American Journal of Plant Sciences
Vol.4 No.5A(2013), Article ID:32245,7 pages DOI:10.4236/ajps.2013.45A004
Preliminary Report of PGR’s Influence to Multiple Shoots Induction and Plant Regeneration on Plumbago auriculata
![]()
Institute of Landscape Architecture, Sichuan Agriculture University, Chengdu, China.
Email: *spgao65@yahoo.cn
Copyright © 2013 Yi Chen, Suping Gao. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 7th, 2013; revised April 15th, 2013; accepted May 1st, 2013
Keywords: Plumbago auriculata; Multiple Shoots; Induction; Plant Regeneration; PGR
ABSTRACT
With the explants of young nodals and leaves of Plumbago auriculata, we studied multiple shoots induction and plant regeneration in the medium with different PGR proportions. The results showed the nodals of Plumbago auriculata could be used as the explant to induce multiple shoots. After culturing young nodals for 30 d, optimum single shoot formation was achieved on MS medium supplemented with 0.3 mg/L 6-BA and the induction rate was 70.79%. After being transferred to the medium containing 1.0 mg/L 6-BA, 1.0 mg/L NAA and 0.2 mg/L IAA for 1 - 2 generation, single shoot was induced to form multiple shoots with the multiplication rate was 506.45%. After multiple shoots grew to 3 cm high, the multiple shoots were cut into single shoots and transferred onto half-strength MS medium supplemented with 0.5 mg/L NAA and the rooted rate was 94.33%. Finally the intact plants were obtained.
1. Introduction
Plumbago auriculata is an evergreen perennial shrub which belongs to the family of Plumbaginaceae. It is originated from South Africa and distributed in tropical and subtropical regions of the world. It is a vigorous plant with fresh green leaves and elegant flowers. Its management is simple and it is tolerant to high temperature, high humidity and diseases. It’s an excellent flowering plant which can be planted indoor and outdoor, such as bedroom, balcony, area around the building and flyover [1]. It is a rich source of an alkaloid, plumbagin (2-methyl-5- hydroxyl,4-naphthoquinone) [2], which can be used as anti-cancer drug and specially recommended for the treatment of rheumatism, piles, diarrhea, anasarca and skin diseases [3]. So Plumbago auriculata is a kind of plant with high ornamental and medicinal value.
The main propagation method of this plant is seed sowing. Due to low fruiting rate, the seed price is expensive. Plant tissue culture is widely used plant micropropagation method to produce plant seedlings and it can offer certain advantages over traditional methods of propagation. So plant tissue culture may be used to enhance breeding rate and large-scale production of Plumbago auriculata.
To date, plant regeneration via tissue culture of Plumbago auriculata was not reported. The present investigation was undertaken to establish a rapid multiplication system from explants of Plumbago auriculata.
2. Materials and Methods
2.1. Materials & Establishment of Asepsis System
1-year plants of Plumbago auriculata were selected as materials. Young nodals and leaves were used as the explant source. Wash the actively growing shoots of Plumbago auriculata with detergent “Tide” for 30 min. Clean the surface with toothbrush and rinse it with running tap water for 2 h. Then the shoots were surface-sterilized in 75.0% alcohol for 45 s and in 0.1% mercuric chloride solution for 10 min. followed by washing with sterile distilled water. The internodal segments were further cut into 1.0 cm pieces and the leaves were cut into segments of 0.4 cm × 0.4 cm to be used as explants.
2.2. Selection of Different Explants
Place the nodals and leaves explants on MS medium supplemented with 0.6 mg/L 6-BA and 0.2 mg/L 2,4-D. Record the results after 30-day culture and select the best explants as the material for single shoot induction.
No. of inducing callus = No. of explants forming callus;
Rate of inducing callus (%) = (No. of explants forming callus/No. of explants) × 100%;
No. of inducing single shoots = No. of explants forming single shoots;
Rate of inducing single shoots (%) = (No. of explants forming single shoots/No. of explants) × 100%.
2.3. Induction of Single Shoots
Place the explants on MS medium supplemented with different concentrations and combinations of PGRs. Twenty cultures were used per treatment and each experiment was repeated for three times. Record the results after 30- day culture.
