Advances in Nanoparticles
Vol. 1 No. 3 (2012) , Article ID: 25166 , 7 pages DOI:10.4236/anp.2012.13006
Preparation and Characterization of Nano-Sized (Mg(x)Fe(1–x)O/SiO2) (x = 0.1) Core-Shell Nanoparticles by Chemical Precipitation Method
Center Nano science and Technology, Institute of Science and Technology, Jawaharlal Nehru Technological University Hyderabad, Hyderabad, India
Email: Mohsenxm@gmail.com, omrah.h486@gmail.com, kalagadda2003@gmail.com
Received September 20, 2012; revised October 21, 2012; accepted November 3, 2012
Keywords: Magnetic Nanoparticle; Core-Shell; TG-DTA; SEM; TEM
ABSTRACT
Magnetic core-shell nanoparticles have been widely studied because of their excellent and convenient magnetic and electrical properties.In this present work core-shell magneticnanoparticles (MNPs) were synthesized by simple chemical precipitation method. Firstly Mg(x)Fe(1–x)O (magnesiwuestite) nano powder samples were synthesised by low temperature chemical combustion method. Secondly the as synthesised Mg(x)Fe(1–x)O nanoparticles are used to synthesis magnetic core-shell Nano particles byusing 2-propanol, poly ethylene glycol (PEG), ammonia solution 30 wt%, tetraethyl orthosilicate (TEOS). Separation of the core-shell magnetic nanoparticles from the aqueous suspension using a centrifuge. The synthesised MNPs and core shell MNP were characterized by X-ray diffraction (XRD), Thermal gravimetric-differential thermal analyzer (TG-DTA), Transmission electron microscopy (TEM), scanning electron microscopy (SEM), (EDAX) for structural, thermal and morphological respectively. It is observed that the particle size of spherical sampleis 32.5 nm.
1. Introduction
In the past decades, considerable attention has been devoted to the preparation of magnetic nanoparticles because of unique magnetic properties microwaves devices, noise filters, recording heading [1-3] and sensors [4].
The core-shell type magnetic nanoparticles can be broadly defined as comprising a core (inner material) and a shell (outer layer material). These are able to consist of a wide range of different combinations in close interacttion, including inorganic/inorganic, inorganic/organic, organic/inorganic, and organic/organic materials which the inorganic/inorganic interaction has utilized in this paper. For these types of particle core and shell both are made of metal, metal oxide, semiconductor, any other organic compound, or silica.The silica coating used on a core particle has several advantages.The Most basic advantage of silica shell compares to other inorganic compounds, it reduces bulk conductivity, increase the suspension stability of the core particle, it can block the core surface without interfering in the redox reaction at the core surface.
In recent years, various chemical techniques have been successfully employed for the synthesis of inorganic/inorganic interaction (Mg0.1Fe0.9O/SiO2) such as sol-gel [5], hydrolysis [6], stober method [7-8].
The choice of shell material of the core-shell nanoparticle is generally strongly dependent on the end application and use. The Developing new synthesis techniques make it possible to synthesize not only the symmetrical (spherical) shape nanoparticles but also a variety of other shapes such as cube, hexagon [9], prism [10], octahedron [11], disk [12], wire, rod and tube [13] etc.magnetic coreshell NPs are also used in important bio applications, including magnetic bio separation and detection of biological entities (cell, protein, nucleic acids, enzyme, bacterial, virus, etc.),clinic diagnosis and therapy (such as MRI (magnetic resonance image) and MFH (magnetic fluid hyperthermia), targeted drug delivery and biological labels [14].
Preparation of core-shell nanoparticle involves multistep synthesis procedure. But the most important step during synthesis is to maintain uniform coating and controls the shell thickness such as precipitation [15], polymerization [16], microemulsion [17], sol-gel condensation [18]. The overall size and shell thickness can be controlled by varying the reactant concentration both the core and shell material.
2. Experimental
2.1. Preparation of Mg(x)Fe(1–x)O Nano Powder
A number of techniques have been used for the preparation of nanoparticles, such as sol-gel [19], micro emulsion method [20], ball milling [21], microwave hydrothermal method [22].
