American Journal of Plant Sciences
Vol.3 No.12(2012), Article ID:25780,6 pages DOI:10.4236/ajps.2012.312205
Linking Climatic Variability with Spatial Performance in Two Varieties of Quinoa Distributed in a Semi-Arid Zone
![]()
1Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Colina El Pino s/n, La Serena, Chile; 2Banco Base de Semillas-Instituto de Investigaciones Agropecuarias (INIA), Centro Regional Intihuasi, La Serena, Chile; 3Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Facultad de Ciencias del Mar, Universidad Católica del Norte, Coquimbo, Chile.
Email: *marco.molina@ceaza.cl
Received September 28th, 2012; revised October 19th, 2012; accepted November 17th, 2012
Keywords: Quinoa; Thermal Amplitude; Plasticity; Ecophysiology; Crop Yield
ABSTRACT
Different crop varieties can respond in different ways to the climatic variations at local scale. Thus, in order to maximize the yield for a determined crop, the response of different varieties submitted to local climatic conditions should be assessed. The main goal of this study was to evaluate the ecophysiological responses of two varieties of Quinoa (PRP and BO78) submitted to different conditions of thermal amplitude. We performed two experiments in both greenhouse and in 3 sites on experimental-field where were evaluated survival, photochemical efficiency, plant growth and dry biomass in both varieties and compared them with the mean of the thermal conditions recorded during the last 16 years in the Coquimbo Region, Chile. Overall, individuals of BO78 showed higher performance in the sites with lower thermal amplitude than those of PRP. By contrast, in sites with higher thermal amplitude individuals of PRP showed better survival, physiological performance and biomass and therefore higher performance. Our results suggest that while BO78 showed an ecotypic strategy, the PRP showed a plastic strategy to maintain higher performance in sites with moderate and high climatic variability. We consider that under an increase in desertification, semi-arid areas would be available for stress tolerant crops like Quinoa, but the success for the food security in these regions may depend upon the variety used.
1. Introduction
Climatic conditions play a pivotal role in the development of agricultural activities. The increase of the population makes it necessary to identify stress-tolerant plants with high nutritional qualities, in order to maintain food security [1,2]. In addition to water availability, the yields of the cultivars distributed in arid and semi-arid zones depend on chilling temperatures and diurnal temperature amplitude [3]. Thus if local characteristics such as the topography of a specific site show high spatial variability, different varieties or cultivars may respond in different ways. Therefore, only unraveling how different varieties or cultivars respond under regional or local climatic conditions will it be possible to find the best combination among varieties and sites in order to maximize performance for a given crop.
The majority of studies evaluating the success of establishment and production in crops have focused mainly on morphological traits [4-6]. Nevertheless, physiological performance is thought to be the major mechanisms allowing organisms to adapt to novel, stressful or changing environments. For instance, physiological traits that control the uptake of carbon dioxide and water use efficiency are highly plastic [7] and are key determinants of growth and crop yield [8].
One of the most adapted plant species to adverse climatic conditions is Quinoa (Chenopodium quinoa Willd). This plant is highly nutritious, with very high protein content and a lot of minerals and vitamins [9]. Accumulating simple carbohydrates and changing in their enzymes, it can grow in a variety of environmental conditions, such as very low chilling temperature and extreme diurnal temperature amplitude [10]. However both harvest factors and nutritional properties depend on environmental conditions. For example, [9] showed that temperature is one of the main determinant factors in the spatial variability of Quinoa productivity. On the other hand, in a recent paper [2] showed that different varieties of Quinoa possess high tolerance to abiotic stress, but the magnitude of the response varies with each variety.
The goal of this article is to evaluate the spatial distribution of the potential performance of two Quinoa varieties in a semi-arid zone. Specifically, we matched the survival, photochemical efficiency and biomass of both varieties submitted to thermal amplitude variation with the records of 16 years of climatic conditions for this semi-arid zone.
2. Material and Methods
This study was carried-out in 3 sites along an East-West gradient: La Serena (30˚02'S, 71˚14'W), Vicuña (30˚16'S, 70˚30'W) and Paihuano (30˚08'S, 70˚42'W) in the semi-arid Coquimbo Region of Chile, south of the hyperarid Atacama desert. It is characterized by a complex topography with altitude that varies from sea level increasing to the east, reaching heights of about 5000 min the Andes (Figure 1(a)). This region has 3 transverse valleys, Elqui, Limarí and Choapa, where agricultural activities can be developed by using the water resources coming from the mountains.
2.1. Climatic Conditions
The climatic conditions were evaluated using the Karlsruhe Atmospheric Mesoscale Model KAMM [11]. More information about the model can be found in [12]. We evaluated the mean meteorological conditions over a period of 16 years, from 1990 to 2006. The methodology is based on cluster analysis [13], which means that all days of the period are divided into groups, called clusters, which are similar in some way. The variables used to define similarity were the zonal and meridional winds, both at 500 hPa, and the temperature amplitude between 850 hPa and 500 hPa. The criteria used for clustering was the Euclidean Distance as the distance coefficient and Ward’s method as the grouping algorithm [14].
