American Journal of Plant Sciences
Vol.5 No.18(2014), Article
ID:48583,10
pages
DOI:10.4236/ajps.2014.518277
In Vitro Germination and Plantlet Establishment of Wild Chamomile of Morocco Cladanthus mixtus (L.) Oberpr. and Vogt
Naima Harras*, Ahmed Lamarti
Laboratoire de Biotechnologie et d’Amélioration des Plantes, Département de Biologie, Faculté des Sciences M’Hannech II, Tétouan, Maroc
Email: *harrasnaima@hotmail.fr
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 June 2014; revised 8 July 2014; accepted 31 July 2014
ABSTRACT
In vitro seeds germination and plantlets establishment of Cladanthus mixtus (L.) Oberpr. and Vogt were studied in this report. A reliable protocol was developed for in vitro seed germination, multiplication and plantlet regeneration of Cladanthus mixtus. The seeds were sterilized and cultured in different media. Among the two basal media evaluated for symbiotic seed germination, gelled distilled water was found to be the best with a high percentage of seeds germination (100%) after 33 hours of culture. Seedlings were further transferred to different types of media. Result observed after 4 weeks showed that MS medium promotes the highest growth with an average of 2.75 ± 0.12 cm shoot length and 2.60 ± 0.29 shoots per explants, and the mean number of roots achieved 3.33 ± 0.17 root per explants with a length of 2.42 ± 0.16 cm. This study showed that macroelements of MS (1962) medium is essential for in vitro shoot multiplication, growth and rooting of shoots of Cladanthus mixtus L.
Keywords:Asymbiotic Seed Germination, In Vitro Propagation, Basal Media, Cladanthus mixtus (L.)

1. Introduction
Tribe Anthémidées includes 109 genus and 1740 species [1] . The phylogenetic studies have confused the names of species Anthemideae, including genus Chamaemelum, Ormenis and Cladanthus [2] .
Genus Cladanthus is clearly related to the genera Anthemis and Chamaemelum and most species were previously included in the last genus. However, a molecular study of Chamaemelum Mill. and related genus showed that traditionally marked as Chamaemelum is paraphyletic [2] .
Cladanthus is a small genus of five species, all native to the Mediterranean region and south-west Europe [3] .
Cladanthus mixtus (L.) Oberpr. and Vogt, wild Moroccan chamomile, is an aromatic plant known as the vernacular name “Hellâla” [4] , and the newly adopted scientific, including the synonyms Anthemis mixta L., Chamomilla mixta Grenier et Godron, Chamaemelum mixtum (L.) All., Chamaemelum mixtum (L.) All. var. mixtum, Chamaemelum mixtum var. aureum (Durieu) Benedi, Chamaemelum mixtum var. glabrescens (Maire) Benedi, Ormenis aurea Durieu, Ormenis mixta (L.) Dumort. subsp. mixta, Ormenis mixta subsp. aurea (Durieu) Batt., Ormenis mixta subsp. multicaulis (Braun-Blanq. & Maire) Maire, Ormenis mixta var. glabrescens Maire [5] .
The Moroccan chamomile, or simple-leaved chamomile, is a tall spontaneous annual plant, fragrant, 1 m in height, with the peripheral ligulate flowers which are white, a golden yellow at the base and the central florets are tubular; achenes are all identical, devoid of pappus three coasts visible on the internal face, smooth outside [6] [7] .
Moroccan Chamomile widespread in Algeria, Morocco, northern and eastern part of the Mediterranean basin [7] , grows in sandy soils in northern Morocco along the Atlantic coast [8] . It is usually found in woodlands, fields and pastures sandy and stony plain and low mountains [9] .
The infusion of Moroccan chamomile leaves and flowers is used in Moroccan traditional medicine for treatment of different ailments [4] [10] .
Moroccan Chamomile is mainly used for the extraction of essential oil of camphor odor; Morocco is the leading provider in the international market; it is sought in cosmetics, medicine and especially in perfumery [11] -[14] .