In the present research paper low temperature glycine based chemical combustionsynthesis [23] is used to prepare Mg(x)Fe(1–x)O nanoparticles. The iron nitrate (Fe(NO3)3.9H2 O) and magnesium nitrate (Mg(NO3)3.6H2O) were dissolved in a beaker with sufficient deionized water with Glycine in appropriate amount and the solution was placed under thermal stirring with the temperature of 60˚C; at this temperature, different molar amounts of the fuel were added. The precursor solutions were maintainedunder thermal stirring for few minutes to ensure homogeneity subsequently these solutions were placed in a hot plate., as the temperature reached 100˚C, water started to boil and evaporate from the solution, which increased solution viscosity substantially, during which the compound caught fire. Precursor is annealed for 2 hrs at 300˚C the as-obtained precursor and annealed precursor were both characterized.
2.2. Preparation of Core-Shell Mg(x)Fe(1–x)O/SiO2
Appropriate amount of Mg(x)Fe(1–x)O Nano powder was added to 120 ml of 2-propanol and sonicated for about 1 hr. Under continuous stirring, 2.0 g of PEG (poly ethylene glycol), 10 ml of deionize water, 10 ml of ammonia solution (30 wt%) and 0.2 ml of TEOS (tetraethyl orthosilicate) as source of silica were consecutively added into the above suspension, the reaction was allowed to continue for 24 h under stirring. After the reaction was completed, the products were collected through centrifugation at 4000 rpm, followed by washing with deionized water and ethanol several times, anddried at 80˚C overnight in an oven. The core-shell (Mg(x)Fe(1–x)O/SiO2) magnetic nanoparticles were obtained. The shell and core thickness were controlled by experimental parameters such as coating time, feed concentrations, catalyst and other precursor.
The core-shell magnetic nanoparticle and Mg(x)Fe(1–x)O nanoparticles also was characterized by X-ray diffraction (Bruker D8 Advance, Germany), Thermal gravimetric analysis, (SII EXSTAR 6300R, Japan), Scanning Electron Microscope and Energy-dispersive X-ray spectroscopy, S-3400N-hitachi-Japan), Transmission Electron Microscope (JEM-2100, Jeol).
3. Result and Discussion
3.1. X-ray Diffraction Analysis
The powder x-ray diffraction measurements (XRD) of the samples were obtained on a Bruker D8 Advance systems were taken from 20˚ to 80˚ (2θ value) using Cu-Kα (λ = 0.154 nm) radiation. The crystallite size (D) of the synthesized nanoparticles was measured using the Scherrer’s formula [24]
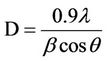 (1)
(1)
where D is the crystallite size, λ is the x-ray wavelength, the Bis equal to full width half maximum, and θ is the Bragg angle.
The existence of a peak around the diffract ion angle (2θ) equal to 35.66˚C corresponding to (311) plane confirms the formation of spinel ferrites. The calculated average crystallite size (D) is 52 nm. Figure 1 shows the XRD pattern of the synthesized Nano powder. It is clear that a cubic structure of Mg(x)Fe(1–x)O that is described in JCPDS 89-4924 is detected in all samples. Seven peaks centered at 2θ = 30.28˚C, 35.66˚C, 43.33˚C, 53.77˚C, 57.31˚C, 62.93˚C and 74.44˚C, which correspond to diffraction planes of (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0) and (5 3 3) respectively are detected. According to JCPDS 89-4924, the obtained phase has a cubic structure. In the X-ray diffraction pattern of Mg(x)Fe(1–x)O/SiO2 five peaks at 24˚, 33˚, 41˚, 49˚ and 64˚ appeared. The first three peaks were from crystalline SiO2, and next two peaks might be from α-Fe2O3. In addition to these peaks, the diffractogram of showed a sharp peak of crystalline SiO2 at 33˚. It can be concluded that not pure Mg(x)Fe(1–x)O nano crystals were formed and the silica matrix could be crystallized.
3.2. Thermal Gravimetric Analysis (TGA)
Decomposition path of Mg(x)Fe(1–x)Oand Mg(x)Fe(1–x)O/ SiO2 core-shell magnetic nanoparticle were studied by TGA. Figure 2 shows the weight loss percentage for the magnetic particles during heat treatment under constant air flow which is placed on the graph. Thermo gravimetric measures the mass of a sample as the temperature increases. This method is useful for determining sample purity, moisture content of sample, carbonate, organic content and for studying decomposition reaction.