No. of inducing single shoots = No. of explants forming single shoots;
Rate of inducing single shoots (%) = (No. of explants forming single shoots/No. of explants) × 100% (Tables 1 and 2).
2.4. Induction of Multiple Shoots
Cut the single shoots after they grew to 2 - 3 cm long and place them on MS medium supplemented with different concentrations and combinations of PGRs. Twenty cultures were used per treatment and each experiment was repeated for three times. Record the results after 30-day
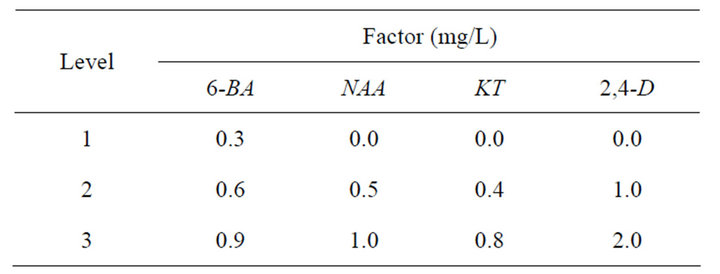
Table 1. Factor level table of single shoot induction.
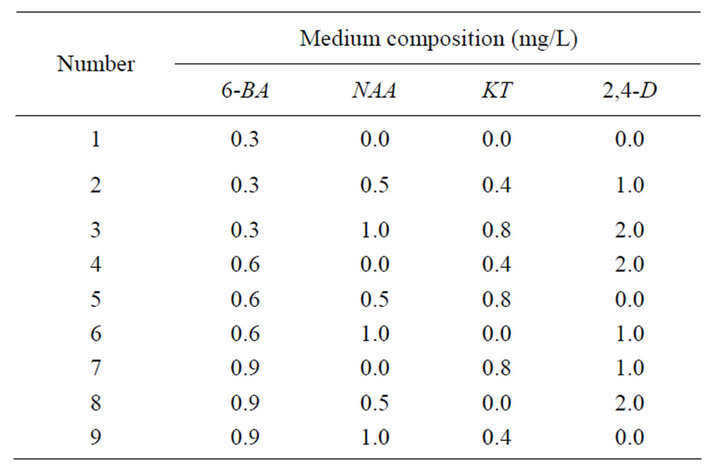
Table 2. Orthogonal table of single shoot induction.
culture.
Rate of multiplication (%) = (No. of inducing multiple shoots/No. of single shoots) × 100% (Tables 3 and 4).
2.5. Induction of Roots
For root induction, excised microshoots (3 - 4 cm) were cultured on half-strength MS basal salts supplemented with different concentrations of PGR. Twenty cultures were used per treatment and each experiment was repeated for three times. Record the result after 35-day culture.
Rates of shoot rooted (%) = (No. of shoot rooted/No. of inoculated) × 100% (Tables 5 and 6).
2.6. Culture Medium & Culture Condition
For single shoot induction, explants were cultured on MS medium supplemented with different concentrations and combinations of 6-BA, NAA, KT and 2,4-D. For multiple shoots induction, single shoots were cultured on MS medium supplemented with different concentrations and combinations of 6-BA, NAA, KT and IAA. For root induction, excised microshoots (3 - 4 cm) were cultured on half-strength MS basal salts supplemented with different concentrations of IAA and NAA. All the media contained 3% sucrose and 0.7% agar. The pH was adjusted to 5.8 with 1 N NaOH or 0.1 N HCl and media were autoclaved for 15 min at 121˚C·d 14 h/d photoperiod was provided by cool fluorescent tubes (1500 - 2000 Lux).
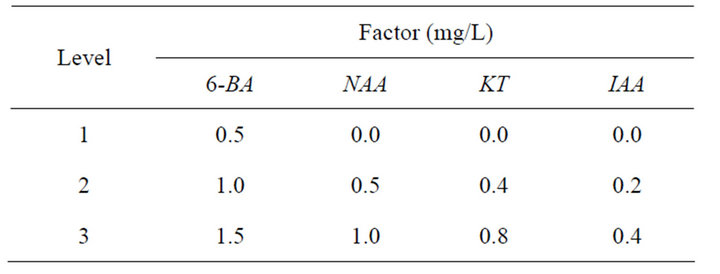
Table 3. Factor level table of multiple shoot induction.