In Morocco, wild Chamomile recommended as an anxiolytic and rebalancing the central nervous system, is recommended in nervous breakdowns, for shortcomings liver and stomach light and colibacillaires colitis [12] .
The essential oil of Cladanthus mixtus is active in vitro against Escherichia coli, Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus and fungi Penicillium parasiticus, Aspergillus niger and Trametes pini [15] .
Extract of Cladanthus mixtus inhibits the corrosion of steel in acidic medium. A critical concentration of extract provides the maximum protection [16] .
The plants used in the phyto-pharmaceutical preparations are obtained mainly from the natural growing areas. With the increase in the demand for the extract of these plants, the plants are being overexploited, threatening the survival of many species. Also, many medicinal plant species are disappearing at an alarming rate due to rapid agricultural and urban development, uncontrolled deforestation, and indiscriminate collection. Advanced biotechnological methods of culturing plant cells and tissues should provide new means for conserving and rapidly propagating valuable, rare, and endangered medicinal plants.
Although Cladanthus mixtus presents a significant medicinal and aromatic value, no study focuses on the micropropagation of this species; in vitro culture is the subject of a new study. So we are interested in the vegetative propagation by in vitro culture (micropropagation) of chamomile Morocco: C. mixtus L.
The aim of this paper is to test the effect of different culture media on in vitro seed germination of Cladanthus mixtus (L.), and micropropagation, multiplication and rooting of seedling germination.
2. Materials and Methods
2.1. Plant Material and Sterilization Method
Achenes of Cladanthus mixtus (L.) were provided by INRA Kenitra (Morrocco) under the name Ormenis mixta (L.) Dumort. They were collected from the Maamoura (Morrocco) in 2007.
Achenes are sorted and cleared the rest of the capitulum. They are put in transparent plastic bags and stocked in the dark at room temperature.
Sterilization was performed according to the following protocol: The seeds were submerged in 7% (v/v) calcium hypochlorite for 15 min with additional of three drops of Tween 20, then immersed in 0.1% (v/v) mercuric chloride (HgCl2) for 1 min. and finally rinsed three times (5, 10 and 15 min) with sterile distilled water to remove traces of chlorine.
2.2. Effect of Basal Media on in Vitro Seed Germination
The sterilized seeds was inoculated into sterilized culture into Petri dishes (9 mm) containing 20 ml of different basal media. Thirty seeds were incubated for each treatment and repeated three times, and the seedlings used in subsequent experiments.
The basal media screened, to identify the suitable medium for seed germination were: Ghautheret (1959) [17] (Table 1) and gelled distilled water.
All media were supplemented with 3% sucrose and gelled with 0.7% agar Bacteriological and adjusted to a pH of 5.6 - 5.8 with NaOH and HCl prior to autoclaving at 120˚C for 20 min. All the cultures were incubated at 25˚C under white fluorescent light intensity (800 lux) with a 16 hours photoperiod and 80% relative humidity.
The percentage (%) of seeds germination was visually monitored on each hour, and it was recorded in Figure 1. The seeds considered to be germinated by the emergence of radicles from the seeds [18] . Percentage of seed germination was calculated according to the following equation (adapted from [19] ):
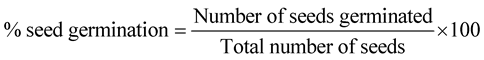
2.3. Effet of Basic Culture Media on Plantlet Development from Shoot Tips from Cladanthus mixtus (L.)
The young seedlings used of 2 - 3 mm were obtained from in vitro germination of seeds (2 leaf stage cotyledonary) of 100 seedlings. They are disposed vertically to the surface of a culture medium containing the macroelements, microelements and vitamins of Murashige and Skoog (1962), myo-inositol (meso-inositol) 100 mg/l, 3% sucrose (Table 2) and solidified with 0.7% bacteriological agar type E.