3.3. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
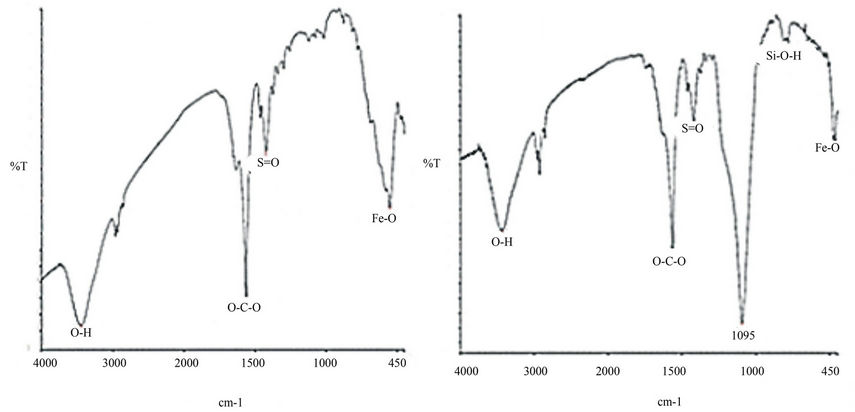
The formation of Nano crystalline Mg(x)Fe(1–x)O and Mg(x) Fe(1–x)O/SiO2 core-shell nanoparticle are supported by FTIR spectra.The FT-IR spectra for variable GN−1 ratios were recorded in the range 400 - 3000 cm−1 (Figure 3). A broad absorption spectrum is seen around 3430 cm−1 (Figures 3(a) and (b)) which is a characteristic stretching vibration of hydroxylate group (O-H). Peaks localized at 1560 cm−1 and 1411 cm−1 are assigned to asymmetrical

Figure 1. XRD patterns of Mg(x)Fe(1–x)O nanoparticles and Mg(x)Fe(1–x)O/SiO2 core-shell.
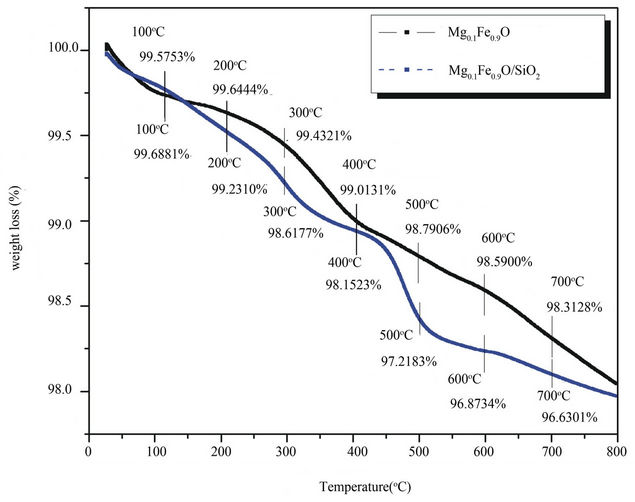
Figure 2. TGA curvefor Nano crystalline Mg(x)Fe(1–x)O and Mg(x)Fe(1–x)O/SiO2 core-shell nanoparticle.
and symmetrical stretching vibration of carboxylate (O-C-O) respectively (Figures 3(a) and (b)). The peak around 1400 cm−1 (broad) characterizes the formation of surface sulfate species with S=O bond (Figures 3(a) and (b)).Absorption bands due to the iron-oxygen (Fe-O) are observed in the 450 - 550 cm−1 (Figures 3(a) and (b)). The band at 955 cm−1 (Figure 3(b)), Probably caused by Si-O-H stretching vibrations [25,26]. The high frequency band is around 1561 cm−1 (Figure 3(a)) which is common feature to all the ferrites [27] and 1095 cm–1 (Figure 3(b)) which is characteristic of the silica [28].
3.4. SEM, TEM and EDAX Analysis
Figures 4-6, shows the detailed morphology and crystalline structure of the Mg(x)Fe(1–x)O nano powder and
 (a) (b)
(a) (b)
Figure 3. (a) FT-IR spectra of the Mg(x)Fe(1-x)O powders produced by combustion method at 300˚C; (b) FT-IR spectra Mg(x)Fe(1-x)O/SiO2 core-shell nanoparticle.




Figure 4. (a-d) SEM micrograph and EDAX of sample; (a) Mg(x)Fe(1-x)O; (b) Mg(x)Fe(1-x)O/SiO2 core-shell, scale bar 20 μm.
Mg(x)Fe(1–x)O/SiO2 core-shell nanoparticle, then they were further investigated by SEM, TEM and selected-area electron diffraction (SAED).
