American Journal of Plant Sciences
Vol.3 No.10(2012), Article ID:24056,4 pages DOI:10.4236/ajps.2012.310165
Disease Development Caused by Ascochyta rabiei on Chickpea Detached-Leaves in Petri Dishes
![]()
1Department of Biology, Brawijaya University, Malang, Indonesia; 2Department of Botany, LaTrobe University, Melbourne, Australia.
Email: *harijati@ub.ac.id
Received August 26th, 2012; revised September 23rd, 2012; accepted October 1st, 2012
Keywords: Disease Severity (DS); Disease Incident (DI); Ascochyta rabiei; Chickpea; Detached-Leaves
ABSTRACT
A study using detached-leaves aimed to improve selection method. The improving method was done by scoring both disease symptom and lesion size. The research was begun by selecting agar concentration and dose of conidia that could distinguish response of very susceptible or resistant chickpea genotype. The result was used to determine disease severity (DS) and disease incident (DI) of eight genotypes that were previously tested in the field. Results of the tested agar concentration and dose of conidia showed that 1.5% and 2% agar were good to determine susceptible or resistant genotype; while 1 × 105 or 5 × 104 conidia dose was suitable for inoculation. The formula of DS (no. of leaflets in category × category value/Total no. of leaflets × 10) × 100, and DI (no. leaflets with pycnidial lesions + no. leaflets with necrotic lesions)/Total no. of leaflets × 100 successfully measured genotype response. The lesions development on detached leaves of the susceptible cultivar (Lasseter) began as circular, pale-colored areas, extending to the area covered by the drop of inoculum, then became light brown and finally dark brown. However, the response of resistant line (FLIP508) was restricted in area (and often confined to a tiny speck) surrounded by chlorosis or drying of the tissue.
1. Introduction
Screening for disease resistance in crop plants requires an efficient screening technique, which enables the researcher to distinguish field resistant and susceptible genotypes with a minimum of effort. A field trial conducted under natural conditions was the common method and was often the most effective method for screening because it entailed exposure of the plant genotypes to natural epidemics under the sort of conditions that were commonly encountered in the real cropping situation. However, field trials did not allow control over conditions such as temperature and humidity, the incidence and severity of the pathogen, or the presence of others fungi [1]. They did not allow control of the pathotype to which the plants were exposed. They were also very time consuming and expensive. Glasshouse trials allowed more control over the infection process, the time of inoculation and the environmental conditions. Methods involving inoculation of detached leaves in Petri dishes allowed an even greater degree of control of the conditions of the inoculation. For example, a detached leaf assay was used successfully to distinguish resistant and susceptible genotypes of durian (Durio zibethinus) to Phytophthora palmivora [2]. Routine work to test resistance of wheat and barley to powdery mildew was also performed by using detached leaves [3]. Detached leaves were also used effectively in a study of the resistance of wheat to Mycosphaerella graminicola [4]. Application detached leaves of rice in studies of Xanthomonas oryzae pv and oryzicola had satisfactory result [5]; detached leaves of rice were also used successfully to study the resistance of rice cultivars to Magnaporthe grisea [6].
In studies of resistance of chickpea in ascochyta blight, assessment disease incidence and severity on detached leaflets placed on water in Petri dishes was applied [7]. Disease incidence was measured as the percentage of leaflets infected, and disease severity was calculated from the estimated size of the lesions. Lesion size was scored on a 0 - 5 scale, where 0 indicated no lesion, and 1, 2, 3, 4, 5 indicated 10%, 25%, 50%, 75%, and 100% of leaflet area affected, respectively. Overall disease severity was calculated according to given formula [8]—disease severity = {no. plants in a category × category value} divided by {total no. plants × max. category value} × 100. Cultivars were considered resistant if the score for diseases severity was less than 50, and susceptible if it was in the range of 50 - 100. Similar results were obtained from a detached leaf assay and an assay using intact plants when using young leaves; yet, when using older leaves, sometimes the resistance categories were altered. The phenomenon of inconsistent results in studies of the resistance of tomato germplasm to early blight (Altenaria solani) using a detached leaf assay was showed [1].
