American Journal of Plant Sciences
Vol.07 No.17(2016), Article ID:72708,17 pages
10.4236/ajps.2016.717213
Evapotranspiration and Above Ground Biomass of Acer rubrum from Liners to 8 m Tall Trees
Richard C. Beeson Jr.
Mid-Florida Research and Education Center Apopka, University of Florida, Gainesville, FL, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 9, 2016; Accepted: December 10, 2016; Published: December 13, 2016
ABSTRACT
To meet minimum spring flows, water management districts in Florida sought to make both agriculture and urban landscapes water efficient, which includes tree farms. Acer rubrum L. (red maple) trees are endemic to Central Florida and native to the eastern portion of the United States. Urban and suburban expansion has increased use of A. rubrum in landscape plantings and their production in nurseries. In Florida A. rubrum is planted around stormwater retention areas, but also in urban landscapes. To provide a basis for irrigation allocations both during production and in landscapes, daily actual evapotranspiration (ETA) for three red maple trees were measured with weighing lysimeters, beginning with rooted cuttings and continuing until trees averaged 8 m in height. Empirical models were derived to calculate ETA based on crown horizontal projected area or trunk caliper, adjusted daily by changes in reference evapotranspiration (ETo). Water use efficiency, based on carbon sequestered in above ground wood mass, was calculated at the end of five growing seasons. Average ETA to produce these maples was 29,107 L over 4.75 years, with an average water use efficiency of 1 kg dry mass of wood per 709 L of water lost by transpiration.
Keywords:
Acer rubrum, Irrigation Scheduling, Irrigation Modelling, Container Production, Field Production, Landscape Irrigation

1. Introduction
Trees add value to managed landscapes through aesthetics and ecosystem services such as storm water management, pollution abatement, and increased cooling [1] [2] [3] [4] . Trees increase property values in residential and urban communities [5] [6] . Landscape trees often require irrigation during all stages of life: especially during production in containers or as large specimens [7] [8] [9] ; during root system establishment post transplanting [10] ; in arid climates [11] ; or where rooting volume constrains access to water. Maintaining landscape tree health and efficient water use requires estimates of water demand in each of these situations in order to schedule irrigation amount and timing.
Transpiration among tree species, and within species, varies widely based on location, tree size and evaporative demand. Whole tree transpiration can range from 10 to 200 L day−1, mostly based on size differences of canopies [12] . Most studies have quantified tree evapotranspiration (combined surface evaporation and tree transpiration, ETA) for relatively short periods of times, rarely longer than a month and mostly focused on forest trees. Yet ETA of isolated trees, a common arrangement in landscapes, tends to be greater than trees in forests due to higher ventilation of foliage and more sunlit leaf area [13] [14] [15] [16] . Beeson [17] quantified the effect increased ventilation and illumination on isolated trees, showing maximum ETA was maintained to 67% canopy closure, and likely beyond, then declining at higher densities approaching 100% due to mutual shading and less ventilation. Research on landscape tree water use has been consolidated into a national standard for estimating water demand of landscape plants [18] . A key element of the standard was defining tree water demand estimates as a fraction of local reference evapotranspiration (ETo) that are lower in dry climates compared to humid climates (Kjelgren et al., 2016). This climate difference in tree water use estimates is due to reduced transpiration from stomata at high ETo/VPD levels compared to humid climates. Tree sensitivity to dry air in arid climates has been widely documented, but studies of tree water use and hence ability to estimate demand in humid climates are much more limited.
Previous studies in a humid climate have examined large tree water use over periods of a year or more. Ruiter [19] quantified ETA of Pinus radiata (radiata pine) using large drainage lysimeters with volumes of 7 m3. Lysimeters were planted singly with 9 mon- th-old seedlings. After 3 years, trees irrigated throughout the period averaged 3 to 4 m in height, with daily average ETA over four weeks of 21 L·day−1. Edwards [20] measured daily ETA of single trees of four species grown in southern New Zealand for a year. At the end trees ranged from 3.3 to 5.6 m in height. ETA exceeded 120 L·day−1 in summer for Eucalyputus fastigata, and was near zero for deciduous species during winter. In addition to guiding efficient irrigation of landscape and nursery trees in high rainfall climates, quantitative studies of tree water use can be used to estimate demand in water balance at varying tree densities in watersheds for increasing water for domestic use [21] , or decreasing runoff in urban areas [1] , and combined with biomass measurements, tree water use efficiency [22] .
Here is present actual water use (ETA) of three individual Acer rubrum trees measured by weighing lysimeters, representing a nursery setting, from rooted cuttings to 8 m tall trees over nearly five years. The objective was to develop simple correction factors to estimate tree ETA (volume units) based on ETo and easily measured tree traits than control transpiration: projected canopy area and trunk cross sectional area. Modeling the volume of tree water use volume will aid managers and policy makers in humid regions, especially in subtropical climates with lengthy dry seasons, in estimating water demand in nursery production and tree-dominated landscapes in guiding irrigation scheduling of nursery and urban landscape trees, and determining water allocations.
