International Journal of Medical Physics, Clinical Engineering and Radiation Oncology
Vol. 1 No. 3 (2012) , Article ID: 24899 , 7 pages DOI:10.4236/ijmpcero.2012.13012
The Application of Fat-Suppression MR Pulse Sequence in the Diagnosis of Bone-Joint Disease
Department of Radiology, Nanjing Hospital, Nanjing Medical University, Nanjing, China
Email: 393110388@qq.com
Received October 11, 2012; revised November 13, 2012; accepted November 20, 2012
Keywords: Magnetic Resonance Imaging; Fat Suppression Sequences; Bone Joint
ABSTRACT
Fat-suppression technology of magnetic resonance is very important in clinical practice. This article is written to interpret the principle, advantages/disadvantages and clinical applications of some regular fat-suppression sequences in the diagnosis of Bone-Joint Disease, including: 1) frequency-selective saturation (FS); 2) short-TI inversion recovery (STIR); 3) frequency selective inversion pulse; 4) fat suppression water or fat selective excitation technique; 5) Dixon technology and 6) magnetization transfer contrast (MTI).
1. Introduction
Fat tissue contains amount of hydrogen protons which show high signal in both T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) sequence, which not only cover the signal of other tissues, but also produce chemical shift artifact. Fat suppression MR pulse sequence can suppress the signal from adipose tissue, so it can be used in routine magnetic resonance imaging in order to reduce chemical shift artifact and improve visualization of uptake of contrast material. Now this imaging sequence is also significant in the diagnosis of musculoskeletal diseases. This article is written to describe the principles of these different kinds of fat-suppression MR sequences and their applications for the musculoskeletal system.
2. Commonly Used Fat-Suppression MR Sequences
2.1. Frequency-Selective Saturation (FS)
Procession frequency of protons in fat is different from that in water molecules. A frequency-selective saturation radio-frequency pulse with the same resonance frequency as that of lipids is applied at first, then a homogeneity spoiling gradient pulse is applied immediately. Thus, the signal we get contains no contribution from lipid [1]. This method can be used in any MR imaging sequence, such as TIWI, T2WI, and PDWI (FS-T1WI, FS-T2WI, FS-PDWI). FS-T2WI has advantage in spine, joints of extremities and Minor Joints, especially has high value in the diagnosis of vertebral body occult fractures, thus can used as a routine sequence for spinal trauma [2]. While FS-PDWI is commonly used in joints of extremities, such as knee joint. It has advantage in the detecting the occult fractures of extremities, especially with Bone Contusion, ligament, tendon and menisci injury [3,4]. This fat suppression is specific, for it can get complete suppression of lipid signal, so signal in nonadipose tissue is practically unaffected as long as the saturation pulse frequency and bandwidth are properly selected. But this method has a high demand for magnetic field homogeneity, so it can only be used MR instruments with highfield-strength magnets; in the large FOV scan, the effect of fat suppression is poor in peripheral region. It is only applied to small-FOV examinations such as head, neck, spine imaging and minor joints of extremities imaging. Also it increases the scanning time and specific absorption rate (SAR) value as well.
Figure 1 FS-T2WI has advantage in knee joint, which can detect the occult fractures of knee, especially with bone contusion, ligament, tendon and menisci injury.
Figure 2 FS-T2WI also has advantage in minor joints, which can detect the occult fractures of minor joints, especially with bone contusion, articular effusion and ligament injury.
Figures 3 and 4 FS-PDWI is commonly used in knee joint. It has advantage in the detecting the occult fractures of extremities, especially with bone contusion, liga ment, tendon and menisci injury.
2.2. Short-TI Inversion Recovery (STIR)
Short TI inversion recovery (STIR) sequence can suppress fat signal based on differences in the TI of the tissues. The TI of fat tissue is shorter than that of water.