Because of the inconsistent results obtained previously with the use of a detached leaf assay to study the resistance of chickpea to ascochyta blight [7], the present study was performed to improve detached-leaves method by prior looking for agar concentration of Water Agar media that could differentiate resistance and susceptible genotype as well as dose of conidia. This was, then, followed by the characterization of both disease symptom and lesion size by using score category for eight genotypes that were previously known their response in the field. The expected result was the detached leaf assay would give the same ranking of resistance/susceptibility as the assessment of plants subject to natural epidemics of ascochyta blight in the field and the inoculation of intact plants.
2. Materials and Methods
2.1. Detached Leaf Cultures
Leaves were cut from the plants with a short section of stem attached and dipped for 30 seconds in 70% ethanol, followed by 1 minute in 2% sodium hypochlorite, and three rinses for 1 minute each in SDW (Sterilised Distillated Water). The surface-sterilised leaves were placed in sterile Petri dishes and blotted dry with sterile cloth. The leaves were then placed on agar media in Petri dishes with the lower part of the attached stem immersed in the agar.
All agar media (Water Agar and amended agar), all glasswares, glass spreader rods, forceps, knife holders, fine sieving cloth, Mira-cloth and all pipette tips were sterilised by autoclaving at 110 kPa and 125˚C for 20 minutes. Agar media were poured into standard disposable Petri dishes (9 cm diameter, 1.3 cm deep) or rectangular dishes (15 × 15 cm, 2.5 cm deep) (100 ml per eight circular dishes or per three rectangular dishes). All agar plates were sealed using Parafilm (Pechiney Plastic Packaging, Chicago).
2.2. Development of a Method of Detached Leaf Culture
2.2.1. Agar Concentration Test
The Water Agar could provide water for the maintenance of the leaves if the petiole or attached stem section was embedded in the agar. The maintenance was also conducted by allowing the leaflets sit flat on the water or Water Agar. Different concentrations of Water Agar were tested to obtain optimum condition for the maintenance of leaflet. Also, different spore concentrations were applied in order to get a clear difference between susceptible and resistant leaves in the detached leaf culture. Four concentrations of agar in water (1%, 1.5%, 2% and 2.5% in distilled water) were tested for their ability to support disease development on the third leaf from the tip of 6-wk-old plants of the susceptible cultivar (Lasseter) and the resistant line (FLIP508). Each agar concentration had four replicates. All leaves were surface-sterilized by using 70% ethanol for 30 sec, followed by 1 min in 2% sodium hypochlorite, and three rinses for 1 min each in SDW. The percentage of leaflets infected was determined 6 days after inoculation.
2.2.2. Spore Dose Test
Conidia produced on PDA were harvested by inundating the cultures with SDW, then scraping the surface with a sterilised glass rod. Spore suspensions were filtered through four layers of Miracloth to remove agar debris and mycelium, and their concentration was determined by a haemocytometer (Neubauer) as a stock of conidia. Five doses (1 × 105; 5 × 104; 1 × 104; 1 × 103; 1 × 102 conidia·ml–1) were prepared by adjusting the concentration of the harvested stock of conidia. The third leaf from the tip of 6-wk-old plants of Lasseter and FLIP508 was detached, surface-sterilised and maintained on the selected concentration of Water Agar. Leaves of Lasseter and FLIP508 were placed side-by-side in each square Petri dish. Each leaflet was inoculated by placing a drop of spore suspension on the adaxial surface. Each treatment had four leaf replicas leaves. The percentage of leaflets infected on each leaf was determined from days 4 to 11 after the inoculation.
2.3. Disease Development on Eight Chickpea Genotypes
Seeds of Kabuli types (Bumper, Kaniva, FLIP114, FLIP90, FLIP92) and Desi types of chickpea (Lasseter, FLIP508, FLIP510) were planted in pots and grown in a glasshouse for 6 weeks (pre-flowering), then brought to the laboratory where leaf no. 5 (counting from the tip) on the main stem was harvested and surface-sterilised as described above. The leaves were placed on sterilised selected concentration of Water Agar in 90-mm Petri dishes. Each leaflet was inoculated with a 5 µl droplet of a conidia suspension of A. rabiei (the dose from the result of the previous step). There were four plant replicas of each genotype. Observations were conducted at 12 days post inoculation (dpi) using the following two methods for determining disease severity and disease incidence:
1) Disease severity (DS) on individual leaflets were determined on a scale of 0 - 10, where 0 indicated no lesions; 2, 3, 4, and 5 indicated 1 necrotic speck, 2 - 3 necrotic specks, a few necrotic specks and about 10% leaflet area affected, respectively; 6, 7, 8, 9 and 10 indicated 25%, 30% - 40%, 50%, 60% - 70% and 80% - 100% leaflet area affected with evidence of pycnidia formation in concentric zones (Figure 1).