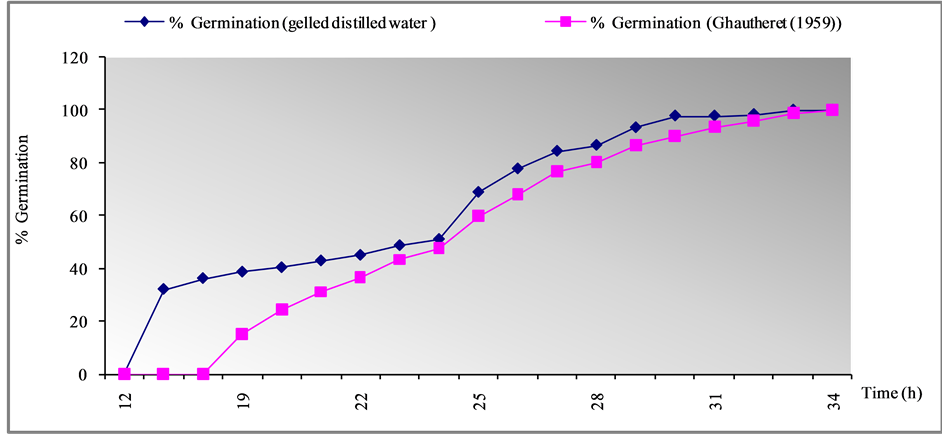
Figure 1. Effect of medium composition on the germination of seeds Cladanthus mixthus L.
Table 1. Macroelements of Gautheret (1959) used during germination of seeds of Cladanthus mixtus L.
Table 2. Vitamins and sugars MS medium (1962).
Transplanting is done every 30 days, three clones A (small size), B (medium size) and C (long size) were selected from the 9th transplant. The development of the culture medium was carried out on the clone C (long size).
To determine the most favorable to the growth of explants nutrient conditions we chose settings the salt solutions of MS: Murashige and Skoog (1962) [20] , SD: Sahah and Dalal (1978) [21] , SH: Schenk and Hildebrant (1972) [22] , MSm: Murashing and Skoog modified (Badoc, 1982) [23] , and B5: Gamborg and Eveleigh (1968) [24] , (Table 3), with microelements and vitamins of Murashige and Skoog (1962), myo-inositol (meso-inositol) 100 mg/l, agar (0.7%) and 3% sucrose, were tested on the apex of cladanthus mixtus L. (clone A).
These macronutrients are different by their nitrogen (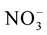 and
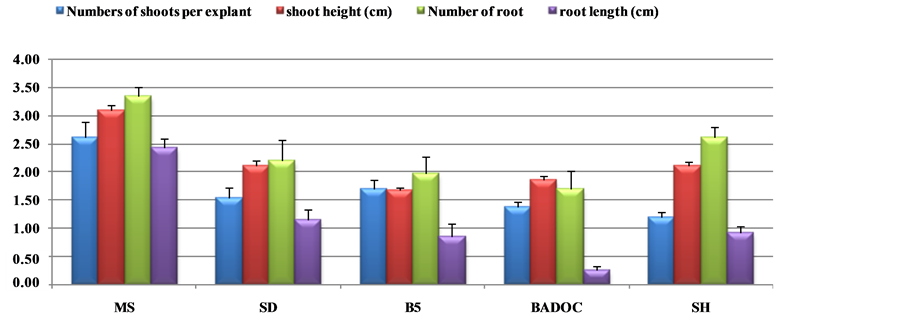
and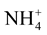 ) and potassium content (Table 4). After 30 days in culture the response was evaluated. The results presented in Table 5, and with examples in Figure 2 and Figure 3, Numbers of shoots, root as well as shoot height and root length, percentage of hypérhydrie and callogenesis were determined after 4 week of culture.
) and potassium content (Table 4). After 30 days in culture the response was evaluated. The results presented in Table 5, and with examples in Figure 2 and Figure 3, Numbers of shoots, root as well as shoot height and root length, percentage of hypérhydrie and callogenesis were determined after 4 week of culture.