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Transplanting
Rooted cuttings of a red maple cultivar (Acer rubrum “Florida Flame”) were obtained late winter 2001 from a nursery in north Central Florida. Five uniform rooted cuttings were transplanted into 26 L containers using a 70% composted pine bark: 30% Florida sedge peat: 10% coarse sand substrate amended with 0.68 Kg per·m3 of micronutrients and 2.3 Kg per·m3 of dolomite limestone to buffer to a pH of 6.0. The same substrate components and amendments were blended new by the same commercial potting mix company (Florida Potting Soil Inc., Orlando, FL) in February in 2001, 2002 and 2003 as trees were repotted into larger containers each spring. In 2004 and 2005, the sedge peat used in previous years was replaced by “NuPeat” (Florida Potting Soil Inc.) which was comprised of 1/3 composted yard waste, 1/3 composted & screened hardwood bark and 1/3 Florida sedge peat. The quantities of micronutrients and limestone per substrate volume were similar to previous years. These five containers were painted inside with a copper hydroxide mixture (Spin Out, Griffin Corp. Valdosta, GA) to inhibit root circling [7] [8] , and covered outside with aluminum foil to reduce evaporation by heat loading. These containers were also covered with a shallow convex dome to exclude most rainfall and to reduce evaporation. Each subsequent study year, trees were transplanted during late winter into sequentially larger containers. Each of these larger containers were also painted on the inside with Spin-out and covered with aluminum foil on the outside. In 2002, trees were transplanted in a 95 L (0.55 m diameter) container, then in each subsequent year 2002-2005 trees were progressively moved into 361, 760, and 1140 L containers. In 2003 an appropriately-sized wire basket (32-COT, Cherokee Manufacturing Inc., St. Paul, MN) was placed in containers to maintain root ball integrity and to lift trees without damage to trunks.
2.1.2. Tree Care
Trees were staked and fertilized as needed and pruned as needed during the growing period. Trees were fertilized with controlled release fertilizer (Polygon 19N-4.2P-11.6K, Harrell’s Fertilizer Co. Lakeland, FL) each year. Mid-to-late winter each year starting in 2002, overall tree canopies were pruned to promote tree structure in accordance with Florida Grades and Standards for Nursery Crops [23] . In 2003 after transplanting, trees were pruned to raise the bottom of tree canopies to 1.2 m above the root ball. Beginning in June of 2002, foliar sprays of Kocide 3000 (DuPont, Wilmington, DE) and Dithane (Dow AgroSciences, Indianapolis, IN.) were applied biweekly through October each year to control a leaf bacteria). Foliar fungicide sprays applied biweekly in later years to prevented leave loss.
2.1.3. Experimental Layout
The first year, the five study trees were suspended from a 2 m high tripod lysimeter [24] , consisting of a basket to hold the container suspended from a load cell (SSM-100, Interface Force Inc., Scottsdale, AZ) underneath the tripod. Load cells were connected to a data logger (CR10X, Campbell Scientific, Inc., Logan, UT) and multiplexer system (AM-416 and AM-32) that collected lysimeter mass every half hour and controlled irrigation [24] . In 2002 the three largest trees were placed singly in large weighting lysimeters [24] in a row oriented east-west. Each triangular lysimeter basket was suspended from three 341 kg load cells (SSM-750, Interface Force Inc., Scottsdale, AZ) attached to steel pillars at apices. A basket accommodated up to a 1.55 m diameter polyethylene container. Study trees remained in the triangular lysimeter 2002-2005.
Spacing of border trees each spring was representative of nursery production at each stage of growth. In 2001 trees were 0.4 m on center using a square arrangement with 95 border trees handled and transplanted the same as the study trees. Lysimeters were randomly placed within a middle row of the block of 4 rows of 25 containers. In 2002 18 border trees were transplanted into similar containers as the study trees and placed around each triangular lysimeter study tree to maintain tree canopy cover that approximated that of a commercial nursery, with an initial canopy density of approximately 50%. In 2003 border trees were reduced to 12 per lysimeter at approximately the same 50% density. In 2004, border trees around each lysimeter were reduced to 6 with again approximately 50% initial spacing. In 2005 one border tree was placed in the four cardinal directions around each lysimeter, with one tree between lysimeters within the row.
2.2. Irrigation
In 2001, all trees were irrigated concurrently with a micro-irrigation spray stake (light green, 25.2 L·hr−1, Roberts Irrigation, San Marcos, CA) as needed at midnight. ETA from each lysimeter was calculated from daily changes in mass between 600 h and 2200 h (EST; earliest sunrise at the site was 6:29 am, with sunset at 8:26 pm), to avoid corrections for dew condensation and allow excess irrigation to drain. Irrigation initiated at midnight if the minimum cumulative ETA exceeded 544 g, equivalent to 6.2 mm of water over a substrate surface. Trees were irrigated to excess at night to insure complete saturation of the substrate to achieve maximum ETA each day. Irrigation volume was based on the greatest mass change among the five weighed trees, multiplied by 1.15 to account for irrigation non-uniformity and for increases in plant mass due to growth. Irrigation was applied until the slowest increase in mass gain among the five weighed trees achieved the target mass increase to insure all trees were at 100% container capacity the following morning. Daily ETA volumes less than 544 g were retained and added to the following day’s ETA. During rain events, container mass often increased due to accumulation in a container or clinging to foliage, especially near sunset. These increases negated some daily ETA volumes and occasionally prevented an irrigation event. ETA consisted mostly of transpiration, although some evaporation likely occurs through the trunk opening in the covers.