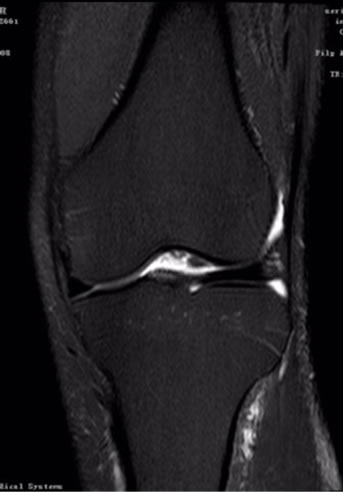
Figure 1. coronal T2-FS MRI image of knee joint.
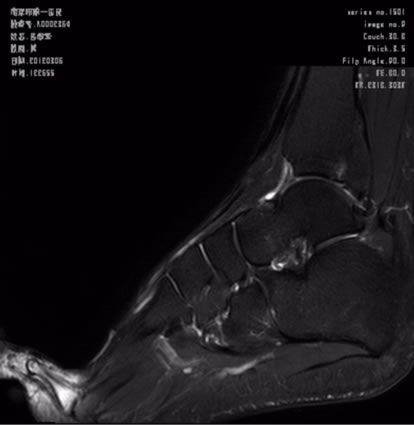
Figure 2. Sagittal T2-FS image of foot.
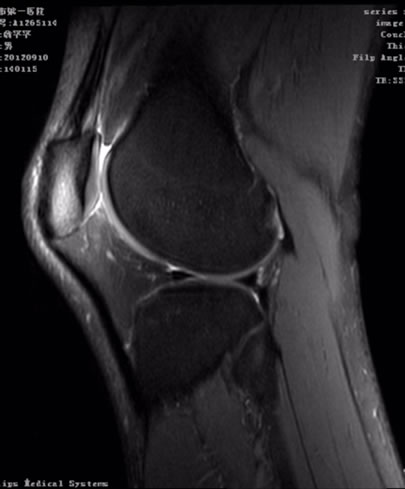
Figure 3. Sagittal PDWI-FS image of knee joint.
So, after 180˚ inversion pulse, the longitudinal magnetization of fat tissue will recover faster than that of water. Then a 90˚ pulse is applied at the null point of fat tissue, we can get the signal produced by water without fat tissue. STIR suppress the fat signal by this way [1]. Advantages of this method are insensitive to magnetic field inhomogeneity and can be used in low-field-strength magnets, and can also be applied for the large FOV scan-
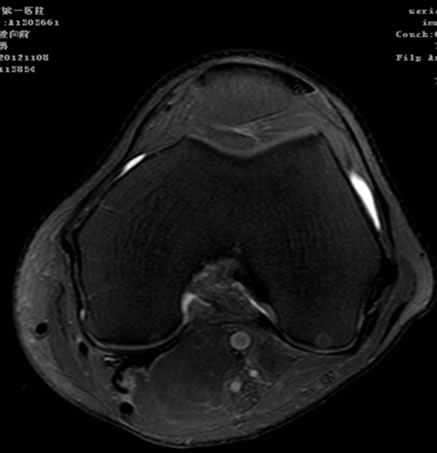
Figure 4. Axial PDWI-FS image of knee joint.
ning such as spine,Limb Long Bones and trunk imaging. Disadvantages include 1) This fat suppression is nonspecific, for signal from tissue or fluid with a TI similar to that of fat will also be suppressed; 2) This method increases the scanning time, especially the TR time; 3) It can not be used with contrast-enhanced magnetic resonance angiography.
The clinical applications of this technique as follows:
1) STIR has the advantage in the diagnosis of bone marrow diseases. It can evaluate multiple sclerosis of the cervical spinal cord [5] and is useful in the rib chondrosarcoma with intramedullary progression completely resected [6]. In bone marrow abnormalities of foot and ankle, STIR images and T1-weighted contrast-enhanced fat-suppressed MR images demonstrate almost identical imaging patterns, and diagnoses determined with these findings show little difference. Because of low cost and no intravenous injection required, we prefer to the use of the STIR sequence [7].
2) STIR, widely used in spine MRI, has a lot of advantages in displaying spinal cord injury, in interpreting whether acute vertebral compression fracture or chronic, and in detecting the occult fractures. This pulse sequence has been reported as an ideal tool to determine the integrity of the posterior ligamentous complex (PL-C) which has been proposed to be an integral aspect in the treatment algorithm for spinal trauma [8]. It also has a high value in the diagnosis of osteoporotic vertebral fractures.