The disease severity on a whole leaf was determined by using the following formula:

A chickpea genotype was regarded as highly susceptible, moderately susceptible, moderately resistant, or highly resistant if the disease severity value was >40, 25 - 39, 20 - 24, or 5 - 19, respectively.
2) Disease incidence (DI) (% leaflets infected) was determined from the total number of leaflets with pycnidial lesions (ignoring the size of the lesions) and necrotic lesions (with a diam, at least ~0.5 mm), according to the following formula:

A genotype was considered highly susceptible, moderately susceptible, moderately resistant, or highly resistant if the disease incidence value was >50%, 30% - 49%, 20% - 29%, or 5% - 19%, respectively.
3. Results and Discussion
Of the four concentrations of Water Agar (WA) tested, only the intermediate agar concentrations (1.5% and 2%) allowed differentiation between Lasseter (susceptible cultivar) and FLIP508 (resistant line) (Figure 2). Those concentrations apparently gave optimum range for maintenance at near 100% relative humidity. As known, spore germination and infection of leaves require a certain leaf wetness or humidity level. Didymella rabiei gave maximum disease severity on chickpea when the plants received 18 h leaf wetness after inoculation [9]. In further experiments, these concentrations were used to maintain detached leaves for testing of conidia dose (1.5% of WA) and screening resistant genotypes of chickpea (2% of WA).
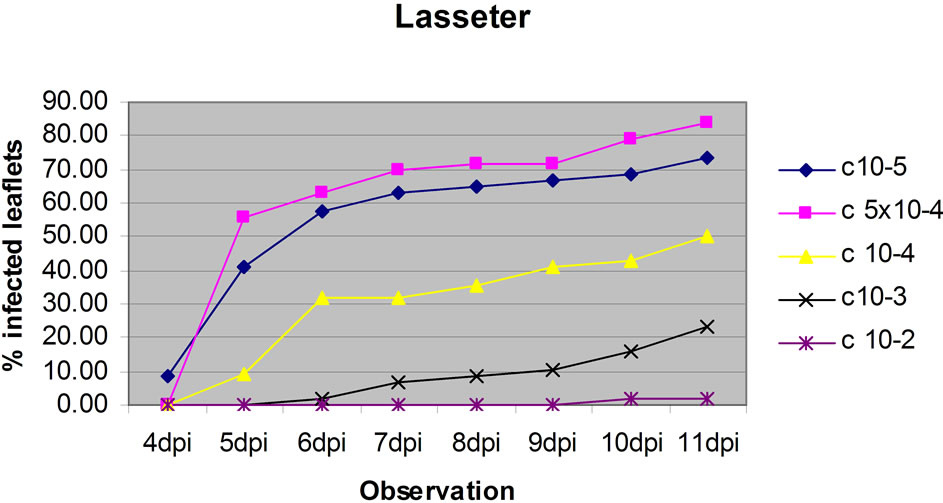
The dose test showed that conidia doses of 1 × 105 and 5 × 104 gave similar results and Lasseter clearly had a higher percentage of leaflets infected than FLIP508 at doses 1 × 105, 5 × 104 and 1 × 104 per ml (Figure 3), particularly at up to 6 dpi. At the two highest doses, relatively high percentages of leaves of FLIP508 became infected; yet, the lesions developed more slowly than on Lasseter (60% leaflets infected at 10 dpi on FLIP508, compared with 6 dpi on Lasseter). The intermediate dose (1 × 104 conidia·ml–1) best differentiated between Lasseter and FLIP508, resulting in 50% of leaflets infected in Lasseter, and 20% in FLIP508 at 11 dpi. The low inoculum doses did not cause enough disease to differentiate among the genotypes. Because the two highest doses showed similar result in resistant line (FLIP 508), one of the dose was adopted for testing the level of resistance /susceptibility of eight chickpea genotypes.