For each cluster, a representative simulation with the KAMM model was performed. The initial atmospheric profiles required by the model were evaluated by taking the mean values of the members of each cluster. The necessary data for clustering and initialization were taken from the National Center for Environmental Prediction/
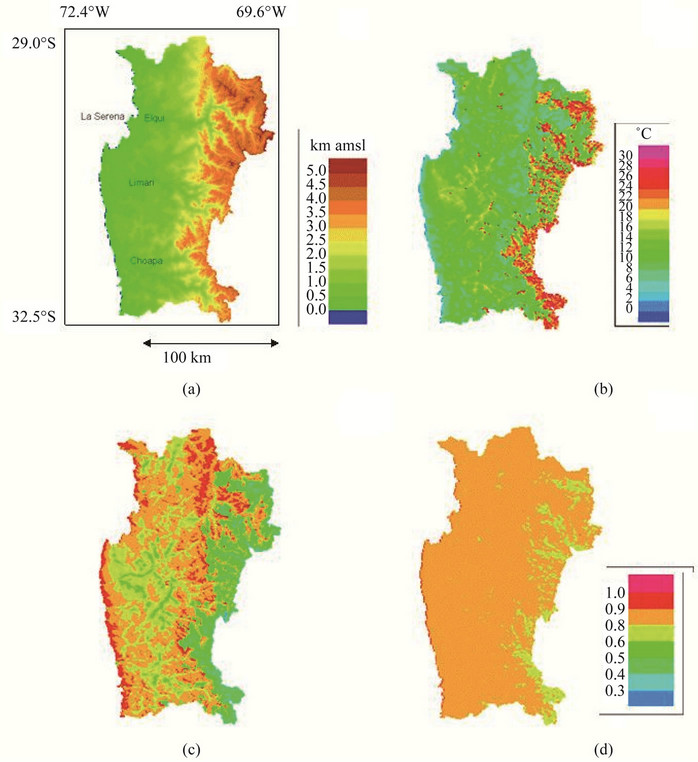
Figure 1. Topography of the Coquimbo region (a), mean diurnal temperature amplitude (b), performance of BO78 variety (c) and performance of PRP variety (d).
NCAR Reanalysis [15]. The profiles are used by the KAMM to construct the initial state of the atmosphere as start data for the simulations.
The simulations of the clusters were averaged taking into account the statistical weight of the clusters. The resulting mean diurnal cycles of the meteorological variables were evaluated and compared with observations taken from 30 ground meteorological stations distributed in the Coquimbo Region. Since the model needs as input fields of topography, soil type and soil cover,we used the soil map based on the survey performed by CONAMA [16].
2.2. Germination and Growth Conditions
We used individuals of two Quinoa varieties (PRP and BO78) grown from seeds obtained from the National Seed Bank of Chile managed by INIA-Intihuasi (Vicuña, Chile). Seeds were surface-sterilized with 70% (v/v) ethanol for 5 min followed by 10% (v/v) commercial bleach for 5 min, and rinsed five times in sterile water. After stratification at 4˚C for three days to synchronize germination, seeds were sown in 12 × 12 cm Petri dishes containing autoclaved half-strength Murashige and Skoog medium (MS; [17]). Plates containing 25 seeds each were then arranged vertically in growth chambers at 21˚C (±2˚C) under a 16/8 h light/dark photoperiod (100 μmol·m–2·s–1 irradiance) for up to 12 days.
2.3. Greenhouse Experiment
One hundred and twenty one-month-old seedlings of each variety were transplanted to plastic pot (0.5 l) filled with commercial soil and distributed in growth chambers with different temperature amplitudes (Table 1). The manipulative experiment of thermal amplitude lasted for 6 months and during this period we recorded the survival every day; at end of the experiment both physiological performance (Fv/Fm) and dry biomass were measured.
Plants were irrigated (50 ml/ind.) and supplemented with 0.2 g·l–1 of Phostrogen® (Solaris, NPK, 14:10:27) once every 3 and 15 d, respectively.
We devised an ad hoc equation to provide an overall estimate of the performance of the two varieties using the values of survival, Fv/Fm and biomass, using a scale of 0 to 1 for each. Fv/Fm was measured in this scale; percent survival was converted to decimal values. Since the maximum biomass was produced with 0 thermal amplitude, we divided the observed biomasses by this value for each strain. Because survival is the most important factor for performance in the semi-arid Coquimbo Region, we weighted this character by 0.5 and used 0.25 as the weight for the other two variables. The performance measure with the scaled values was thus:
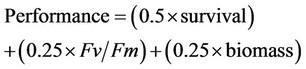
We considered this weighting because the most critical process to obtain a successful yield in the field of the semi-arid Coquimbo region is the survival of seedlings, followed by physiological performance and accumulation of biomass. The information obtained from this manipulative experiment was superimposed on the map of thermal amplitudes of the Coquimbo region, obtaining this way a map of potential performance for the Quinoa varieties.