From 2002-2005, irrigation was governed by the lysimeter tree’s ETA. During May to early November, irrigation algorithms applied water equivalents of 50% of mass change between 600 and 1300 HR (EST) at 1300 hrs. This midday irrigation was to maximize growth [25] without leaching from a container. Changes in irrigation regimes occurred consistently each year. Nightly re-saturation of a substrate was accomplished by applying 125% to 135% of the mass change between 600 HR and 2200 HR in three equal sub- volumes at midnight, 100 and 200 hr. Water was applied in excess of 100% ETA to insure sufficient resources for maximum ETA each day. Minimal leaching occurred before the third irrigation cycle. In mid-November irrigation reverted back to applications only at night.
2.3. Data Collection
2.3.1. Reference ET
Reference evapotranspiration (ETo) was calculated each day from a Campbell Scientific weather station located in a grassy field located 25 m west of lysimeters. The weather station consisted of a pyranometer (Li-200; Li-Cor Inc., Lincoln, NE), a tipping bucket rain gauge (TE525, Texas Instruments, Dallas, TX), temperature/humidity sensor (CS-215, Campbell Scientific Inc., Logan, UT) and a wind sensor (Model 014, Met One Instruments, Grants Pass, OR) and a CR10X data logger that used Application Note 4 (Campbell Scientific Inc.) to calculate ETo with resistance as described by Allen et al. [26] .
2.3.2. Growth
Growth measurements of tree height, branch spread of widest width and width perpendicular, and maximum trunk caliper at 0.15, 0.30 and 1.2 m above the substrate were recorded on lysimeter trees every three weeks during each growing season. Beginning year three trunk circumference was measured with a metal tape measure. Horizontal Projected Canopy Area (PCA, m2) was calculated by multiplying consistent perpendicular measurements of branch spread. Trunk cross sectional area (TCSA, cm2) was calculated for each of the three trunk measurements. Total tree leaf areas were quantified for each tree using five individual branches representative of the range of branch diameters late each growing season just prior to leaf senescence. Leaves were removed and leaf area was measured for each tree (Model 3100 leaf area meter, Li-Cor Inc., Lincoln, NE). Remaining leaves on a tree were then removed and dried at 68˚C until a constant dry mass was obtained. Specific leaf area (g·cm−2) was calculated for each branch, with the mean multiplied by total leaf dry mass to calculate total leaf area per tree. To determine total leaf and aboveground biomass, border trees were harvested just prior to leaf senescence. In 2001, 10 border trees were harvested, and then 2002-2004 one border tree per lysimeter tree was harvested for total biomass and leaf area for a total of three trees each year. In 2005, similar leaf area measurements were recorded for each lysimeter tree, but to terminate the project, for each lysimeter tree branches and trunks remaining after leaf removal were placed in a drying oven at 68˚C and dried to constant mass for each year to determine total aboveground biomass.
2.2.3. Water Use
Usually ETA was calculated daily as differences between mass recorded at 600 hrs minus mass recorded at 2200 hrs. When partial midday irrigation was in effect, increases in mass from midday irrigation was calculated by the datalogger and added to in the daily sum. However if rare loss of power (hurricanes) or rain events occurred between 600 to 2200 hr, actual daily cumulative ETA was estimated as described by Beeson [27] . For power lost, each tree’s daily ETA before and after the loss was normalized to a water volume per unit ETo (Normalized ETo; L·mm−1). This assumed leaf area was constant and normalized values varied minimally over short periods of 4 to 7 days without precipitation. Daily ETA for each missing day was estimated by multiplying the Normalized ETo volume by the measured ETo for each missing day. When rainfall occurred between 600 and 2200 hr, half hour mass data was plotted to indicate rainfall events. Periods of decreases in mass were summed to estimate ETA. This was then vetted by normalizing by ETo, then comparing the rain day normalized ETA to normalized ETA of recent rainless days.
In late August 2001, after end of shoot elongation but before leaf senescence, trunk diameter was measured on 10 border trees at 0.30 m above soil level. Leaves were removed and leaf area was measured for each tree (Model 3100 leaf area meter, Li-Cor Inc., Lincoln, NE). In 2002 and 2003 measurements of trunk circumference at 0.15, 0.30 and 1.2 m above a root ball were also recorded on three border trees in November. Leaf areas were quantified for each tree using five individual branches representative of the range of branch diameters. Remaining leaves on a tree were then removed and dried at 68˚C until a constant dry mass was obtained. Specific leaf area (g·cm−2) was calculated for each branch, with the mean multiplied by total leaf dry mass to calculate total leaf area per tree. Total leaf area was divided by respective trunk cross sectional area (TCSA) calculated from trunk circumference at 30 cm and averaged across the 3 replications to assess if leaf area was constant or varied with xylem increases. In 2005, similar measurements were recorded for each lysimeter tree. Branches and trunks remaining after leaf removal were placed in a drying oven at 68˚C and dried to constant mass for each year.
2.4. Analysis
Daily volumetric water use was plotted over the growing season for each year. The measures of tree cross sectional areas that control transpiration (PCA and TCSA at three heights, in m2) averaged for seven consecutive days every three weeks over each season were regressed against to the corresponding Normalized ET (ETA ÷ ETo in liters mm-1) centered on day 4 of the 7-day period. At the end of the study whole plant water use efficiency was calculated for each tree by dividing total seasonal ETA by final above ground biomass. All statistical analysis was conducted using SAS (ver. 8.0) Proc GLM. Total tree leaf area was divided by respective trunk cross sectional area (TCSA) calculated from trunk circumference at 30 cm and averaged across the 3 replications to assess if the ratio of leaf area to xylem area was constant or varied with yearly xylem increment growth. Whole plant water use efficiency was calculated at the end of year 5 by dividing above ground biomass by sum total ETa.