Similar to intact plants kept in the glasshouse, lesions development on detached leaves of the susceptible cultivar Lasseter began as circular, pale-coloured areas, extending to the area covered by the drop of inoculum, then became light brown (Figure 4) and finally dark brown. The lesions developed a concentric zonation of mucilaginous exudates of conidia of A. rabiei (Figure 5) which tended to dry out, followed by the blackening of pycnidia. Lesions ranged in size from less than 25% to 100% of the leaflet area (Figure 6). However, some lesions showed a dark necrotic reaction that was restricted in area (and often confined to a tiny speck) surrounded by chlorosis or drying of the tissue (Figure 7). Lesions were also commonly formed on petioles (Figure 8). From the result of
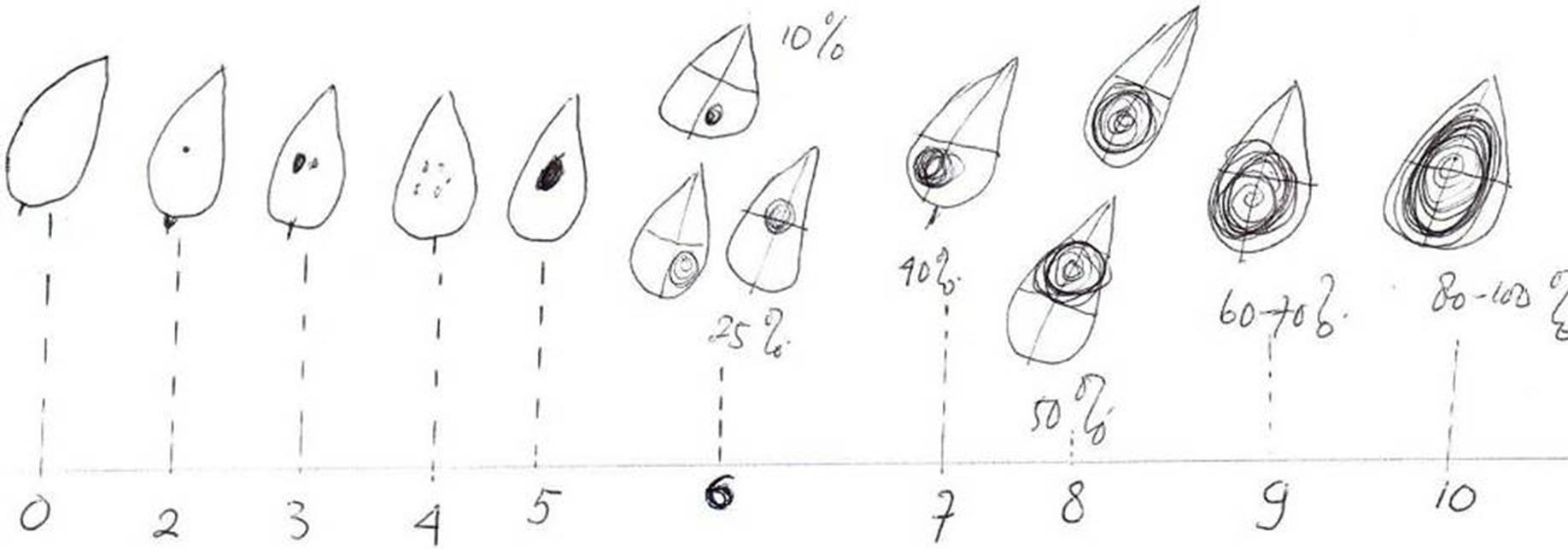
Figure 1. Diagram showing relative areas of necrotic and pycnidial lesions used to assess disease severity (DS) on a leaflet on a scale of 0 to 10.