2.4. Statistical Analysis
Thirty explants were used per treatment and the experiment was repeated twice. Data collected in all experiments were analyzed by SPSS version 17 and subjected to analysis of variance (ANOVA). The mean differences were tested using Duncan multiple range test (DMRT) with significant value of P < 0.05.
3. Results and Discussion
3.1. Asymbiotic Seed Germination
The disinfecting method was 100% successful in eliminating any seed contamination.
Germination tests are performed under well-defined conditions where all factors are controlled. The germination of Cladanthus mixtus was carried on distilled gelled water and Ghautheret (1959) medium. Trace output is shown in the following Figure 1:
The two basal medium which has been tried with different nutritional composition, gelled distilled water After 33 hours, 100% of the seeds germinated, as considered by emission of the first radical in gelled water.
The maximum rate of germination (100%) is reached just after 34 hours in the medium containing the Gautheret (1959) medium.
The similarity of results can be explained by the ability of achenes Cladanthus mixtus to germinate in different environments.
Seeds of C. mixtus exposed to gelled distilled water medium showed higher germination percentage than for exposure to Gautheret (1959) medium.
This varied response in seed germination in different media might be due to the media compositions as both the media contains very low amount of macro and the micro elements and vitamins compared to Gautheret (1959) and gelled distilled water.
There is no significant effect of the composition of the medium on the germination of seeds Cladanthus mixthus L. So seeds germination does not require specific nutrient solutions to germinate, and Gautheret (1959) positively influenced seed germination and subsequent seedlings development (Figure 1).
Table 3. Macroelements composition (mg/l and mM) of different media used during cultivation of shoot of Cladanthus mixtus (L.)
Table 4. Ionic composition (mEq/l) of five macro-solutions tested.
Table 5. Effects of five standard macrosalt formulations on shoot growth, rooting, and percentage of hypérhydrie and callogenesis of the wild chamomile Morocco: Cladanthus mixtus L. after 30 days. Numbers of shoots, root as well as shoot height and root length, percentage of hyperhydrie and callogenesis.
Means followed by the same letter along rows are not significantly different by Duncan’s test (P ≤ 0.05). MS: Murashige and Skoog (1962); SD: Shah and Dalal (1980); B5: Gamborg and Eveleigh (1968); MSm: Murashige and Skoog modified (Badoc, 1982); SH: Schenk and Hildebran (1972).
 (a)
(a)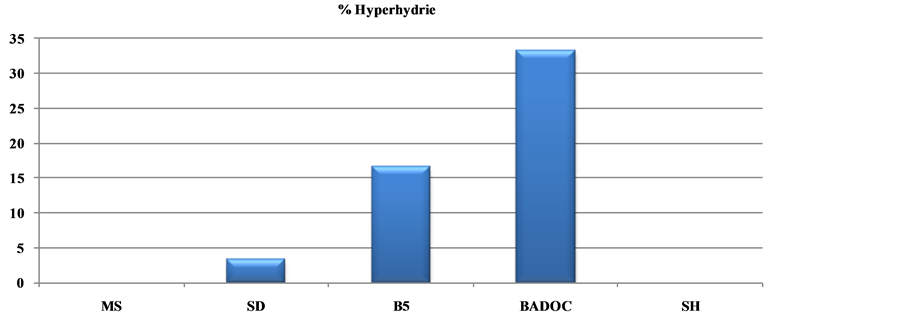 (b)
(b)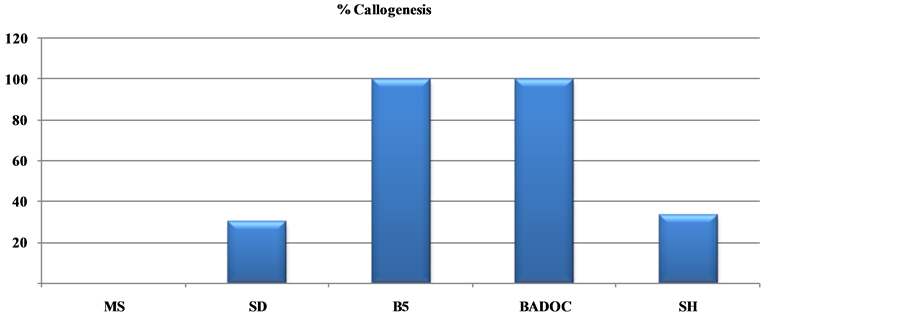 (c)
(c)
Figure 2. Influence of five macroelements on development, growth and rooting (a), the hyperhydrie (b) and the callus formation (c) of shoot (n = 30) collected explants of long clone (A) cladanthus mixtus L., after 4 weeks of culture.