It can be seen,Mg(x)Fe(1–x)O have high porous structure which was shown in (Figure 4(a)) and also can be seen the structure of Mg(x)Fe(1–x)O/SiO2 core-shell magnetic nanoparticle in (Figure 4(b)). Energy dispersive x-ray spectroscopy (EDAX) equipped within the SEM was
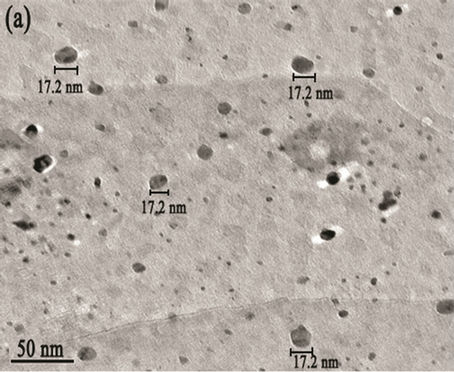
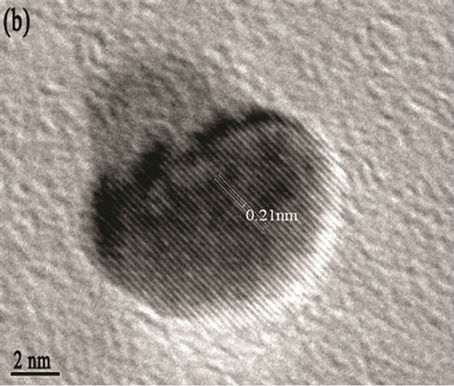
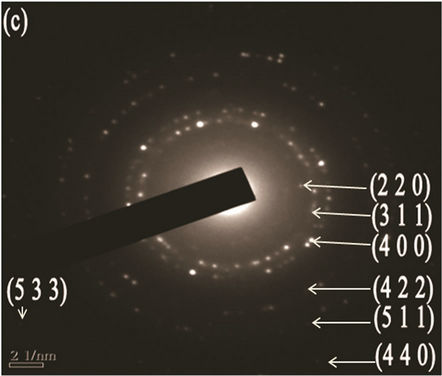
Figure 5. (a) TEM image with scale bar 50 nm; (b) d spacing with scale bar 2 nm (High resolution TEM (HRTEM); (c) SAED patterns of the Mg(x)Fe(1-x)O with scale bar 21nm
used to determine the chemical composition of the synthesized nanoparticles. EDAX revealed the chemical composition of synthesized nanoparticles, as shown in Figures 4(c) and (d). No contaminating elements from re
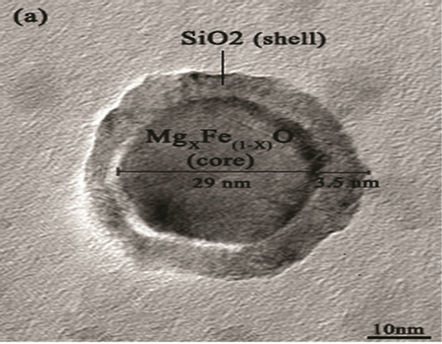
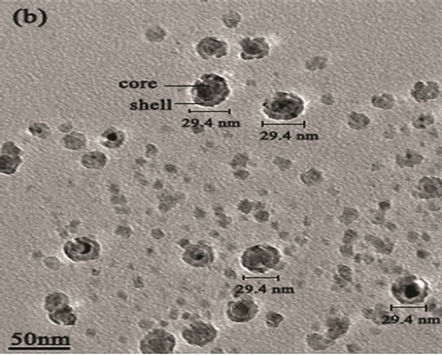
Figure 6. (a) TEM image of MgxFe(1-x)O/SiO2 core-shell; (b) high resolution TEM (HRTEM) of core-shell.
agents were detected Transmission electron microscopy (TEM) shows the particle’s morphology, distribution and its size Figure 5(a). Figure 5(c) is selected-area electron diffraction (SAED) showing(h k l)values which are exactly matching with XRD pattern which is put on white ring in SAED and good uniform dispersion also we observed from Figure 5(b) says Mg(x)Fe(1–x)O has Nano sphere shape with d spacing of 0.21nm. Figure 6(a) shows high magnification TEM images of average SiO2 shell thickness of nanoparticle is 3.5 nm and Mg(x)Fe(1–x) O core diameter is 29nm.The darker core and the lighter shell of the particles in routine transmission electron microscopy (TEM) images suggest the core-shell structure as shown in Figure 6(b) in which the contribution of the atomic number to TEM contrast is also important.