Table 4. Orthogonal table of multiple shoot induction.
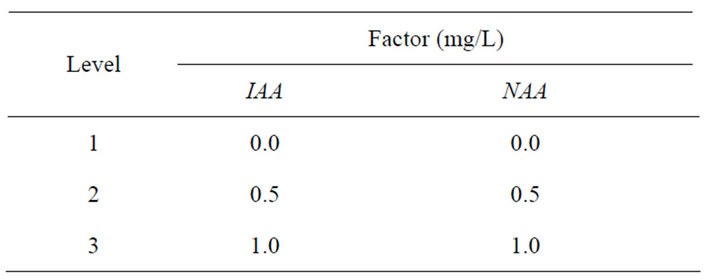
Table 5. Factor level table of root induction.
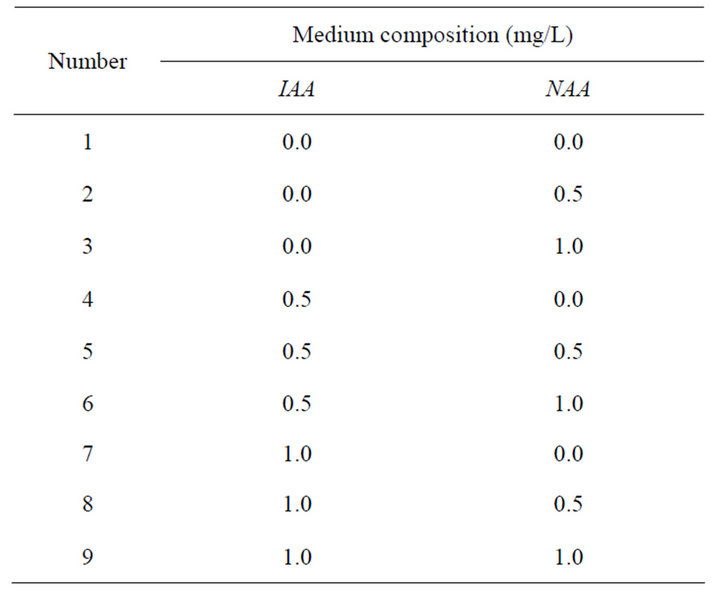
Table 6. Orthogonal table of root induction.
All the cultures were incubated at 25˚C ± 2˚C.
3. Results and Analysis
3.1. Selection of Different Explants
A amount of callus produced from the cut region of nodal explants after 7-day culture on MS medium supplemented with 0.6 mg/L 6-BA and 0.2 mg/L 2,4-D and the callus was light green or light yellow and friable (Figure 1). After 10-day culture, single shoots can be observed at the internodal region of the nodals. After 17-day culture, single shoots grew to 2 - 3 cm long with 5 - 8 young leaves (Figure 2). After 10-day culture, the leaves explants curled and a few amount of callus can been seen from the edge part and the cut region of leaves. The callus was light green and friable (Figure 3).
Results after 30-day culture (Table 7) showed that nodal explants of Plumbago auriculata could induce callus easier than leave explants and the amount of callus induced from nodal explants was more than that of leave explants. Well-developed single shoots can be induced from nodal explants and can be used as explant to induce multiple shoots. Compared with young leaves, young nodals was a better explant for tissue culture of Plumbago auriculata.