3.2. Effet of Culture Media on Plantlet Development from Shoot Tips
We tested the effect of the mineral solution on explants of Morocco wild chamomile; five standard macrosalt formulations MS (1962), SD (1978), SH (1972), Badoc (1982) and B5 (1968) were tested for their effect on shoot multiplication; the results are shown in Table 5, Figure 2 and Figure 3.
Statistically significant differences (P ≤ 0.05) were observed in shoot multiplication from explants cultured in five multiplication media containing different macroelements (Table 5).
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e)
Figure 3. Plantlet of long clone (a) cladanthus mixtus L. developed on five macroelements media observed after 4 weeks of culture, (a) MS medium; (b) SH medium; (c) B5 medium; (d) SD medium; (e) BADOC medium.
Significant differences on shoot number between media were obtained in five multiplication media, with the highest multiplication rate obtained on MS medium. However, shoot number did not significantly increase in other media, as observed in MS medium (Figure 2(a)).
On the MS medium, the mean number of shoots per explants achieved 2.60 with a length of 2.75 cm, the mean number of roots achieved 3.33 per explants with a length of 2.42 cm, and the percentage of hyperhydrie and callus is absent (Figure 2(b), Figure 3(a)).
However, a significant reduction on the mean numbers of shoots, roots as well as shoot height and root length, was observed in other media as compared to MS medium.
Among the five solutions macroelements tested (Table 5), those of B5 and MSm prove harmful to the explants; they induce a higher percentage of callus (100%) (Figure 2(c)) and the percentage of hyperhydrie is 16.33% and 33.33% respectively (Figure 3(c)).
MS, B5 and MSm media do not induce callus against in the midium SD representing 30% of callus which will reach 100% in the SH medium (Figure 3(b)).
MS and SH media do not induce hyperhydric plants against by the SD medium which causes a low percentage of hyperhydrie (3.33%), which increases to 16.33% on B5 and 33.33% on MSm medium.
So according to our results macronutrients MS (1962) is more favorable to the multiplication, growth and rooting of shoots of Cladanthus mixtus L. and is used for the following tested growth regulators.
The success of in vitro culture of plant species is influenced by several factors including the culture medium [25] [26] ; we can say that it is the concentration of nitrate that gave the difference between the five culture media. The decrease of N and K in other media and especially MSm (Badoc) results in a low shoot elongation of clone Studied.
The difference between the number and length of the shoots and roots of the clone A in five culture media can be explained by the difference between the concentrations of these minerals elements.
This difference is explained by [27] which showed that the MS (1962) medium is mainly characterized by very high nitrogen content that the third party is brought in reduced form (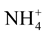 ); this chemical element is the fundamental component of the living matter.
); this chemical element is the fundamental component of the living matter.
The presence of a high concentration of potassium (K+) in the MS medium plays a role in the induction of organogenesis, especially for the neoformation of buds, stem elongation and the production of biological materials.
These same results were observed with [28] -[36] , who have successfully observed a micropropagation of Matricaria recutita and d’Anthémis nobilis L. on Murashige and Skoog (1962) medium, with a very high percentage of shoots.
Although more than 50 different devised media formulations have been used for the in vitro culture of tissues of various plant species [37] [38] , the formulation described by Murashige and Skoog (1962) (MS medium) is most commonly used, often with relatively minor changes ([39] -[46] ).
This explains the ability of this species to grow better in the MS (1962) medium as another medium.