4. Conclusion
Magnetic Core-shell nanoparticle with pure Mg0.1Fe0.9O that is coated with silica has successfully been prepared by chemical precipitation method. The average crystallite size of Mg0.1Fe0.9O nanoparticles were estimated from the full width half maximum of the X-ray diffraction peaks. XRD pattern showed that Mg0.1Fe0.9O have cubic spinel Fd-3m structure with average crystalline size 52 nm. The weight loss percentage of the synthesized particles is calculated with TG-DTA. FTIR conform formation of the Mg0.1Fe0.9O/SiO2. It is observed from SEM that the core is porous in nature with uniform dispersion. From TEM it is very clear that core-shell MNPsMg0.1 Fe0.9O/SiO2 formation with uniform shell thickness. The results reported in this study could be applied for establishing a simple method for the preparation of Mg0.1Fe0.9 O/SiO2 Magnetic Core-shell nanoparticle.
5. Acknowledgements
The authors would like to thank the School of Engineering Sciences & Technology, Hyderabad University for providing XRD, EDS, SEM, Regional Sophisticated Instrumentation Centre for TEM, Mr Masoud Ahmadipour, department of Electrical and Computer science, Semnan University, Iran for Cooperation.
REFERENCES
- V. Sepelak, D. Baabe, D. Mienert, et al., “Evolution of Structure and Magnetic Properties with Annealing Temperature in Nanoscale High-Energy-Milled Nickel Ferrite,” Journal of Magnetism and Magnetic Material, Vol. 257, No. 2-3, 2003, pp. 377-386. doi:10.1016/S0304-8853(02)01279-9
- M. Pavlovic, C. Jovalekic, A. S. Nikolic, D. Manojlovic and N. Sojic, “Mechanochemical Synthesis of Stoichiometric MgFe2O4 Spinel,” Journal of Material Science, Vol. 20, No. 8, 2009, pp. 782-787.
- P. Holec, J. Plocek, D. Niznansky and J. P. Vejpravova, “Preparation of MgFe2O4 Nanoparticles by Microemusion Method and Their Characterization,” Journal of Sol-Gel Science and Technology, Vol. 51, No. 3, 2009, pp. 301- 305. doi:10.1007/s10971-009-1962-x
- S. Q. Liu and Z. Y. Tang, “Nanoparticle Assemblies for Biological and Chemical Sensing,” Journal of Material Chemistry, Vol. 20, No. 1, 2010, pp. 24-35. doi:10.1039/b911328m
- L. XU, W. Q. Zou and J. M. Hong, “Preparation and Characterization of Nanocomposite MgFe2O4/SiO2,” Preparation and Characterization of Nano Composite, Vol. 24 No. 3, 2008, pp. 354-359.
- J. Chen, Y. G. Wang, Z. Q. Li, C. Wang, J. F. Li and Y. J. Gu, “Synthesis and Characterization Of Magnetic Nanocomposites with Fe3O4 Core,” Journal of Physics: Conference Series, Vol. 152, No. 1, 2009, p. 012041
- E. Taboadacabellos, “Synthesis of Fe2O3-SiO2 Composite Nanoparticles Targeting Magnetic Resonance Imaging and Magnetic Hyperthermia Application,” Ph.D. Dissertation, University of de Barcelona, Barcelona, 2009.
- M. Gao, W. Li, J. Dong, Z. Zhang and B. Yang, “Synthesis and Characterization of Superparamagnetic Fe3O4 @SiO2 Core-Shell Composite Nanoparticles,” World Journal of Condensed Matter Physics, Vol. 1, No. 2, 2011, pp. 49-54
- J. Ahmed, S. Sharma, K. V. Ramanujachary, S. E. Lofland and A. K. Ganguli, “Microemulsion-Mediated Synthesis of Cobalt (Pure FCC and Hexagonal Phases) and Cobalt-Nickel Alloy Nanoparticles,” Journal of Colloid and Interface Science, Vol. 336, No. 2, 2009, pp. 814- 819,
- J. S. Hu, Y. G. Guo, H. P. Liang, L. J. Wan and L. Jiang, “Three-Dimensional Self-Organization of Supramolecular Self-Assembled Porphyrin Hollow Hexagonal Nanoprisms,” Journal of American Chemical Society, Vol. 127, No. 48, 2005, pp. 17090-17095. doi:10.1021/ja0553912
