks of the experiment though there were no significant differences amongst treatment means at the finisher phase. Significant differences were not observed in feed intake of birds fed with the experimental diets including those on the control diet both at the starter and finisher phases. Though there were slight differences in mean value at the finisher phase this largely might be due to increase in the body’s energy demand for maintenance and growth, and also individual body makeup.
The varying responses observed for the calcium and phosphorus contents might be due to the imbalance between calcium and other minerals. In poultry, calcium deficiency does not seem to be a problem; however there can be problems of malnutrition that can impair intestinal calcium absorption [6]. Other contraindicative factors are high level of phytate in diet which can interfere with calcium absorption. It was however, observed that there was a concomitant increase in the phosphorus content up to 1.5% inclusion of fossil shell powder after which the phosphorus level dropped. It could be that inclusion levels beyond 1.5% may create imbalance in the absorption and utilization of phosphorus as a result of competition for carrier molecules. Concerns have been raised in the past that a high phosphorus intake would possibly interfere with calcium nutrition by reducing its absorption [7]. Research to evaluate the effect of higher phosphorus content (and lower calcium ratio) of the diet showed that the calcium absorption was not lowered, as long as the calcium intake level is adequate even higher phosphorus levels will not interfere with the calcium absorption [8].
5. Conclusion
This study showed that fossil shell powder can be used successfully in broiler diets to correct nutritional mineral imbalance since it supplies more than 14 trace elements and other elements, it also improves the efficiency of feed utilization. For bone composition, feeding broilers with fossil shell powder at varying amount had little or no effect on calcium but slightly affected phosphorus levels. However further studies could give clearer pictures on possible interactions between fossil shell and basal mineral contents of broiler diets.
REFERENCES
- G. O. Adeyemo, “Effects of Exposure Duration to Cottonseed Cake-Based Diets on Broiler Performance,” International Journal of Poultry Science, Vol. 9, No. 2, 2010, pp. 162-166. doi:10.3923/ijps.2010.162.166
- M. E. Cook, “Skeletal Deformities and Their Causes: Introduction,” Poultry Science, Vol. 79, No. 7, 2000, pp. 982-984.
- E. M. Onyango, P. Y. Hester, R. Stroshine and O. Adeola, “Bone Densitometry as an Indicator of Percentage Tibia Ash in Broiler Chicks Fed Varying Dietary Calcium and Phosphorus Levels,” Poultry Science, Vol. 82, No. 11, 2003, pp.1787-1791.
- P. A. E. Pym, “Selection for Feed Utilization in Broilers,” Proceedings of World’s Congress, Vol. 2, Amsterdam, 1994, pp. 177-182.
- J. D. Janet, “A Few Notes on Safe Solution Inc. Food Grade,” 2009.
- R. W. Perry, G. N. Rowland, T. L. Foutz and J. R. Glisson, “Poult Malabsorption Syndrome. III. Skeletal Lesions in Market Age Turkeys,” Avian Diseases, Vol. 35, No. 4, 1991, pp. 707-713. doi:10.2307/1591599
- M. S. Calvo and Y. K. Park, “Changing Phosphorus Content of the US Diet: Potential for Adverse Effect on Bone,” The Journal of Nutrition, Vol. 126, Suppl. 4, 1996, pp. 1168S-1180S.
- R. P. Heany and A. Recker, “Effect of Nitrogen, Phosphorus and Caffeine on Calcium and Phosphorus Balance in Women,” The Journal of Laboratory and Clinical Medicine, Vol. 99, No. 1, 1982, pp. 46-45.