References
- Watson, L.E., Evansb, T.M. and Boluartec, T. (2000) Molecular Phylogeny and Biogeography of Tribe Anthemideae (Asteraceae), Based on Chloroplast Gene ndhF. Molecular Phylogenetics and Evolution, 15, 59-69.
- Oberprieler, C. (2002) A Phylogenetic Analysis of Chamaemelum Mill. (Compositae: Anthemideae) and Related Genera Based upon nrDNA ITS and cpDNA trnL/trnF IGS Sequence Variation. Botanical Journal of the Linnean Society, 138, 255-273. http://dx.doi.org/10.1046/j.1095-8339.2002.00030.x
- Oberprieler, C., Himmelreich, S. and Vogt, R. (2007) A New Subtribal Classification of the Tribe Anthemideae (Compositae). Willdenowia, 37, 89-114. http://dx.doi.org/10.3372/wi.37.37104
- Bellakhdar, J. (1997) La pharmacopée marocaine traditionnelle-Médecine arabe ancienne et savoirs populairs. Editions Ibis Press, Paris, XXXXX, 208.
- Greuter, W. (2012) Compositae (pro parte majore). In: Greuter, W. and von Raab-Straube, E., Eds., Compositae. Euro + Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity.http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameId=119930&PTRefFk=7000000
- Nègre, R. (1962) Petite flores des régions arides du Maroc. Tome II, 1, 281-282.
- Pottier Alapetite, G. (1981) Flore de la Tunisie. Angiospermes-dicotylédones, Gamopétales. Programme flore et végétation tunisiennes, 655-1190.
- Quezel, P. and Santana, S. (1963) Nouvelle Flore de l’Algérie et des Régions Désertique Méridionales. Editions Centre National de la Recherche Scientifique, Paris, II, 977.
- Jahandiez, E. and Maire, R. (1934) Catalogue des plantes du Maroc. Tomes 1, 2 et 3. Lechevalier, Paris, 913 p.
- Merghoub, N., Benbacer, L., Amzazi, S., Morjani, H. and El Mzibri, M. (2009) Cytotoxic Effect of Some Moroccan Medicinal Plant Extracts on Human Cervical Cell Lines. Journal of Medicinal Plants Research, 3, 1045-1050.
- Franchomme, P., Jollois, R., Pénoël, D. and Mars, J. (1990) L’aromathérapie exactement: Encyclopédie de l’utilisation thérapeutique des huiles essentielles. Roger Jollois Editeur, 44-47.
- Buckle, J. (2003) Basic Plant Taxonomy, Chemistry, Extraction, Biosynthesis, and Analysis (Chapter 3), Clinical aromatherapy. Essential Oils in Practice, 2nd Edition, 38-75.
- Haddad, P.S., Depot, M., Settaf, A., Chabli, A. and Cherrah, Y. (2003) Comparative Study on the Medicinal Plants Most Recommended by Traditional Practitioners in Morocco and Canada. Journal of Herbs, Spices Medicinal Plants, 10, 25-45. http://dx.doi.org/10.1300/J044v10n03_04
- Benjilali, B., Zrira, S. and Aboulkassem, H. (2005) Plantes Aromatiques et Médicinales, Atouts du Secteur et Exigences Pour Une Valorisation Durable. Edition Actes, Rabat.
- Satrani, B., Ghanmi, M., Farah, A., Aafi, A., Fougrach, H., Bourkhiss, B., Bousta, D. and Talbi, M. (2007) Composition Chimique et Activité Antimicrobienne de l’Huile Essentielle de Cladanthus mixtus. Bienvenue sur le site de la Société de Pharmacie de Bordeaux, 146, 85-96.
- Abdel-Gaber, A.M., Abd-El-Nabey, B.A., Sidahmed, I.M., El-Zayady, A.M. and Saadawy, M. (2006) Inhibitive Action of Some Plant Extracts on the Corrosion of Steel in Acidic Media. Corrosion Science, 48, 2765-2779. http://dx.doi.org/10.1016/j.corsci.2005.09.017
- Gautheret, R.J. (1959) La culture des tissues végétaux. techniques et réalisations. Masson et Cie, Paris, 863 p.