3) A new technology combining STIR with wholebody DWI is called Diffusion-Weighted Imaging with Background Suppression (DWIBS), which has the potential in the detection of focal bone marrow lesions from multiple myeloma and provides additional biological information by ADC quantification [9].
Figures 5 and 6 STIR can be applied for the large FOV scanning such as spine from cervical vertebra to sacral vertebra. It has a lot of advantages in displaying spinal cord injury, in interpreting whether acute vertebral
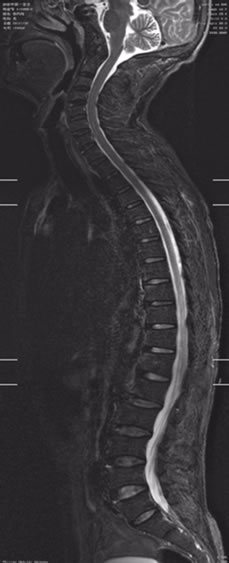
Figure 5. Sagittal STIR image of spine.
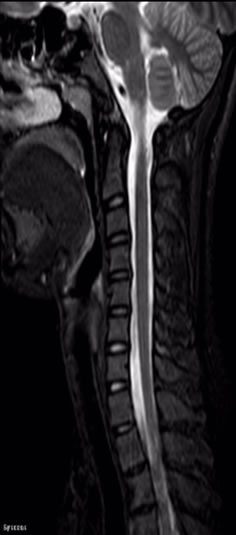
Figure 6. Sagittal STIR image of spine.
compression fracture or chronic, and in detecting the occult fractures.
2.3. Frequency Selective Inversion Pulse (Spectral Presturation with Inversion Recovery SPIR, Spectral Presaturation Attenuated Inversion Recovery SPAIR)
This technology can be seen as a combination of FS and STIR, which is based not only on the resonance frequency of fat tissue, but also on its short TI. Now, SPIR and SPAIR are commonly used in clinic. Compared with traditional STIR, SPIR method adds a 100˚ - 140˚ inversion pulse, and then the real pulse is given. It is the advantage of SPIR that it shortens the scanning time for the angle of inversion pulse decrease. But it has a high demand for magnetic field homogeneity, it can only be used in high-field-strength magnets. Based on SPIR, a new technology called SPAIR is produced, in which a 180˚ inversion pulse is given at first, in that way, fat tissue will be completely saturated. Its advantages are insensitive to inhomogeneity of B1 field and more effecttive and complete fat suppression. This method increases the scanning time and SAR value as well, because its flip angle is larger than that of SPIR.
Both SPIR and SPAIR have advantage in Musculoskeletal System. SPAIR has a high value to show the soft tissue as knee articular cartilage, ligament and articular capsule, thus can be used in the diagnosis of knee disease before the operation. STIR technology usually suffers from artifact caused by spinal cord, while SPAIR does better in the spine imaging, especially in the spinal cord imaging, because it can get the picture with higher quailty with better SNR and less spinal cord artifact. So in the spinal cord injury, SPAIR technique can be used to take the place of traditional STIR sequence.
Figures 7 and 8 SPAIR does better in the spine imaging, especially in the spinal cord imaging .SPAIR brings higher quality picture with better SNR and less spinal cord artifact. Figure 8 clearly shows vertebral body contusion with prominent high signal.
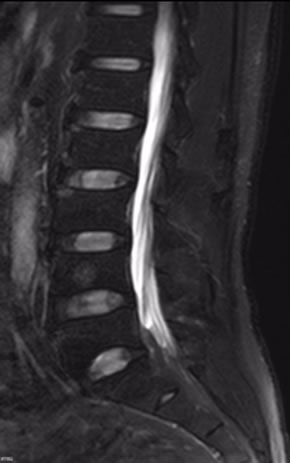
Figure 7. Sagittal SPAIR image of sine.