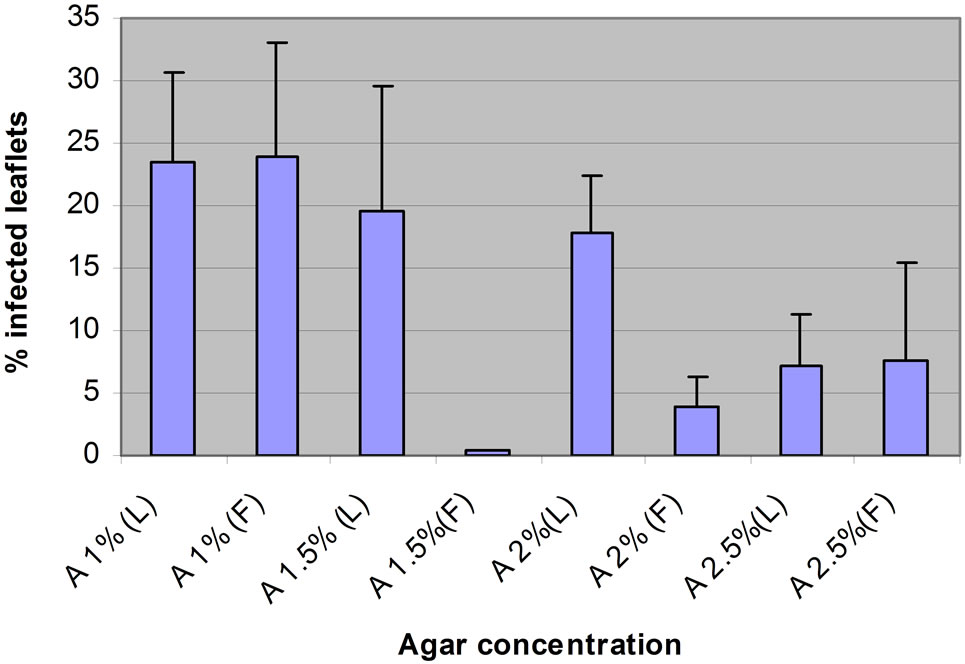
Figure 2. The percentage of leaflets infected on detached leaves of Lasseter (L) and FLIP508 (F) kept on Water Agar at various agar concentrations (1%, 1.5%, 2% or 2.5%).
 (a)
(a)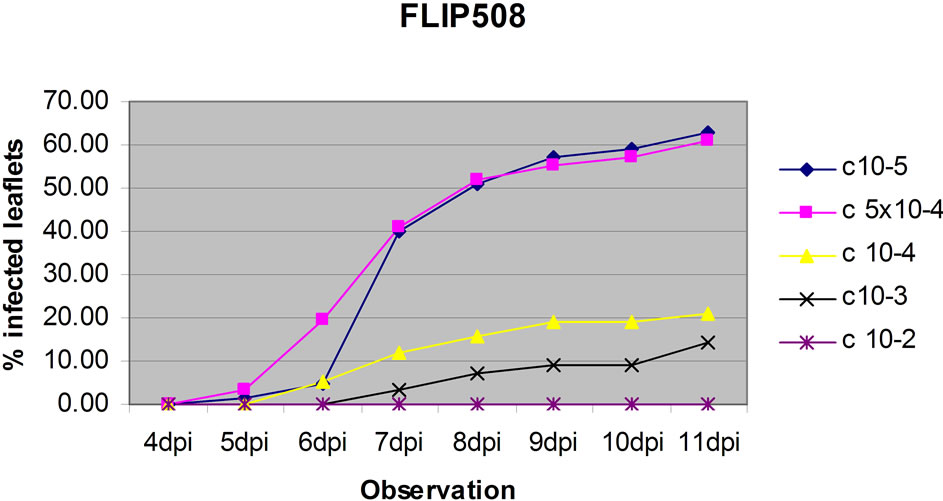 (b)
(b)
Figure 3. The effect of five concentrations (1 × 105, 5 × 104, 1 × 104, 1 × 103, 1 × 102 conidia·ml–1) of conidia of A. rabiei on percentage of leaflets infected on leaves of Lasseter (a) or FLIP508 (b) maintained on 1.5% Water Agar.
 (a)
(a) (b)
(b)
Figure 4. Initial lesions at 4 dpi (a) and at 6 dpi (b) on laminae of leaflets of Lasseter and FLIP508 kept on Water Agar in a Petri dish.
the lesion development, it could be concluded that the development of symptoms of ascochyta blight on detached leaves of chickpea maintained on Water Agar in Petri dishes was similar to that seen on intact plants in the glasshouse. The stage of exudation of brown mucilaginous masses of conidia from pycnidia was ideal for picking conidia off under the binocular microscope as a source of pure conidia of A. rabiei following inoculation of leaves in order to maintain the pathogenicity of the fungus.
However, in the Petri dishes the individual lesions on leaflets became much larger than in the glasshouse or in the field, in many cases extending to 100% of the leaflet area. This was probably because A. rabiei encountered an
 (a)
(a) (b)
(b)
Figure 5. The development of mucilaginous exudates of conidia from pycnidia of A. rabiei (a) and the formation of pycnidia in concentric rings (b) on leaves of Lasseter kept on Water Agar in Petri dishes.