3. Results
3.1. Prevailing Microclimate
The research site was located at latitude 28.693 N and longitude 81.533 W, approximately 35 Km from Orlando, FL, USA in the USDA Hardness Map Zone 9A. The dry season normally begins in mid-October and last through the middle of May. Rainfall during this period in generally less than 7 cm per month, with average temperatures during this period range from 20˚C to 29˚C in October, to 10˚C to 21.7˚C in January. The rainy season starts in late May and last until early October. Temperatures during this period range from 23˚C to 35˚C, with average rainfalls of 19 cm per month. At bud break, the photoperiod is about 12 hours, peaking to 14 hr in late June and declining to 10.5 hrs at complete leaf senescence.
3.2. Quantification of ETA
Tree growth was rapid under long growing seasons and optimum irrigation. Leaf bud break consistently initiated in early March, with senescence completed in late December. Spring increases in ETA were rapid with increasing leaf area and ETo, especially beginning with the third spring (Figure 1). During the fifth spring, ETA increased by 90 L over a 75 day period of bud break and shoot elongation. Conversely, declines in ETA occurred at a much slower pace once rapid shoot elongation ceased. In 2001 peak ETA averaged1.5 L·day−1 (Figure 1(a)). Trees grew from 0.4 to 1.8 m tall the first season, with ETA peaking in mid-August, then declining rapidly from mid-September (Day 260) to early October (Day 280). The decline corresponded with a leaf bacterial infection causing premature senescence by October. Mean cumulative ETA from March to December for 2001 was 157 L.
Spring ETA in 2002 was similar to that of spring 2001 at bud break, about 0.2 L·day−1. ETA increased from near 0 to 4 L·day−1 over the next 60 days (Day 140, late May); and continued to increase with shoot elongation through to late October. ETA frequently achieved 12 L·day−1 from late July (Day 205) to mid-October (Day 290). Height increased from 1.8 to 3.4 m and from 0.77 to 1.84 m in average canopy spread by the end of December, with daily ETA declining to less than 2 L·day−1. Mean cumulative ETA of the three trees in 2002 was 1756 L.
Year three (2003), trees flowered before leaf expansion (Figure 1(c)). Flowering began in mid-February (Day 45), with leaf and shoot growth beginning in late March (Day 90). Although shoot or leaf growth had not yet begun, ETA doubled with flowering to 2 L·day−1, then jumped from 2 to 21 L·day−1over 45 days (Figure 3(b)). ETA peaked




Figure 1. Daily ETA of Acer rubrum from rooted cutting beginning in March 2001 until trees were harvested before leaf drop in 2005. Each point is the mean of three tree replicates. Letters correspond to years; (a) 2001, (b) 2002, (c) 2003, (d) 2004 and (e) 2005.
at 26.5 L·day−1 in early June (Day 155), remaining between 23 to 27 L·day−1 through first week of July. During this time, stems with new, red leaves were 0.30 to 0.45 m in length on most major branches. In mid-July shoot elongation and leaf expansion greatly slowed, and when coupled with maturation of previously expanding leaves, the result was substantial declines in ETA of 9.5 L to 19 L·day−1 across the three trees. Shoot elongation was minimal thereafter, as height and width were only 0.30 m and 0.4 m, respectively, from late June through late November (Figure 1(c)). Cumulative mean ETA for 2003 was 3769 L as trees grew from 3.4 m to 5.1 m in height and from 1.4 to 2.9 m in width.
Prior to flower initiation in late February 2004 (Day 50), ETA was less than 4 L·day−1, and increased to 7 L·day−1 during flowering (Figure 1(d)). With shoot and leaf expansion beginning in early March (Day 85), ETA increased from 7 to 45 L·day−1 over a 50 day period. From early June until mid-July (Day 200), ETA generally ranged from 49 to 64 L·day−1. As in 2003, shoot elongation slowed dramatically in mid-July, resulting in ETA declining to 38 to 45 L·day−1 until mid-August. Thereafter leaf loss due to hurricanes (beginning Day 226) accelerated declines in ETA until leaf senescence was completed in mid-December (Day 350). The 0 L ETA on Day 270 occurred during the peak of Hurricane Jeanne. Despite fall storms, trees increased in height from 5.0 to 6.8 m and width by 0.8 m. Cumulative mean ETA for 2004 was 8376 L.
Year 5 (2005) started with ETA around 7 L·day−1 before flowering which occurred in mid-February (Day 45, Fig. 1E). As mentioned previously, flowering increased ETA, but only by 4 to 5 L·day−1, versus the doubling in previous years that may have been due to defoliation in the previous fall. With onset of shoot elongation (Day 85), transpiration increased from around 12 to 57 L·day−1 over a 14 day period. From first of June (Day 150) until mid-August (Day 225) ETA ranged from 75 to 102 L·day−1. Again slowing of shoot elongation reduced ETA for remainder of fall as number of expanding leaves slowed. This slowdown in shoot growth occurred a month later than previous years, perhaps in response to stress imposed in 2004. Maples were harvested for leaf area and aerial wood biomass before leaf senescence, starting the first week of November and completed by the third week. The last year trees averaged 1.2 m of height growth and 1.1 m in average canopy spread. Mean tree diameter was 17.6 cm measured 0.15 m above substrate level. Mean cumulative ETA for 2006 was 15,045 L per tree. Cumulative ETA measured over 4.75 yrs of production from 0.35 m tall cuttings to 8 m tall tree averaged 29,107 L per tree.