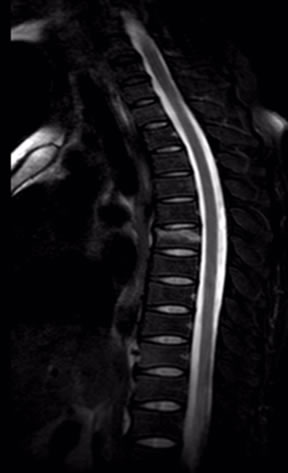
Figure 8. Sagittal SPAIR image of spine.
2.4. DIXON
Because protons in lipid and water molecules have different resonance frequencies, the phases of these protons relative to each other change with time after the initial excitation. Immediately after excitation, the lipid signal and water signal are in phase (the phase difference between them is zero). However, protons in water molecules process fractionally faster than those in lipid; therefore, after a few milliseconds, the phase difference between them is 180˚ (the phase is opposed). With the Dixon method, both in-phase and opposed-phase images are acquired. Summation and subtraction of these two phase images were shown to produce a water-only image and a fat-only image. In this way, Fat suppression will be realized by DIXON technology. It is the advantage of this technology that we can get two groups images each time; whereas the disadvantages of this method are sensitive to magnetic field inhomogeneity and susceptible to the motion artifact and with long scan time .But, Fast spin-echo triple echo Dixon (TED) and iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) of two new technology based on DIXON can overcome these limitations.
In FTED, water and fat signals for the three corresponding echoes have a relative phase shift of –180˚, 0˚, and 180˚ respectively. A fully automated postprocessing algorithm is then used to generate separate water-only and fat-only images for each slice. The new FTED technique enables both uniform water/fat separation and fast scanning. And this method can be used with 2D-FSE, 3D FSE, 2D-STIR and 3D FSPGR [10,11].
FTED has the advantage in spine imaging, because it can offer the picture with high quality, clear histological anatomy, less motion artifact and chemical shift artifact caused by spinal cord and less scan time.
IDEAL sequence, as a new modified DIXON technique, can eliminate the side effects from both B0 and B1 magnetic field inhomogeneity and provide images with the maximum high SNR. Meanwhile, IDEAL is compatible with essentially any pulse sequence, including fast spin echo, steady-state free precession (SSFP), and T1- weighted spoiled gradient-recalled echo (GRE). In all, IDEAL imaging provides uniform and reliable fat suppression [12,13].
IDEAL has the advantage in spine imaging. As compared with fat-saturated T2W FRFSE, IDEAL can provide a higher image quality, higher SNR-efficiency, and consistent, robust and uniform fat suppression. T2W IDEAL FRFSE is a promising technique for MR imaging of the spine at 3.0 T [14,15]; IDEAL technique can quantify tumor infiltration into the lumbar vertebrae in myeloma patients without visible focal lesions. This quantitative techniques truly exploit the utility of quantitative fat imaging for assessing disease processes affecting the bone marrow [16]; IDEAL GRASS images have high cartilage SNR, high contrast between cartilage and adjacent joint structures, and less scan time. Meanwhile IDEAL is also less sensitive to magnetic field inhomogeneity than other fat-suppression methods, which is especially important in evaluating articular cartilage of postoperative patients with metallic orthopedic hardware, so IDEAL GRASS is highly effective at helping evaluate the articular cartilage of the knee joint in patients. In all, IDEAL GRASS has similar sensitivity, specificity, and accuracy as a routine MR protocol for helping detect cartilage lesions [17,18].
Although IDEAL technique need a long scan time, inphase and opposed-phase images, fat-only and wateronly images are obtained during a single acquisition. So time is no more a limitation for IDEAL technique [12].
2.5. Fat Suppression Water or Fat Selective Excitation Technique
This technology makes use of the effect of the chemical shift of fat and water, with spatial selectivity on the basis of frequency-selective excitation and selective water excitation (obtain water signal by suppressing fat signal) or fat stimulate (obtain fat signal by suppressing water signal). And in clinic, we commonly use PROSET (principle of selective excitation technique), WATS and SPGR (spoiled gradient echo).