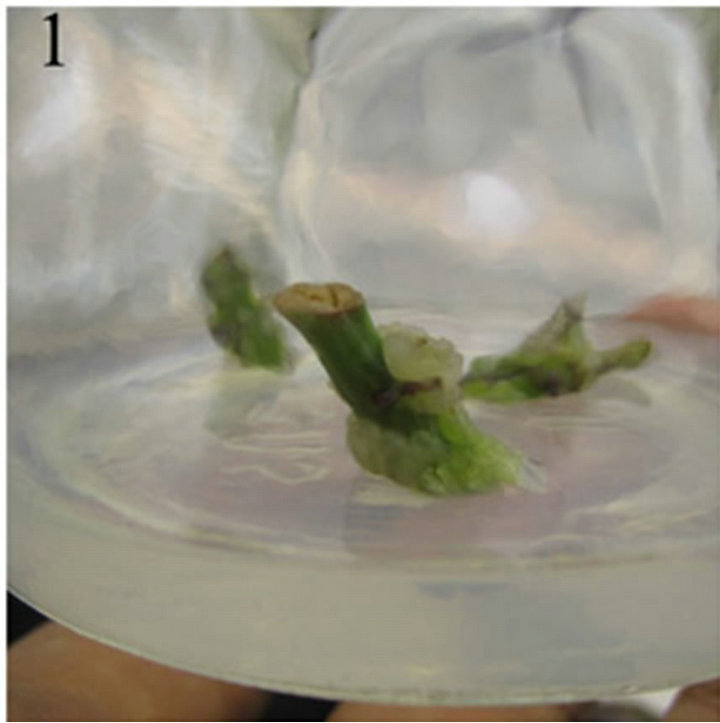
Figure 1. The callus was light green or light yellow and friable.

Figure 2. Single shoot induced from nodal showed good growing status.
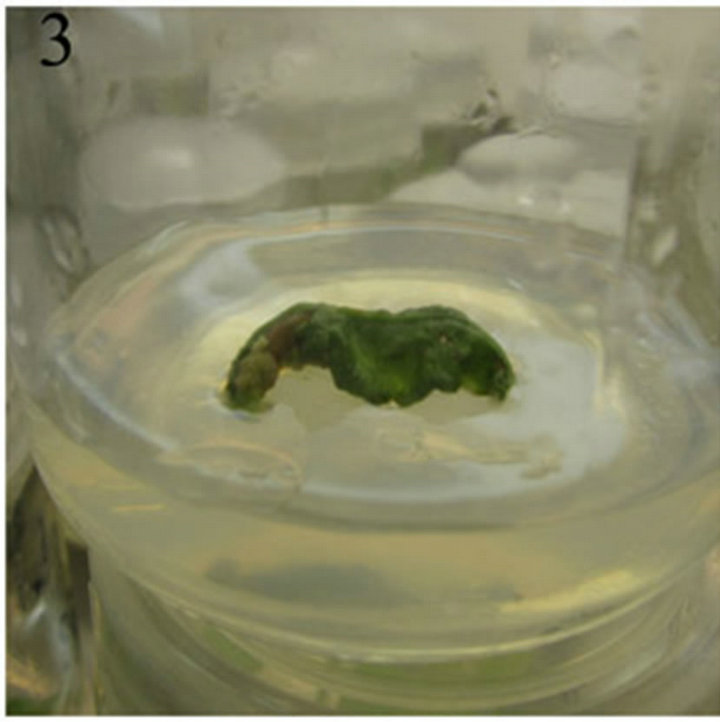
Figure 3. The callus induced from the leaves explants.
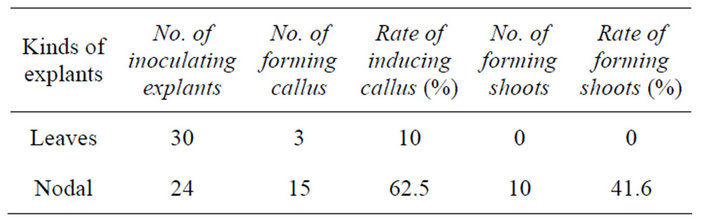
Table 7. Multiple shoots & callus induction from different explants (30 d).
3.2. Effect of Different Concentrations and Combinations of PGR on the Induction of Single Shoots
After 7-day culture, single shoots can be induced from nodal explants on the medium with 6-BA (0.3 mg/L) alone and the rate of single shoots induction was 34.68%. The growing status of single shoots was good (Figure 4). After 30-day culture (Table 8), the rate of single shoot induction was 70.79%.