3.3. Tree ETA during Leaf Change
Since maples are deciduous, changes in ETA during spring bud push and declines in the fall with leaf senescence were not modeled. Yet container grown A. rubrum trees continuously lost water during the early winter to spring bud break periods in the warm climate (Figure 2). To showcase differences in ETA of trees in leaf, compared to barren trees, the estimated N-ETA was plotted for each winter to spring period. Leaf senescence generally occurred around the second week of December and was completed first week of January. At complete leaf senescence (Day 14), N-ETA was generally 17% of that predicted from the 12 inch TCSA algorithm each winter to spring period, though a small amount, without sufficient rainfall or irrigation, trees in containers, or recently transplanted into landscapes, could die during winter months in warm climates.
Trees began flowering in mid-February in 2003 (Day 44, Figure 2(b)), the third year of production. Flowering occurred regularly thereafter each February. Flowering increased ETA about 50% above that of dormant trees and generally persisted until near bud break of new shoots. Bud break that initiated shoot and leaf growth was consistent


Figure 2. Comparison of Normalized ETA (ETA/inch ETo; solid symbols) and Normalized ETA predicted from the 12 inch TCSA WNI model (wide continuous line) for red maple during the period of leaf change (1 Nov. to late spring the following year). Each point is based on the mean of 3 tree replicates.
in its occurrence each year, occurring within a day or two of March 6 (Day 65, Figure 2), likely because trees were clones. Increases in ETA with bud break were extremely rapid and matched the predicted ETA within a week of bud break. Thereafter, increases in ETA were nearly vertical for the next month or two (see Figure 1). Percentages of predicted N-ETA that occurred near bud break varied from year to year and were likely influenced by hurricane damage. Percentages ranged from 20% to 25% for 2002 and 2005, to 44% to 52% in 2003 and 2004, respectively (Figure 2).
3.4. Modeling Daily ETA
Linear regression of N-ETA as functions of the four tree area variables, at the three heights along the tree trunk and the projected horizontal area of the tree crown, was highly linear (r2 > 0.91 all four relationships; P < 0.001; Figure 3). Thus, slopes for each variable can be used as coefficients to predict previous day’s N-ETA on a daily basis; allowing irrigation based on a previous day’s ETo or cumulative days of ETo. The relationship between N-ETA and PCA was slightly closer and more linear over years than with the three measures of trunk cross sectional (Figure 3(a)). The slope coefficient of the N-ETA and PCA relationship is equivalent to the Plant Factor defined in the recent national standard [18] , Water Needs Index as defined by Beeson [27] and crop coefficient as defined for trees in agriculture such as fruit productions [28] , such that the product of this coefficient and ETo estimates the volume of ETA:
Eq. 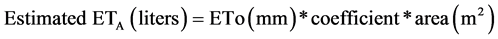 (1)
(1)
The slope coefficient from Figure 1(a), 0.63, is reasonably similar to the 0.7 Plant Factor recommended for humid climates as defined in the national standard for estimating landscape plant water demand [18] . The fit between N-ETA and the three measures of trunk cross sectional area were also high (Figure 3(b)-3(d)), but variation within a year was much greater than for the relationship with PCA.


Figure 3. Relationships of Normalized ETA over five years to four measures of horizontal surface areas of Acer rubrum used to estimate daily ETA: horizontal projected area of tree crowns (a) and surface areas were calculated from trunk circumferences at 0.15 m (b), 0.30 m (c) and 1.2 m (c; first major branch) above soil level.
3.5. Leaf Area-Biomass-Water Use Relationships
Red maple maintained a constant relationship between total leaf area and water supply structures. Final total leaf area was approximately 100 m2 that translated to a leaf area index (LAI) between 4 and 5 (Table 1). The yearly increase in total leaf area was linear with TCSA measured at 30 cm, nearly 500 cm2 leaf area per·cm2 trunk area (r2 = 0.98; Table 2, column 3). Data for 2004 was not included due to leaf area loss from three hurricanes during the late growing season. Similarly, the total leaf area relationship was directly proportional to trunk biomass, (113 cm2·g−1; data not shown), indicating that diffuse porous maple that conducts water over the entire cross sectional sapwood xylem area is transporting water to new leaves. Progressively greater leaf area and sapwood over the five-year study period translated to greater yearly increase in ETA per unit depth of reference evapotranspiration, or 1.12 liters per cm ETo by year (r2 = 0.94; Table 2, column 3). The ability of sapwood to supply water to yearly increases in total transpiring leaf area resulted in whole tree water use efficiency of a yearly average 700 liters of water per kg aboveground biomass (leaves and wood; Table 1). In other words, over the final study growing season (2005), red maple used 7 kg water per kg biomass produced.
Table 1. Final (2005) total leaf area, LAI, leaf area index (final total leaf area ÷ horizontal projected canopy area), above-ground dry biomass, total seasonal water use (ETA), and water use efficiency (liters water per kg biomass) of three individual Acer rubrum “Florida Flame” (red maple) trees 4.75 years after from transplanting from rooted cuttings.