1) In PROSET and WATS, two types of composite pulses can be used routinely: a four-element composite pulse with a 1:3:3:1 amplitude ratio (11.25˚:33.75˚: 33.75˚:11.25˚) and a three-element composite pulse with a 1:2:1 amplitude ratio (22.45˚:45.00˚:22.45˚). With this technique, only water is excited by using section-elective composite pulses, while lipid spins are left in equilibrium, thereby producing no signal [13]. Section-selective water excitation is faster than conventional fat saturation and produces images of better quality.
This technique suppresses the adipose tissue signal of the spinal cord, which reduce the affect of the artifact. And in this technique, slice thickness is thinner, and there is no scan interval, so we can get quantitative measurement of cartilage volume and thickness, and the measureing value is close to actual thickness. It is currently recognized as the optimal scanning sequence to show the articular cartilage. For articular cartilage shows more clearly, a more accurate thickness measurement, a more sensitivity of the diagnosis and the highest specificity can be obtained from this method. And the diagnosis and staging is similar to arthroscopic. So it has significant advantage than traditional fat-suppression methods [18, 19].
In Figure 9, articular cartilage of sacroiliac joint shows clearly in WATS. We can get quantitative measurement
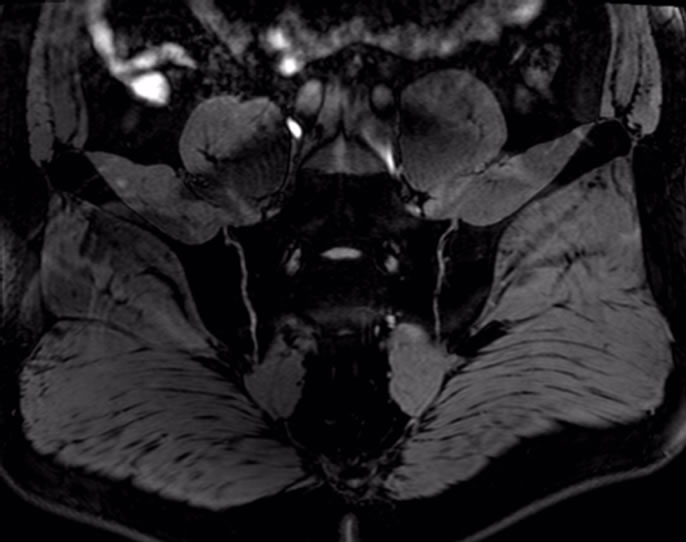
Figure 9. Axial WATS image of sacroiliac joint.
of cartilage volume and thickness, and the measuring value is close to actual thickness.
2) SPGR is a kind of spoiled GRE sequence. In this sequence, only the longitudinal magnetization vector contributes to the MR signal by applying interference RF pulse which make the transverse magnetization becomes zero, because it eliminates the interference of the T2 component and therefore better reflect T1.
SPGR has a few disadvantages which are lack of reliable contrast between cartilage and fluid that outlines surface defects and long imaging time, up to 8 minutes. In addition, SPGR imaging uses gradient and radio-fre quency spoiling to reduce artifacts and achieve near T1-weighting, but this method reduces the overall signal compared with steady-state techniques. Finally, 3D gradient-echo methods are less useful for the diagnosis of ligament or meniscal tears than spin-echo techniques. Despite of these limitations, 3D SPGR imaging is considered as the standard for morphologic imaging of cartilage [20].
Considering of these limitations, a new technology combining SPGR with IDEAL (IDEAL-SPGR) is applied in clinic.Because IDEAL, as a new pulse sequence, can offer uniform and robust water-fat separation, significant SNR, high contrast between cartilage and fluid, and less imaging time to 5 minutes, and it is also insensitive to the inhomogeneity of B0 and B1. These advantages of IDEAL largely corrected the limitation of SPGR. So combining IDEAL with SPGR can provide high-resolution, high-SNR images with less chemical shift artifact within the minimum time. The recombined images are corrected for chemical shift artifact in the readout direction, allowing use of lower receiver bandwidth with an associated increase in SNR [21]. This combined technique is regarded as practice in clinic and has good prospects in the detection and evaluation of joint cartilage of musculoskeletal system MRI [22].