Figure 6. The development of lesions (youngest on the right, oldest on the left) formed by A. rabiei on leaflets of detached leaves of Lasseter kept on Water Agar in Petri dishes, showing the formation of pycnidia, and the expansion of lesions beyond the inoculated area (roughly indicated by the diseased area on the leaflet on the far right).


Figure 7. The range of types of restricted necrotic lesions on leaflets from detached leaves inoculated with conidia suspensions of A. rabiei and kept on Water Agar in Petri dishes.
optimum environment on leaves in the Petri dishes, although it is also possible that the detached leaves expressed less resistance than the attached leaves. Prolonged exposure of plants to humid conditions is known to allow infection of genotypes that are resistant under normal conditions [10]. The restricted necrotic lesions on

Figure 8. Example of a lesion on the stem and petiole of Lasseter kept on Water Agar in a Petri dish, showing the exudation of conidia of A. rabiei.
the resistant genotypes appeared to be just as restricted on the detached leaves as on the attached leaves in the glasshouse.
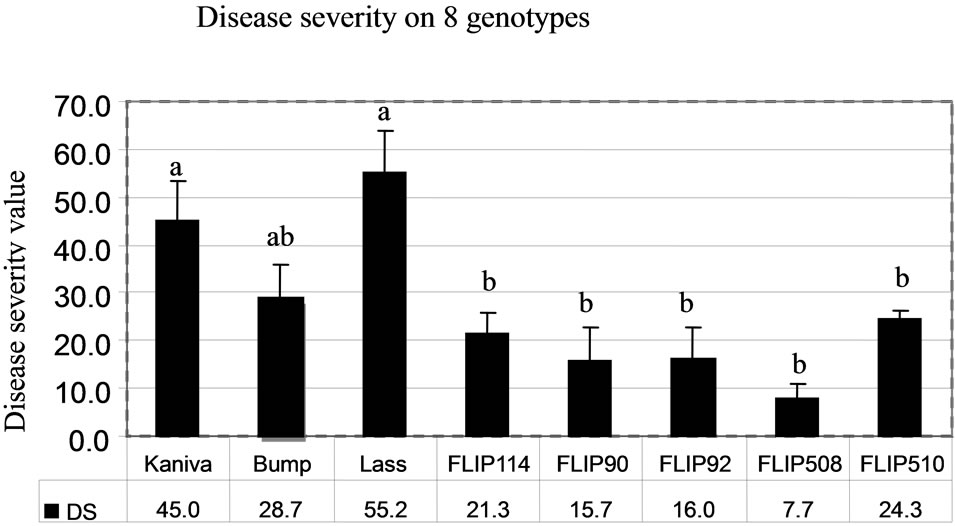
The determinations of disease severity (DS) and disease incidence (DI) gave similar results (Figures 9(a) and (b)). In the desi group, Lasseter (DS, 55.2; DI, 64.4) had the highest disease severity compared with the two other desi types (FLIP508 and FLIP510). In the kabuli group, Kaniva (DS, 45; DI, 56.5) had the highest disease severity and FLIP90 the lowest (DS, 15.7; DI, 9.1). The ranking of the chickpea genotypes from the most susceptible (Lasseter) to the most resistant (FLIP508) was the same with both calculations of disease severity.
The general ranking of susceptible (Lasseter, Kaniva and Bumper) and resistant genotypes (FLIP90, FLIP508 and FLIP510) was similar on the detached leaves in Petri dishes and on intact plants in the field (Harijati, unpublished) and glasshouse (Harijati, unpublished), except for FLIP510 which tended to be ranked similarly to Bumper (susceptible) in the Petri dishes, but was ranked the same as FLIP90 and FLIP508 (resistant) in the field and glasshouse (Table 1). In the field, Lasseter was clearly ranked as being more susceptible than Bumper and Kaniva, based especially on the fact that its stems became broken readily, resulting in death of the tops quite apart from death of tissues caused by infection by A. rabiei. In the protected environment of the glasshouse, the tendency of stems of Lasseter to break was not as evident as in the field, and there was a tendency to rank Bumper as being more susceptible than Lasseter. FLIP114 and FLIP92 were ranked as the more susceptible of the FLIP lines in both the field and the glasshouse. Based on the response of detached leaves in Petri dishes, FLIP510 was ranked as being more susceptible than was evident in the field. This is surprising, and is inconsistent with the reactions of the other resistant genotypes, FLIP90 and FLIP508.