Table 2. Calculated leaf area per unit by trunk cross sectional area (TCSA) for Acer rubrum measured at 0.30 m above the soil line near the end of the growing season. Mean daily Normalized ETA by calculated ETo during periods of trees in leaf, (N = 3). Leaf area was unavailable for 2004 due to leaves stripped from trees by winds of the 3 hurricanes in the fall.
zStandard deviation.
4. Discussion
Tree growth during an 8.5 month growing season results in about 1.5 m of height growth each year. Long growing seasons in Central Florida likely compensated for shorter days (14 hr maximum) and warmer nights compared to northern latitudes. ETA increased rapidly each spring with bud break, obtaining a maximum of 112 L per day in late June the fifth year. After the first year, shoot elongation and leaf production slowed dramatically each July, but often did not terminate until September. Decreases in leaf production due to shoot growth termination and maturation of new leaves often resulted in rapid declines in daily ETA, even though ETo remained fairly consistent into September (data not shown). Stomata of mature leaves are more sensitive to conditions that moderate aperture than young expanding leaves [29] . During winter months, daily water loss from barren trees with covered root balls was measurable up to 7 L for 6.8 m tall trees, and doubled to 14 L when flowers bloomed. Most of this winter water loss was likely evaporation from the root ball, since root ball coverings were not sealed.
ETA values reported here tend to be higher than those previously reported for Acer species in situ. Pausch et al. [30] reported daily ETA rates of 61 and 72 L·day−1 for A. saccharum in a forest near Ithaca NY for 24 cm DBH trees. Maples here were 11 to 13 cm DBH when maximum ETA’s were measured. Similar size understory A. rubrum in Tennessee had maximum ETA rates of only 8.6 L·day−1 [31] . Compared to other deciduous species, Populus “Flevo” and Salix matsudana had similar ETA, but with one third the leaf area and nearly half the height [20] . High ETA reported here is likely due to effects of several factors: effective isolated canopies, abundant irrigation, and generally low vapor pressure deficits (VPD) while in leaf. Previously Beeson [17] reported no effect of canopy closure on woody plant ETA below 67% densities. Border trees were spaced around each lysimeter tree so there was no overlap of canopies with lysimeter trees, thus measured trees were ventilated through data collection but still mimicked local tree farm densities. During most of a growing season, VPD in Central Florida is generally only above 2.5 kPa in April and November (Beeson, data not shown [32] ). Such low VPD are below reported thresholds for stomata closure for most North American temperate tree species [33] [34] .
Algorithms for predicting ETA grossly over-predicted irrigation requirements during leaf senescence and under-predicted during spring leaf flush. Although same trees were observed for nearly 5 years, durations that N-ETA was below predicted lines varied each year. In both 2001-2002 and 2004-2005 winters, predicted ETA was much higher than measured ETA. Trees in both these winters were defoliated the preceding fall; the first year by foliar disease (Figure 2(a)), and the fourth year by hurricane winds (Figure 2(d)). For two winters without unusual events, 2002-2003 and 2003-2004, models predicted N-ETA until near leaf senescence in mid-December. For all springs, models missed rapid increases in ETA after leaf bud break by about 30 days. During shoot bud burst, water loss by transpiration increased daily. To obtain maximum spring growth of red maple, irrigation must be increased rapidly and proportional to leaf flush until development of new leaves slows. Thereafter modelled ETA predicted actual ETA sufficiently for irrigation scheduling.
The consistent ratio of total leaf area to both TCSA and trunk dry mass across trees and years leaf area of studied lysimeter trees was similar to that found in other studies such as for young mountain ash (Sorbus spp.; [21] and Eucalyptus sp. [35] . Increases in ETA in maple was due to increases in sapwood TCSA in young trees [15] [21] , and continued conductivity of previous year’s xylem [36] . Maple xylem is diffuse porous, thus water conductive can remain fully functional for up to 100 years [36] . Final leaf area index of approximately 5 was relatively high compared to another study of isolated urban tree LAI [37] , but maybe be due to the larger, more mature trees here. Another factor may be canopy configuration, as by year 5 the maple crowns were more conical, resulting in more leaf area per PCA, and also possibly explaining the relatively higher ETA per unit PCA in year 5.
Water use efficiency (WUE), quantity of water required to produce a quantity of dry mass, averaged 709 L per kg of above ground wood mass was somewhat more prolific compared to other studies, such a 6.3 kg·kg−1 water for a short rotation Salix viminalis stands [38] and 4.8 g·kg−1 for spruce [37] ; both in Sweden. Water use reported here was far less efficient than for tropical tree species in the Republic of Panama that were reported as 2.52 to 4.35 kg·kg−1 water [22] , although these other studies included root dry mass that was not measured for this research. Had root mass been included red maple WUE would have shown more efficient water use per kg biomass.
5. Conclusion
Daily ETA of A. rubrum can be estimated with high precision based on current methods of calculating ETo and using the appropriate coefficients for a given measure of tree capacity to move and transpire water as given in Figure 3. The three measures using TCSA to estimate water demand (ETA) are suited to nursery production where trunk diameter (caliper) is a routine measure for marketing classification, but can be used for isolated landscape trees with due consideration. Extrapolations beyond red maple tree sizes measured here are possible and would be the most accurate if based on trunk cross sectional area below the first major limb on larger trees for ring porous trees where the trunk is likely to be conducting sapwood. Projected canopy area (PCA) of urban trees would also be suited to estimating water demand (ETA) that would be independent of trunk and conducting sapwood areas, as well as easy to measure for isolated trees. The coefficient (slope) for either PCA or TCSA that corrects calculated ETo to red maple water use is dimensionless, but to estimate in volume units (either liters or gallons) would require both ETo and PCA/TCSA to be in the same class of units, metric or English.