The MS medium with 6-BA (0.3 - 0.6 mg/L) and NAA
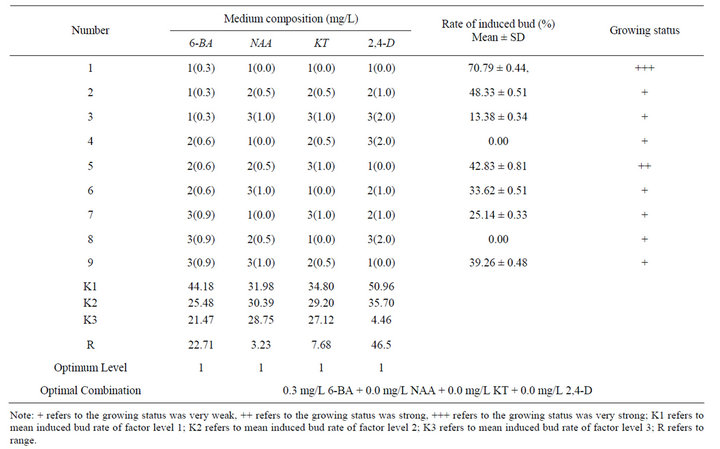
Table 8. Effect of different concentrations and combinations of PGRs on the induction of single shoots.

Figure 4. After 7-day culture, single shoots could be observed on the MS medium contained 0.3 mg/L 6-BA.
0.5 - 1.0 mg/L) and KT (0.0 - 0.8 mg/L) and 2,4-D (0.0 - 1.0 mg/L) induced single shoots after several days of culture, but the inducing rate was lower than that of medium contained 0.3 mg/L 6-BA alone. Besides, cut region of the young nodals placed on the medium containing 2.0 mg/L 2,4-D was highly callusing and the rate of single shoot induction was very low.
Results showed that the effects of different concentrations and combinations of PGR on induction of single shoots were different. Induction rate of single shoot dropped significantly with increasing concentration of 2,4-D. Mean analysis showed that the best medium for single shoot induction was MS plus 0.3 mg/L 6-BA, which was consistent with the testing result.
3.3. Effect of Different Concentrations and Combinations of PGR on the Induction of Multiple Shoots
After single shoots grew to 3 cm long, transfer them on the MS medium contained with different concentrations and combinations of PGR and record the results after 30-day culture (Table 9). The highest multiplication rate were recorded from MS + 1.0 mg/L 6-BA + 1.0 mg/L NAA + 0.2 mg/L IAA and the rate was 506.45%. Besides, multiple shoots induced in this medium were short and compact (Figures 5 and 6) and could be used as a good material for rapid propagation.
Multiplication rate in other medium composition were lower than that of MS + 1.0 mg/L 6-BA + 1.0 mg/L NAA + 0.2 mg/L IAA and the growing status of the multiple shoots induced were not so good as it.
Result recorded after 30-day culture showed that the effect of different concentrations and combinations of PGR on the induction of multiple shoots was different and range analysis showed that 6-BA played the most important role in the multiple shoot induction. Mean analysis showed that the best medium for multiple shoots induction was MS + 1.0 mg/L 6-BA + 1.0 mg/L NAA + 0.2 mg/L IAA, which was consistent with the testing result.
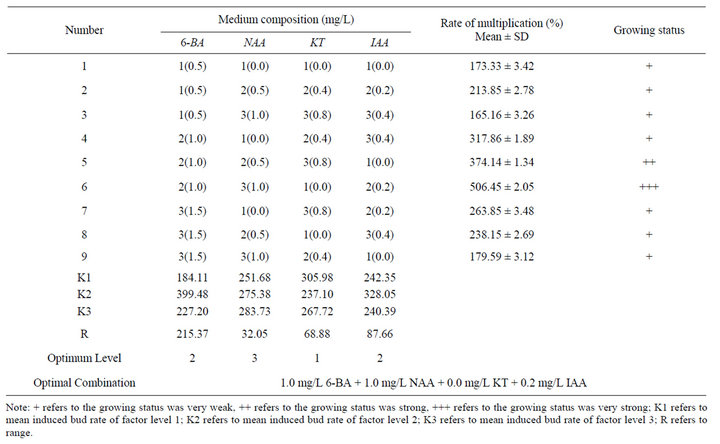
Table 9. Effect of different concentrations and combinations of PGR on the induction of multiple shoots (30 d).
Figure 5. Multiple shoots induced from single shoot.

Figure 6. Induced multiple shoots.