- Côme, D. (1965) L’inhibition de la germination des graines de pommier Pinnus malus L. non dormantes. Rôle possible des phénols tégumentaires. Annales des Sciences Naturelles, 12, 371-478.
- Maliro, M.F.A. and Kwapata, M.B. (2000) Apomictic Embryo Development and Survival in Uapaca kirkiana under in Vitro and in Vivo Seed Germination. Scientia Horticulturae, 83, 139-147. http://dx.doi.org/10.1016/S0304-4238(99)00071-0
- Murashige, T. and Skoog, F. (1962) A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiologia Plantarum, 15, 473-497. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
- Sahah, R. and Dalal, K.C. (1980) In Vitro Multiplication of Glycyrrhiza. Current Science (India), 49, 69-71.
- Schenk, R. and Hildebrandt, A. (1972) Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Canadian Journal of Botany, 50, 199-204. http://dx.doi.org/10.1139/b72-026
- Badoc, A. (1982) Contribution à l’étude des phénomènes d’organogénèses et de callogenèse de tissue de Fenouil vulgaire (Foeniculum vulgare subsp.capillaceum var. vulgare ( mill.) thellung) cultivés in vitro; analyse des constituants de l’huiles essentielles des explants. D. E. A. Univ. Lille Univ., Lille Flandres Artois, 74 p.
- Gamborg, O.L. and Eveleigh, D.E. (1968) Culture Methods and Detection of Glucanases in Suspension Cultures of Wheat and Barley. Canadian Journal of Biochemistry, 46, 417-421. http://dx.doi.org/10.1139/o68-063
- Cozza, R., Turco, D., Bati, C.B. and Bitonti, B. (1997) Influence of Growth Medium on Mineral Composition and Leaf Histology in Micropropagated Plantlets of Olea europaea. Plant Cell, Tissue and Organ Culture, 51, 215-223. http://dx.doi.org/10.1023/A:1005966404642
- Leva, A.R., Petruccelli, R., Goretti, R. and Panicucci, M. (1992) Ruolo di alcuni microelementi e carboidrati nella proliferazione “in vitro” di cv. di olivo (Olea europaea L.). Atti Conv. ‘‘Olive oil quality’’, Firenze, 333-334.
- Margara, J. (1989) Bases de la multiplication végétative, Les méristèmes et l’organogenèse. INRA, Paris, 260.
- Fauconnier, M.L., Jaziri, M., Homés, J., Shimomura, K. and Marlier, M. (1996) Anthemis nobilis L. (Roman Chamomile): In Vitro Culture, Micropropagation and the Production of Essential Oils. Biotechnology in Agriculture and Forestry, 37, 16-37. http://dx.doi.org/10.1007/978-3-662-08618-6_2
- Rout, G.R., Samantaray, S. and Das, P. (2000) In Vitro Manipulation and Propagation of Medicinal Plants. Biotechnology Advances, 18, 91-120. http://dx.doi.org/10.1016/S0734-9750(99)00026-9
- Asai, I., Yoshihira, K., Omoto, T. and Schimomura, K. (1995) Growth and Essential Oil Production in Shoot Culture and Regenerates of Anthemis nobilis L. Plant Tissue Culture Letters, 12, 303-311. http://dx.doi.org/10.5511/plantbiotechnology1984.12.303
- Beiderbeck, R. (1982) Tow Phase Culture: One Way to Isolate Lipophilic Substances from Plant Cell Suspension Cultures. Zeitschrift für Pflanzenphysiologie, 108, 27-30. http://dx.doi.org/10.1016/S0044-328X(82)80087-1