 (a)
(a)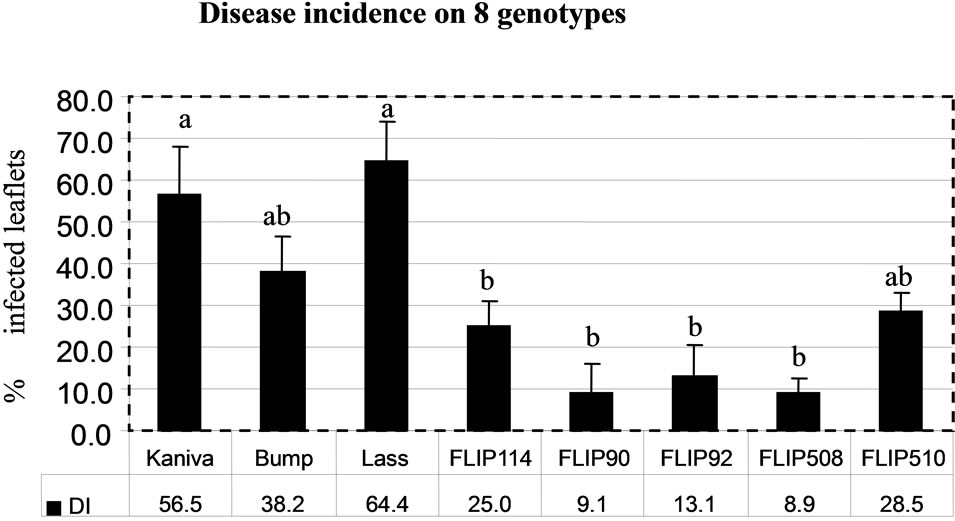 (b)
(b)
Figure 9. Mean disease severity rating (DS) (a) and disease incidence rating (DI) (b). On 8 chickpea genotypes following inoculation with a conidia suspension of A. rabiei on to detached leaves kept on Water Agar in Petri dishes. Bars indicate SE of means. Bars with the same letters are not significantly different at P < 0.05 (ANOVA).
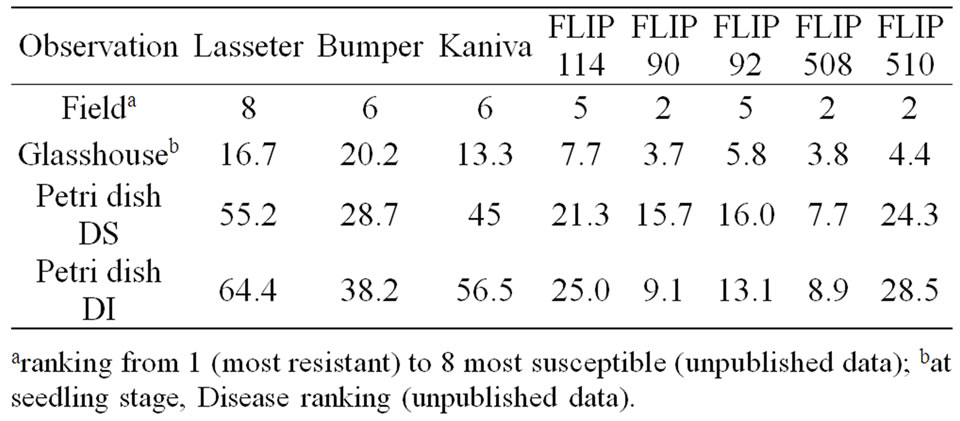
Table 1. Summary of disease ranking of ascochyta blight on a range of chickpea genotypes inoculated as detached leaves in Petri dishes, inoculated as intact plants and kept in a glasshouse (from unpublished data), and following natural infection in the field (from unpublished data).
This result indicated that the detached leaves in Petri dishes need to be treated as preliminary results in assessing disease resistance of genotypes. In the field trial, glasshouse inoculation test and on detached leaves in Petri dishes, FLIP90 and FLIP508 were consistently ranked as the most resistant genotypes. It appears that the use of detached leaves maintained in Petri dishes has potential for the preliminary study of resistance to ascochyta blight in chickpea, although there were some differences in disease expression compared with the field responses.