3.4. Effect of Different Concentrations and Combinations of PGR on the Induction of Root
Elongated multiple shoots (3 cm long) were excised from the parent culture, cut into single shoots and then placed the single shoots on half-strength MS basal salts supplemented with different concentrations of IAA and NAA. After 7-day culture, single shoots placed on medium with 0.5 mg/L NAA began to root and after 15 d, the average length of roots was about 1 cm (Figure 7). After 35-day culture, the average length of roots was about 5 cm and the growing status of the roots was very strong (Figure 8). Single shoots placed on the medium with 0.5 mg/L IAA began to root after 10-day culture. After 21-day culture, single shoots placed on the rest mediums began to root, but the growing status of the root was weak. However, optimal rooting and growth of single shoots were observed on the medium containing 0.5 mg/L NAA after 35-day culture (Table 10). The rate of root induction was 94.33% and the growing status of the root was very strong.
The percentage of shoots forming roots and the time began to root significantly varied with different concentrations of IAA and NAA. At higher concentrations of IAA (1.0 mg/L) and NAA (1.0 mg/L), rooting was inhibited but callusing occurred at the cut ends and the rate of root induction was only 33.65%.
4. Conclusion and Discussion
The induction of multiple shoots, callus and roots sig-
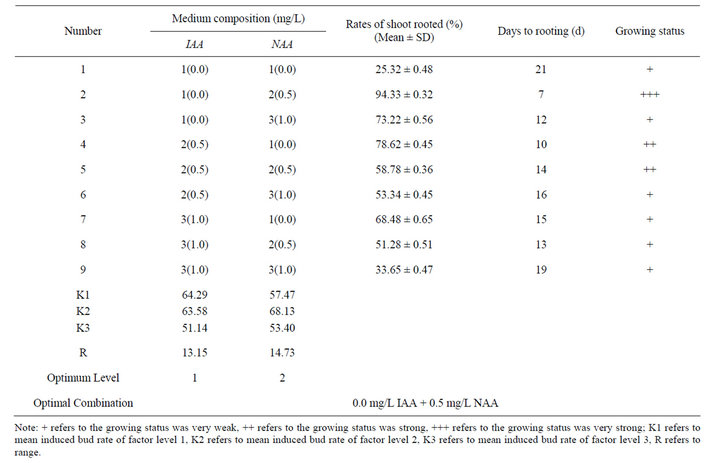
Table 10. Effect of various media on rooting from excised shoots of Plumbago auriculata cultured on 1/2 MS medium (35 d).

Figure 7. After 15-day culture, the average length of roots induced in 1/2 MS medium contained 0.5 mg/L NAA was about 1 cm.

Figure 8. After 35-day culture, the average length of roots induced in 1/2 MS medium contained 0.5 mg/L NAA was about 5 cm and the growing status of the root was very strong.
nificantly varied with the selected explants and concentrations of PGR in tissue culture of Plumbaginaceae [4-8]. Results of this experiment showed that the best explant was young nodals. Similar results were also reported by Smita Chetia [5] on shoot multiplication of Plumbago indica. Nodal contains bud primordium and much branching occurs as a result of loss of apical dominance. It is difficult to induce dedifferentiation because the differentiation grade of leaves is high and ability of regeneration is weak.
For single shoots induction, with increased amount of 2,4-D, induction rate of single shoot was decreased and growing status of single shoots got weaker and weaker. High concentration of 2,4-D induced a few of single shoots and an amount of callus for 2,4-D plays an important role in the process of callus induction and embryoid dedifferentiation. Low concentration of 2,4-D was beneficial to shoots dedifferentiation and growing, but high concentration of inhibited shoot formation [9,10]. For single shoot induction, the concentration of 2,4-D should be reduced and the best induction medium of single shoots was MS plus 0.3 mg/L 6-BA.
The highest induction number of multiple shoots and multiplication rate were obtained with the medium of MS + 1.0 mg/L 6-BA + 1.0 mg/L NAA + 0.2 mg/L IAA, The multiplication rate was 506.5%. The multiplication rate was low when low concentration of 6-BA was used. When it was used together with NAA and IAA, multiplication rate got higher. This was mainly because 6-BA was beneficial to cell division and affected organ differentiation. So the mixed components of 6-BA and auxins could promote cell division and differentiation.