Acknowledgements
Funding for this project was provided by the Southwest Florida Water Management District and the Horticultural Research Institute.
Cite this paper
Beeson Jr., R.C. (2016) Evapotranspiration and Above Ground Biomass of Acer rubrum from Liners to 8 m Tall Trees. American Journal of Plant Sci- ences, 7, 2440-2456. http://dx.doi.org/10.4236/ajps.2016.717213
References
- 1. Dwyer, J.F., McPherson, E.G., Schroeder, H.W. and Rowntree, R.A. (1992) Assessing the Benefits and Cost of the Urban Forest. Journal of Arboriculture, 18, 227-234.
- 2. McPherson, E.G., Simpson, J.R., Peper, P.J. and Xiao, Q. (1999) Benefit-Cost Analysis of Modesto’s Municipal Urban Forest. Journal of Arboriculture, 25, 235-248.
- 3. Nowak, D.J., Hoehn III, R.E., Crane, D.E., Stevens, J.C. and Walton, J.T. (2006) Assessing Urban Forest Effects and Values, Washington DC’s Urban Forest. Resource Bull. NRS-1. U.S. Department of Agriculture, Forest Service, Northern Research Station, Newtown, Square, PA, 24 p.
- 4. Sanders, R.A. (1984) Urban Vegetation Impacts in the Urban Hydrology of Dayton Ohio. Urban Ecology, 9, 361-376.
https://doi.org/10.1016/0304-4009(86)90009-4 - 5. Hardy, J., Behe, B.K., Barton, S.S., Page, T.J., Schutzki, R.E., Muzii, K., Fernandez, R.T., Haque, M.T., Booker, J., Hall, C.R., Hinson, R., Knight, P., McNeil, R., Rowe, D.B. and Safley, C. (2000) Consumers’ Preferences for Plant Size, Type of Plant Material and Design Sophistication in Residential Landscaping. The Journal of Environmental Horticulture, 18, 224-230.
- 6. Stigarll, A. and Elam, E. (2009) Impact of Improved Landscape Quality and Tree Cover on the Price of Single-Family Homes. The Journal of Environmental Horticulture, 27, 24-30.
- 7. Beeson Jr., R.C. (1992) Restricting Overhead Irrigation to Dawn Limits Growth in Container-Grown Woody Ornamentals through Small Increases in Diurnal Water Stress. HortScience, 27, 996-999.
- 8. Beeson Jr., R.C. (1992) Shoot and Root Responses of Eighteen Southeastern Woody Landscape Species Grown in Cupric Hydroxide-Treated Containers. The Journal of Environmental Horticulture, 10, 214-217.
- 9. Beeson Jr., R.C. and Keller, K. (2003) Effect of Cyclic Irrigation on Growth of Magnolias Produced Using Five In-Ground Systems. The Journal of Environmental Horticulture, 21, 148-152.
- 10. Gilman, E.F., Black, R.J. and Dehgan, B. (1998) Irrigation Volume and Frequency and Tree Size Affect Establishment Rate. Journal of Arboriculture, 24, 1-9.
- 11. Lindsey, P. and Bassuk, N. (1991) Specifying Soil Volumes to Meet the Water Needs of Mature Urban Street Trees and Trees in Containers. Journal of Arboriculture, 17, 141-149.
- 12. Wullschleger, S.D., Meinzer, F.C. and Vertessy, R.A. (1998) A Review of Whole-Plant Water Use Studies in Trees. Tree Physiology, 18, 499-512.
https://doi.org/10.1093/treephys/18.8-9.499 - 13. Ringgaard, R., Herbst, M. and Friborg, T. (2012) Partitioning of Forest Evapotranspiration, the Impact of Edge Effects and Canopy Structure. Agricultural and Forest Meteorologyis, 166, 86-97.
https://doi.org/10.1016/j.agrformet.2012.07.001 - 14. Rose, C.W. (1984) Modeling Evapotranspiration: An Approach to Heterogeneous Communities. Agricultural Water Management, 8, 203-221.
https://doi.org/10.1016/0378-3774(84)90054-4 - 15. Breda, N., Granier, A. and Aussenac, G. (1995) Effects of Thinning on Soil and Tree Water Relations, Transpiration and Growth in an Oak Forest (Quercus petraea (Matt.) Liebl.). Tree Physiology, 15, 295-306.
https://doi.org/10.1093/treephys/15.5.295 - 16. Teklehaimanot, Z., Jarvis, P.G. and Ledger, D.C. (1991) Rainfall Interception and Boundary Layer Conductance in Relation to Tree Spacing. Journal of Hydrology, 123, 261-278.
https://doi.org/10.1016/0022-1694(91)90094-X - 17. Beeson Jr., R.C. (2010) Response of Evapotranspiration of Viburnum odoratissimum to Canopy Closure and the Implications for Water Conservation during Production and in Landscapes. HortScience, 45, 359-364.
- 18. ASABE Standards (2015) S623: Determining Landscape Plant Water Demands. ASABE, St. Joseph.