- E. Schmidt, A. Vargas, T. Mallat and A. Baiker, ShapeSelective Enantioselective Hydrogenation on Pt Nanoparticles,” Journal of American Chemical Society, Vol. 131, No. 34, 2009, pp. 12358-12367. doi:10.1021/ja9043328
- X. Qu, L. Omar, T. B. Le, L. Tetley, K. Bolton, K. W. Cjooi, W. Wang and I. F. Uchegbu, “Polymeric Amphiphile Branching Leads to Rare Nanodisc Shaped Planar Self-Assemblies,” Langmuir, Vol. 24, No. 18, 2008, pp. 9997-10004. doi:10.1021/la8007848
- G. Cao, D. Liu, “Template-Based Synthesis of Nanorod, Nanowire and Nanotube Arrays,” Advance Colloid Interface Science, Vol. 136, No. 1-2, 2008, pp. 45-64. doi:10.1016/j.cis.2007.07.003
- W. Wu, Q. G. He and C. Z. Jiang, “Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies,” Nanoscale Research Letters, Vol. 3, No. 11, 2008, pp. 397-415. doi:10.1007/s11671-008-9174-9
- A Imhof, “Preparation and Characterization of TitaniaCoated Polystyrene Spheres and Hollow Titania Shells,” Langmuir, Vol. 17, No. 12, 2001, pp. 3579-3585. doi:10.1021/la001604j
- P. A. Dresco, V. S. Zaitsev, R. J. Gambino and B. Chu, “Preparation and Properties of Magnetite and Polymer Magnetite Nanoparticles,” Langmuir, Vol. 15, No. 6, 1999, pp. 1945-1951. doi:10.1021/la980971g
- G. Hota, S. B. Idage and K. C. Khilar, “Characterization of Nano-Sized CdS-Ag2S Core-Shell Nanoparticles Using XPS Technique,” Colloids Surface A, Vol. 293, No. 1-3, 2007, pp. 5-12. doi:10.1016/j.colsurfa.2006.06.036
- T. Li, J. Moon, A. A. Morrone, J. J. Mecholsky, D. R. Talham and J. H. Adair, “Preparation of Ag/SiO2 Nanosize Composites by a Reverse Micelle and Sol-Gel Technique,” Langmuir, Vol. 15, No. 13, 1999, pp. 4328-4334. doi:10.1021/la970801o
- L. J. Berchmans, R. K. Selvan, P. N. S. Kumar and C. O. Augustin, “Structural and Electrical Properties of Ni1-xMgxFe2O4 Synthesized by Citrate Gel Process,” Journal of Magnetism and Magnetic Materials, Vol. 279, No. 1, 2004, pp. 103-110. doi:10.1016/j.jmmm.2004.01.073
- P. Holec, J. Plocek, D. Niznansky and J. P. Vejpravova, “Preparation of MgFe2O4 Nanoparticles by Microemulsion Method and Their Characterization,” Journal of SolGel Science and Technology, Vol. 51, No. 3, 2009, pp. 301-305. doi:10.1007/s10971-009-1962-x
- M. Rabanal, A. Varez, B. Levenfeld, J. Torralba, “Magnetic Properties of Mg-Ferrite after Milling Process,” Journal of Material Processing Technology, Vol. 143, 2003, pp. 470-474. http://www.sciencedirect.com/science/article/pii/S0924013603004643
- V. Seema, P. A. Joy and Y. B. Khollam, “Synthesis of Nanosized MgFe2O4 Powders by Microwave Hydrothermal Method,” Journal of Material Letters, Vol. 58, No. 6, 2004, pp. 1092-1095. doi:10.1016/j.matlet.2003.08.025
- F. M. H. Zarandi and K. Sadrnezaad, “Thermomechanical Study of Combustion Synthesized Ti-Ni Shape Memory Alloy,” Materials and Manufacturing Processes, Vol. 12, No. 6, 1997, pp. 1093-1105. doi:10.1080/10426919708935206
- B. Dcullity, “Elements of X-Ray Diffraction,” 3rd Edition, Addison-Wesley Publishing Co., Boston, 1976.
- A. V. Rao, P. B. Wagh, D. Haranath, et al., “Influence of Temperature on The Physical Properties of TEOS Silica Xerogels,” Ceramics International, Vol. 25, No. 6, 1999, pp. 505-509. doi:10.1016/S0272-8842(97)00085-0
- A. M. Efimov, “Quantitative IR Spectroscopy: Applications to Studying Glass Structure and Properties,” Journal of Non-Crystalline Solids, Vol. 203, 1996, pp. 1-11.
- R. Dwaldron, “Infrared Spectra of Ferrites,” Physical Review, Vol. 99, No. 6, 1955, pp. 1727-1735.
- L. Li, G. R. Li, L. Smith, et al., “Preparation and Characterization of Nanocomposite MgFe2O4/SiO2,” Chemistry Material, Vol. 12, 2000, pp. 705-714.