2.6. Magnetization Transfer (MTI)
This approach exploits the fact that water rapidly exchanges magnetization with protons in protein and membrane phospholipid of tissue and cells but does not exchange magnetization with fat protons in the tissue. Saturation of the proton signal from protein and membrane phospholipid thus results in partial saturation of the water proton signal, then we get an image including a portion of the water signal and full fat signal. Subtraction of this image from the standard image, containing both water and fat signals, results in an image in which all fat signal is cancelled. This fat-elimination method is more efficient at suppressing fat signal than the conventional fat-suppression and wateror fat-selective imaging methods [23].
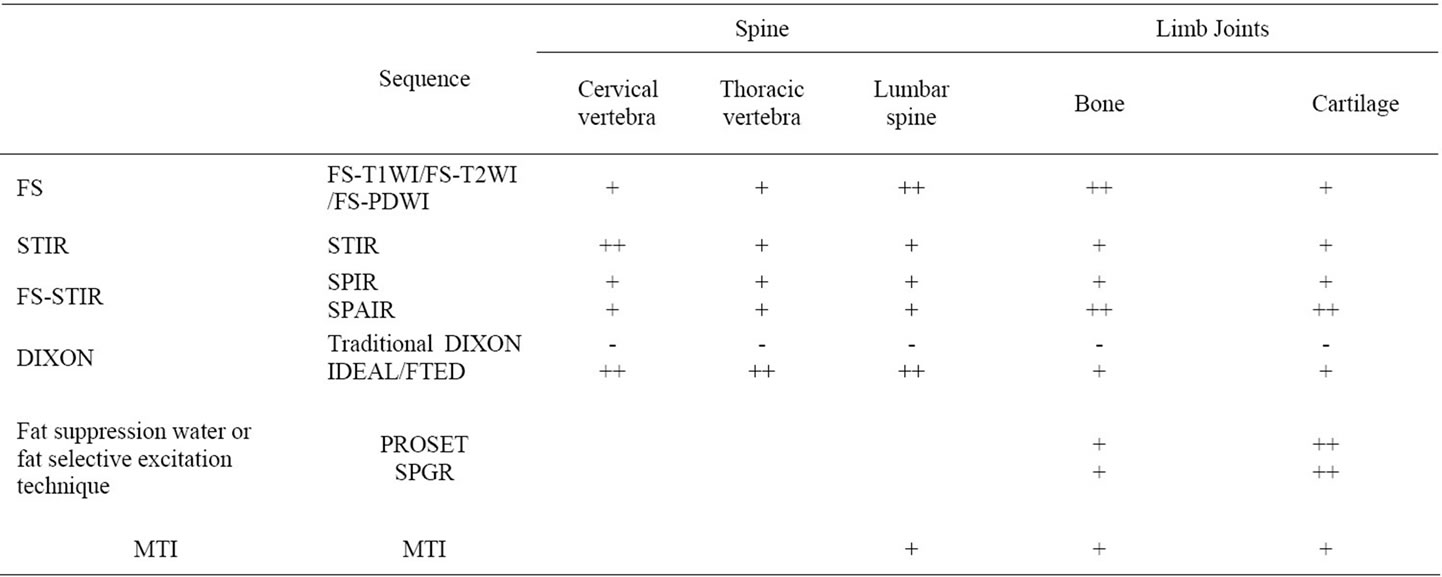
Table 1. The application of different fat-suppression sequences in the diagnosis of bone joint.
This technique is widely used in nervous system MRI, but few report in musculoskeletal system, mainly: on spinal cord disease as spinal cord cavernomas, bleed caused by injury, and syringomyelia; on intervertebral disc protrusion/extursion, which can show the relationship between the intervertebral disc and spinal canal; and on the bone contusion of the knee, which can increase the specificity and sensitivity in the diagnosis [24].