- Máday, E., Tyihák, E. and Szöke, E. (2000) Occurrence of Formaldehyde in Intact Plants, Micropropagated Plants and Hairy Root Cultures of Chamomile (Matricaria recutita L.). Plant Growth Regulation, 30, 105-110. http://dx.doi.org/10.1023/A:1006320905207
- Omoto, T., Asai, I., Ishimaru, K. and Shimomura, K. (1998) Geranyl Isovalerate Accumulation in Adventitious Root Culture of Anthemis Nobilis. Phytochemistry, 48, 971-974. http://dx.doi.org/10.1016/S0031-9422(97)00993-X
- Reichling, J., Bisson, W. and Becker, H. (1984) Various Factors Affecting the Accumulation of Essential Oils in Intact Plants Ant in Callus Cultures of Matricaria chamomilla. Planta Medica, 50, 334-337. http://dx.doi.org/10.1055/s-2007-969724
- Sato, A., Sharon de Lima, S., Affonso, V.R., Esquilbel, M.A. and Lage, C.L.S. (2006) Micropropagation of Chamomilla recutita (L.) Rauschert: A Shock Treatment Model with Growth Regulators. Scientia Horticulturae, 109, 160-164. http://dx.doi.org/10.1016/j.scienta.2006.03.004
- Echeverrigaray, S., Fracaro, F., Andrade, L.B., Biasio, S. and Atti-Serafini, L. (2000) In Vitro Shoot Regeneration from Leaf Explants of Roman Chamomile. Plant Cell, Tissue and Organ Culture, 60, 1-4. http://dx.doi.org/10.1023/A:1006368210670
- Gamborg, O.L., Murashige, T., Thorpe, T.A. and Vasil, I.K. (1976) Plant Tissue Culture Media. In Vitro, 12, 473-478. http://dx.doi.org/10.1007/BF02796489
- Huang, L. and Murashige, T. (1977) Plant Tissue Culture Media: Major Constituents, Their Preparation and Some Applications. TCA Manual/Tissue Culture Association, 3, 539-548. http://dx.doi.org/10.1007/BF00918758
- Chand, S., Sahrawat, A.K. and Prakash, D.V.S.S.R. (1997) In Vitro Culture of Pimpinella anisum L. (Anise). Journal of Plant Biochemistry and Biotechnology, 6, 91-95. http://dx.doi.org/10.1007/BF03263017
- Jha, S. and Sen, S. (1985) Regeneration and Rapid Multiplication of Bowiea volubilis Harv. in Tissue Culture. Plant Cell Reports, 4, 12-14. http://dx.doi.org/10.1007/BF00285494
- Koblitz, H., Koblitz, D., Schmauder, H.P. and Groger, D. (1983) Studies on Tissue Cultures of the Genus Cinchona L. Alkaloid Production in Cell Suspension Cultures. Plant Cell Reports, 2, 122-125. http://dx.doi.org/10.1007/BF00269334
- Koblitz, H., Koblitz, H., Schmauder, H.P. and Groger, D. (1983) Studies on Tissue Cultures of the Genus Cinchona L. Plant Cell Reports, 2, 95-97. http://dx.doi.org/10.1007/BF00270175
- Ravishankar, G.A. and Venkataraman, L.V. (1988) Plant Cell Culture for High Value Metabolites. Science Reporter, 7, 379-384.
- Saxena, C., Rout, G.R. and Das, P. (1998) Micropropagation of Psoralea Corylifolia Linn. Journal of Medicinal and Aromatic Plant Sciences, 20, 8-15.
- Wakhlu, A.K., Nagari, S. and Barna, K.S. (1990) Somatic Embryogenesis and Plant Regeneration from Callus Cultures of Bunium persicum Boiss. Plant Cell Reports, 9, 137-138. http://dx.doi.org/10.1007/BF00232089
- Zhou, J., Ma, H., Guo, F. and Luo, X. (1994) Effect of Thidiazuron on Somatic Embryogenesis of Cayratia japonica. Plant Cell, Tissue and Organ Culture, 36, 73-79. http://dx.doi.org/10.1007/BF00048317
NOTES

*Corresponding author.