- 19. Ruiter, J.H. (1987) Growth, Crop Conductance and Prediction of Stem Volume Increment of Irrigated and Non-Irrigated Young Radiata Pine in Non-Weighing Lysimeters. Forest Ecology and Management, 20, 79-96.
https://doi.org/10.1016/0378-1127(87)90151-4 - 20. Edwards, W.R.N. (1986) Precision Weighing Lysimetry for Trees, Using a Simplified Tared-Balance Design. Tree Physiology, 1, 127-144.
https://doi.org/10.1093/treephys/1.2.127 - 21. Vertessy, R.A., Benyon, R.G., O’Sullivan, S.K. and Gribben, P.R. (1995) Relationships between Stem Diameter, Sapwood Area, Leaf Area and Transpiration in Young Mountain Ash Forest. Tree Physiology, 15, 559-567.
https://doi.org/10.1093/treephys/15.9.559 - 22. Cernusak, L.A., Winter, K., Aranda, J., Turner, B.L., and Marshall, J.D. (2007) Transpiration Efficiency of a Tropical Pioneer Tree (Ficus insipida) in Relation to Soil Fertility. Journal of Experimental Botany, 58, 3549-3566.
https://doi.org/10.1093/jxb/erm201 - 23. Gaskalla, R. (1998) Grades and Standards for Nursery Plants. 2nd Edition, Florida Department of Agriculture and Consumer Services, Division of Plant Industry Tallahassee, 237 p.
- 24. Beeson Jr., R.C. (2011) Suspension Lysimeter Systems for Quantifying Water Use and Modulating Water Stress for Crops Grown in Organic Substrates. Agricultural Water Management, 98, 967-976.
https://doi.org/10.1016/j.agwat.2011.01.005 - 25. Beeson Jr., R.C. and Haydu, J. (1995) Cyclic Microirrigation in Container-Grown Landscape Plants Improves Plant Growth and Water Conservation. Journal of Environmental Horticulture, 13, 6-11.
- 26. Allen, R.G., Jensen, M.E., Wright, J.L. and Burman, R.D. (1989) Operational Estimates of Reference Evapotranspiration. Agronomy Journal, 81, 650-662.
https://doi.org/10.2134/agronj1989.00021962008100040019x - 27. Beeson Jr., R.C. (2006) Relationship of Plant Growth and Actual Evapotranspiration to Irrigation Frequency Based on Managed Allowable Deficits for Container Nursery Stock. Journal of the American Society for Horticultural Science, 131, 140-148.
- 28. Steduto, P., Hsiao, T.C., Fereres, E. and Raes, D. (2012) Crop Yield Response to Water. Irrigation and Drainage Paper 66, United Nations FAO, Rome.
- 29. Jordan, W.R., Brown, K.W. and Thomas, J.C. (1975) Leaf Age as a Determinant in Stomatal Control of Water Loss from Cotton during Water Stress. Plant Physiology, 56, 595-599.
https://doi.org/10.1104/pp.56.5.595 - 30. Pausch, R.C., Grote, E.E. and Dawson, T.E. (2000) Estimating Water Use by Sugar Maple Trees: Considerations When Using Heat-Pulse Methods in Trees with Deep Functional Sapwood. Tree Physiology, 20, 217-237.
https://doi.org/10.1093/treephys/20.4.217 - 31. Wullschleger, S.D., Hanson, P.J. and Tschaplinski, T.J. (1998) Whole-Plant Water Flux in Understory Red Maple Exposed to Altered Precipitation Regimes. Tree Physiology, 18, 71-79.
https://doi.org/10.1093/treephys/18.2.71 - 32. Beeson Jr., R.C. and Brooks, J. (2008) Modeling Actual Evapotranspiration of Acer rubrum from a Rooted Cutting to an 8m Tall Tree. Acta Horticulturae, 792, 91-97.
https://doi.org/10.17660/ActaHortic.2008.792.8 - 33. Waring, R.H. and Franklin, J.F. (1979) Evergreen Coniferous Forest of the Pacific Northwest. Science, 29, 1380-1386.
https://doi.org/10.1126/science.204.4400.1380 - 34. Whitcomb, C.E. (1978) Know It and Grow It. Oil Capital Printing Co., Tulsa, 500 p.
- 35. Dye, P.J. and Olbrich, B.W. (1993) Estimating Transpiration from 6-Year-Old Euclyptus grandis Trees: Development of a Canopy Conductance Model and Comparison with Independent Sap Flux Measurements. Plant, Cell & Environment, 16, 45-53.
https://doi.org/10.1111/j.1365-3040.1993.tb00843.x - 36. Gebauer, T., Horna, V. and Leuschner, C. (2008) Variability in Radial Sap Flux Density Patterns and Sapwood Area among Seven Co-Occurring Temperate Broad-Leaved Tree Species. Tree Physiology, 28, 1821-1830.
https://doi.org/10.1093/treephys/28.12.1821 - 37. Cienciala, E., Lindroth, A., èermák, J., Hallgren, J.-E. and Kuèera, J. (1994) The Effects of Water Availability on Transpiration, Water Potential and Growth of Picea abies during a Growing Season. Journal of Hydrology, 155, 57-71.
https://doi.org/10.1016/0022-1694(94)90158-9 - 38. Lindroth, A. and Cienciata, E. (1995) Water Use Efficiency of Short-Rotation Salix viminalis at Leaf, Tree and Stand Scales. Tree Physiology, 16, 257-262.
https://doi.org/10.1093/treephys/16.1-2.257