3. Summary
Fat-suppression pulse sequence is very important in musculoskeletal system MRI.FS-T2WI is commonly used in spine and minor joints of extremities; FS-PDWI in joints like knee. For low-field-strength magnets or large-FOV scan, we can choose STIR, which can be used in the head, neck and spine imaging, especially in cervical vertebra, for it has a high value in showing spinal cord. For highfield-strength magnets, SPIR and SPAIR are commonly used in the articular cartilage and spinal imaging. Fat suppression water or fat selective excitation technique has the advantage in nervous root imaging. IDEAL technique, as a modification of DIXON does well in the spinal imaging. The combined IDEAL-SPGR sequence has good prospects in musculoskeletal system MRI. The clinical applications of these fat-suppressed techniques can be referred to Table 1. In all, these fat-suppressed techniques have their own advantages. So we need further explore their new advantages and new combinations in order to improve and optimize our MR fat suppression to make outstanding contributions for the MRI diagnosis.
REFERENCES
- E. M. Delfaut, J. Beltran, G. Johnson, et al., “Fat Suppression in MR Imaging: Techniques and Pitfalls,” RadioGraphics, Vol. 19, No. 2, 1999, pp. 373-382.
- L.-Q. lu, X.-D. Yin, Q.-Z. Wu, et al., “The Value of MR Imaging of T2 WI-FS Sequence in the Diagnosis of Vertebral Body Occult Fractures,” Journal of Medical Imaging, Vol. 21, 2011, pp. 255-258.
- L.-Q. lu, M.-S. Xu, Q.-Z. Wu, et al., “The Value of MR Imaging of PDFASAT Sequence in the Diagnosis of Extremities Occult Fractures,” Chinese Journal of Radiology, Vol. 38, No. 12, 2004, pp. 1324-1328.
- L.-Q. lu, M. Feng, S.-Z. Wang, et al., “The Value of MRI PDW-FS Sequence in the Diagnosis of Bone Contusion,” Journal of Medical Imaging, Vol. 15, 2005.
- C. Philpotta and P. Brotchieb, “Comparison of MRI Sequences for Evaluation of Multiple Sclerosis of the Cervical Spinal Cord at 3 T,” European Journal of Radiology, Vol. 80, No. 3, 2011, pp. 780-785. doi:10.1016/j.ejrad.2010.09.031
- K. Ohata, F. Chen and H. Date “Rib Chondrosarcoma with Intramedullary Progression Completely Resected by Magnetic Resonance Imaging: Useful Short Inversion Time Inversion Recovery Sequence,” Interactive Cardiovascular and Thoracic Surgery, Vol. 12, No. 5, 2011, pp. 853-854. doi:10.1510/icvts.2010.262212
- M. R. Schmid, J. Hodler, P. Vienne, et al., “Bone Marrow Abnormalities of Foot and Ankle: STIR versus T1-Weighted Contrast-Enhanced Fat-Suppressed Spin-Echo MR Imaging,” Radiology, Vol. 224, No. 2, 2002, pp. 463-469. doi:10.1148/radiol.2242011252
- C. G. Crosby, J. L. Even, Y. Song, et al., “Diagnostic Abilities of Magnetic Resonance Imaging in Traumatic Injury to the Posterior Ligamentous Complex: The Effect of Years in Training,” The Spine Journal, Vol. 11, No. 8, 2011, pp. 745-755. doi:10.1016/j.spinee.2011.07.005
- G. Sommer, M. Klarhöfer, C. Lenz, et al., “Signal Characteristics of Focal Bone Marrow Lesions in Patients with Multiple Myeloma Using Whole Body T1w-TSE, T2WSTIR and Diffusion-Weighted Imaging with Background Suppression,” European Radiology, Vol. 21, No. 4, 2010, pp. 857-862.
- J. Ma, B. S. Jong, Y. Zhou, et al., “Fast Spin-Echo TripleEcho Dixon (FTED) Technique for Efficient T2-Weighted Water and Fat Imaging,” Magnetic Resonance in Medicine, Vol. 58, No. 1, 2007, pp. 103-109. doi:10.1002/mrm.21268
- N. Russell, M. D. Low, J. Matthew and M. D. Austin, “Fast Spin-Echo Triple Echo Dixon: Initial Clinical Experience with a Novel Pulse Sequence for Simultaneousfat-Suppressed and Nonfat-Suppressed T2-Weighted Spine Magnetic Resonance Imaging,” Journal of Magnetic Resonance Imaging, Vol. 33, No. 2, 2011, pp. 390-400.