Determination of disease severity (DS) and disease incidence (DI) gave similar results. As the latter is easier and more straightforward to determine, it would probably be a suitable method for further studies. Whether a lesion is “pycnidial” (i.e. produces pycnidia of A. rabiei in a typical concentric zonation on the necrotic lesion) or “necrotic” (i.e. a small, dark necrotic spot without production of pycnidia) is the indication of susceptibility and resistance, respectively. Also, the method used here is an improvement on that used before [7] because whole leaves and a small piece of stem were placed on the Water Agar, rather than on separate leaflets. Placing the whole leaf with a small piece of attached stem on the Water Agar, with the stem embedded in the agar, is likely to maintain the physiological functioning of the leaflets better than when individual leaflets are cut from the leaf and floated on water [7].
4. Conclusion
The agar concentration that is suitable for culturing leaves of detached-leaves method is 1% and 2%. In those concentrations, response susceptible and resistance genotype are clear. Susceptible genotype shows more severe than resistance genotype at 1 × 105 or 5 × 104 dose of conidia. Disease symptoms and lesion size that are formulated into disease severity (DS) and disease incidence (DI) on detached-leaves method are successful to give similar ranking with resistance/susceptibility level of field trial.
REFERENCES
- M. R. Foolad, N. Ntahimpera and B. J. Christ, “Comparison of Field, Greenhouse and Detached-Leaflet Evaluation of Tomato Germ Plasm for Early Blight Resistance,” Plant Disease, Vol. 84, No. 9, 2000, pp. 967-972. doi:10.1094/PDIS.2000.84.9.967
- L. L. Vawdrey, T. M. Martin and J. Faveri, “A Detached Leaf Bioassay to Screen Cultivars for Susceptibility to Phytophthora palmivora,” Australasian Plant Pathology, Vol. 34, 2005, pp. 251-253. doi:10.1071/AP05005
- J. K. M. Brown and M. S. Wolfe, “Structure and Evolution of a Population of Erysiphe graminis f.sp. Hordei,” Plant Pathology, Vol. 39, No. 3, 1990, pp. 376-390. doi:10.1111/j.1365-3059.1990.tb02514.x
- L. S. Arraiano, P. A. Brading and J. K. M. Brown, “A Detached Seedling Leaf Technique to Study Resistance to Mycosphaerella graminicola (anamorph Septoria tritici) in Wheat,” Plant Pathology, Vol. 50, No. 3, 2001, pp. 339-346. doi:10.1046/j.1365-3059.2001.00570.x
- G. L. Xie and T. W. Mew, “A Leaf Inoculation Method for Detection of Xanthomonas oryzae pv. oryzicola from Rice Seed,” Plant Disease, Vol. 82, No. 9, 1998, pp. 1007-1011. doi:10.1094/PDIS.1998.82.9.1007
- Y. Jia, B. Valent and F. N. Lee, “Determination of Host Response to Magnaportha grisea on Detached Rice Leaves Using a Spot Inoculation Method,” Plant Disease, Vol. 87, No. 2, 2003, pp. 129-133. doi:10.1094/PDIS.2003.87.2.129
- F. S. Dolar, A. Tenuta and V. J. Higgins, “Detached Leaf Assay for Screening Chickpea for Resistance to Ascochyta Blight,” Canadian Journal of Plant Pathology, Vol. 16, No. 3, 1994, pp. 215-220. doi:10.1080/07060669409500756
- K. Xi, R. A. A. Morral, R. J. Baker and P. R. Verma, “Relationship between Incidence and Severity of Blackleg Disease of Rapeseed,” Canadian Journal of Plant Pathology, Vol. 12, No. 2, 1990, pp. 164-169. doi:10.1080/07060669009501020
- O. Jhorar, D. R. Butler and S. S. Mathauda, Effect of Leaf Wetness Duration, Relative Humidity, Light and Dark on Infection and Sporulation by Didymella rabiei on Chickpea,” Plant Pathology, Vol. 47, No. 5, 1998, pp. 586-594. doi:10.1046/j.1365-3059.1998.0280a.x
- B. C. Y. Collard, P. K. Ades, E. C. K. Pang, J. B. Brouwer and P. W. J. Taylor, “Prospecting for Sources of Resistance to Ascochyta Blight in Wild Cicer Species,” Australasian Plant Pathology, Vol. 30, 2001, pp. 271-276. doi:10.1071/AP01036
NOTES
*Corresponding author.