The percentage of shoots forming roots and the time began to root significantly varied with the concentrations of IAA and NAA and the optimal rooting and growth of single shoots were observed on medium containing 0.5 mg/L NAA. Similar observations were reported by I. Sivanesan on shoot regeneration of Plumbago zeylanica Linn [6]. At the same concentration of auxins, root induction rate of NAA was higher than that of IAA for the activity of NAA was greater than that of IAA [11]. And root induction rate of single auxin was higher than that of various auxins. With the increasing concentration of NAA, root induction rate was raised firstly and then decreased. Similar observations were made on tissue culture of Plumbaginaceae Limonium Mill by Feng Xiaoying [12], Tang Guijun [13], Lu Linli et al. [14] and Yang Chunmei et al. [15]. But the results were different from the observations by Fan Xiaofeng [16]. So the optimal root induction medium was half MS medium containing 0.5 mg/L NAA.
REFERENCES
- H. Qin, “Plumbago Auriculata, Rookie of the Landscape,” China Flowers & Horticulture, Vol. 24, 2010, p. 24.
- I. J. Crouch, J. F. Finnie and J. van Staden, “Studies on the Isolation of Plumbagin from in Vitro and in Vivo Grown Drosera Species,” Plant Cell, Tissue and Organ Culture, Vol. 21, No. 1, 1990, pp. 79-82. doi:10.1007/BF00034496
- Q. R. Zhang, “Research on the Chemical Composition of Plumbago zeylanica,” Traditional Chinese Medicines, Vol. 30, No. 5, 2007, pp. 558-560.
- J. M. Li, “Tutorial of Plant Tissue Culture,” China Agricultural University Press, Beijing, 1996.
- S. Chetia. “High Frequency in Vitro Shoot Multiplication of Plumbago indica, a Rare Medicinal Plant,” Phytochemistry, Vol. 62, 2003, p. 619.
- I. Sivanesan, “Shoot Regeneration and Somaclonal Variation from Leaf Callus Cultures of Plumbago zeylanica Linn,” Asian Journal of Plant Sciences, Vol. 6, No. 1, 2010, pp. 83-84.
- G. R. Rout, “Rapid Clonal Propagation of Plumbago zeylanica Linn,” Plant Growth Regulation, Vol. 28, No. 1, 1999, pp. 1-4. doi:10.1023/A:1006146713143
- M. Carola, “High Frequency of Plant Regeneration in Plumbago zeylanica Linn from Cotyledons Via-Somatic Embryogenesis,” Plant Cell Reports, Vol. 16, 2006, pp. 295-298.
- Z. X. Liu and X. H. Liao, “Technology of Plant Tissue Culture,” Chemical Industry Press, Beijing, 2007.
- S. C. Chen, “Plant Tissue Culture,” Chongqing University Press, Chongqing, 2006.
- J. F. Wang, “Tissue Culture and Rapid Propagation Technology of Flowers,” China Forestry Publishing, Beijing, 2006.
- X. Y. Feng, “Rapid Propagation of L. sinuatum by Tissue Culture,” Guizhou Agricultural Sciences, Vol. 30, No. 1, 2002, pp. 9-13.
- G. J. Tang, “Technology of Rapid Propagation of L. sinuatum,” China Flowers & Horticulture, Vol. 12, 2003, p. 16.
- L. L. Lu, Z. L. Li and Q. Jiang, “Rapid Propagation of L. sinuatum by Tissue Culture,” Acta Agriculturae Jiangxi, Vol. 21, No. 6, 2009, pp. 40-42.
- C. M. Yang, L. F. Wu and Zhang, “Selection of VirusFree Seedling Medium of L. sinuatum,” Yunnan Agricultural Science and Technology, Vol. 1, 2005, pp. 778-784.
- X. F. Fan and Y. L. Yang, “Callus Induction and Plant Regeneration of Limonium aureum,” Acta Botanica Boreali-Occidentalia Sinica, Vol. 27, No. 2, 2010, pp. 0257- 0261.
NOTES
*Corresponding author.