- D. N. Costa, I. Pedrosa, C. McKenzie, et al., “Body MRI Using IDEAL,” American Journal of Roentgenology, Vol. 190, No. 4, 2008, pp. 1076-1084. doi:10.2214/AJR.07.3182
- O. Hauger, E. Dumont and J. F. Chateil, “Water Excitation as an Alternative to Fat Saturation in MR Imaging: Preliminary Results in Musculoskeletal Imaging,” Radiology, Vol. 224, No. 3, 2002, pp. 657-663. doi:10.1148/radiol.2243011227
- M. Murakami, H. Mori, A. Kunimatsu, et al., “Postsurgical Spinal Magnetic Resonance Imaging with Iterative Decomposition of Water and Fat with Echo Asymmetry and Least-Squares Estimation,” Journal of Computer Assisted Tomography, Vol. 35, No. 1, 2011, pp. 16-20. doi:10.1097/RCT.0b013e3181f8d30d
- A. J. Ren, Y. Guo, S. P. Tian, et al., “MR Imaging of the Spine at 3.0T with T2-Weighted IDEAL Fast Recovery Fast Spin-Echo Technique,” Korean Journal of Radiology, Vol. 13, No. 1, 2012, pp. 44-52. doi:10.3348/kjr.2012.13.1.44
- M. Takasu, C. Tani, Y. Sakoda, et al., “Iterative Decomposition of Water and Fat with Echo Asymmetry and Least-Squares Estimation ( IDEAL) Imaging of Multiple Myeloma: Initial Clinical Efficiency Results,” European Radiology, Vol. 22, No. 5, 2012, pp. 1114-1121. doi:10.1007/s00330-011-2351-8
- R. Kijowski, D. G. Blankenbaker, M. A. Woods, et al., “3.0-T Evaluation of Knee Cartilage by Using ThreeDimensional Ideal Grass Imaging: Comparison with Fast Spin-Echo Imaging,” Radiology, Vol. 255, No. 1, 2010, pp. 117-127. doi:10.1148/radiol.09091011
- D. G. Disler, M. P. Recht and T. H. McCauley, “MR Imaging of Articular Cartilage,” Skeletal Radiology, Vol. 29, No. 7, 2000, pp. 367-377. doi:10.1007/s002560000222
- H. Yoshioka, M. Alley, D. Steines, et al., “Imaging of the Articular Cartilage in Osteoarthritis of the Knee Joint: 3D Spatial Spectral Spoiled Gradient-Echo vs Fat-Suppressed 3D Spoiled Gradient-Echo MR Imaging,” Journal of Magnetic Resonance Imaging, Vol. 18, No. 1, 2003, pp. 66-71. doi:10.1002/jmri.10320
- G. E. Gold, C. A. Chen, S. Koo, et al., “Recent Advances in MRI of Articular Cartilage,” American Journal of Roentgenology, Vol. 193, No. 3, 2009, pp. 628-638. doi:10.2214/AJR.09.3042
- S. B. Reeder, C. A. McKenzie, A. R. Pineda, et al., “Water-Fat Separation with IDEAL Gradient-Echo Imaging,” Journal of Magnetic Resonance Imaging, Vol. 25, No. 3, 2007, pp. 644-652. doi:10.1002/jmri.20831
- D. B. Siepmann, J. McGovern, J. H. Brittain, et al., “HighResolution 3D Cartilage Imaging with IDEAL SPGR at 3 T,” American Journal of Roentgenology, Vol. 189, No. 6, 2007, pp. 1510-1515. doi:10.2214/AJR.07.2661
- J.-H. Chen, C. Le, et al., “Fat-Free MRI Based on Magnetization Exchange,” Magnetic Resonance in Medicine, Vol. 63, No. 3, 2010, pp. 713-718. doi:10.1002/mrm.22208
- C.-N. Mao, S.-Z. Wang, Q.-Z. Wu, et al., “Magnetization Transfer Contrast Gradient Echo T2WI Sequence in the Diagnosis of Bone Contusion of Knee Joint,” Chinese Journal of Medical Imaging Technology, Vol. 25, 2009, pp. 1262-1264.