2.4. Field Experiment
Ninety one-month old seedlings of each variety were transplanted to plastic pot (30 l) filled with commercial soil and distributed in each study site (N = 15 individual/ variety/site). Individuals of both varieties of Quinoa were chosen for measurements of photosynthetic performance using an Infra-red gas analyzer (CIRAS-2; PP-Systems, Have-hill, MA, USA). Measurements were made on 12 adult individuals in each site, during two consecutive days,
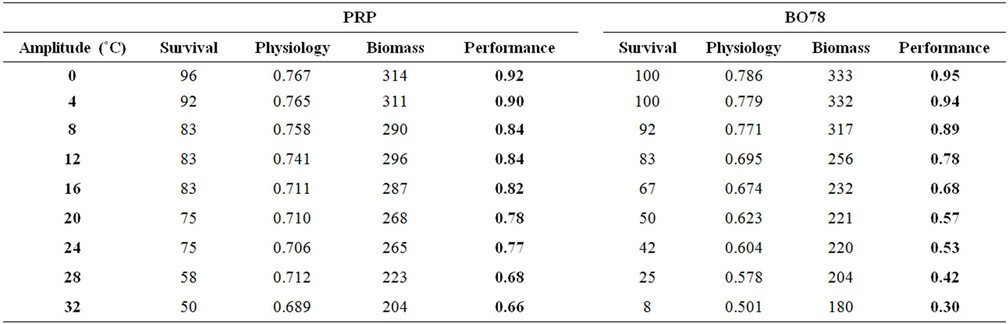
Table 1. Survival percentage, physiological performance (Fv/Fm) and dry biomass (g) in two varieties (PRP and BO78) of quinoa submitted to a manipulative experiment of thermal amplitude variation.
in the late growing season (November). Measurements were performed on leaves fully exposed to the sun, on a typical daily cycle (sunny day), because this condition is likely to be most representative of daily carbon gain. Photosynthetic performance was compared with a twoway ANOVA, where the varieties and sites were independent variables. On the other hand, individuals of Quinoa were randomly assigned to each of 3 sites and were measured prior to the start of experimental treatments (initial height). A one-way ANOVA showed no differences in initial height among groups (data not shown). Pots were randomly placed on bare ground exposed to natural environmental conditions. After 6 months, plant height was measured again and plant growth was determined as the difference between this measure and initial height for each individual. Plant growth was compared with a two-way ANOVA, where the varieties and sites were independent variables.
3. Results
3.1. Climatic Conditions
The mean temperature amplitudes are shown in Figure 1(b). It can be seen that the diurnal temperature amplitude increases eastwards, with coastal values of about 8˚C and values up to 26˚C in the high Andes (Figure 1(b)). This effect may be due to the humidity coming from the ocean, which increases the humidity near the coast and moderates the heating of the air, and the higher solar radiation and extreme drought condition in the Andes Range. On the other hand, minimum and maximum temperatures at the coast are about 12˚C and 20˚C, respectively, while in the Andes the minimum and maximum temperatures values range between 12˚C and 14˚C, respectively, mainly in the high summits.
3.2. Greenhouse Experiment
Survival was higher in the BO78 variety than PRP below 8˚C thermal amplitude, at larger values this trend was reversed (Table 1). Similarly, photochemical efficiency (Fv/Fm) and biomass were greater in individuals of BO78 than those belonging to variety PRP but only up to 8˚C, at greater thermal amplitude the trend was reversed (Table 1). Performance of both varieties decreased with increase of thermal amplitude, being more notorious in BO78 than PRP (Table 1). Spatial variability in the performance of Quinoa showed differences among varieties. Individuals of variety BO78 showed higher performance in the coast zones (lesser thermal amplitude) with a sharp decrease inland (Figure 1(c)). Inversely, those individuals of variety PRP showed better overall performance than BO78, maintaining their performance relatively constant from coast to inland with a smooth decrease in the foothills where the thermal amplitude exceeds 30˚C (Figure 1(d)).
3.3. Field Experiment
The photosynthetic performance of Quinoa individuals was significantly higher in the coastal site (F1, 66 = 83.93; p < 0.001) than others most continental (Figure 2(a)). On the other hand, interaction was significant (F2, 66 = 111.57; p < 0.001) because those individuals of BO78 variety showed higher photosynthetic performance in the coastal site decreasing toward inland, but PRP variety showed similar photosynthetic performance along the gradient (Figure 2(a)). Similarly, plant growth was significantly higher in the coastal site (F1, 66 = 5.21; p < 0.001) compared with the other two sites (Figure 2(b)). Interaction factor was significant (F2, 66 = 9.82; p < 0.001) because while BO78 variety showed a steeper decrease from coastal site to inland site, those of PRP variety showed a smooth increase on the same gradient (Figure 2(b)).
4. Discussion
Quinoa is one of the most consumed crops worldwide and is very tolerant to abiotic stress [18]. Nevertheless, it
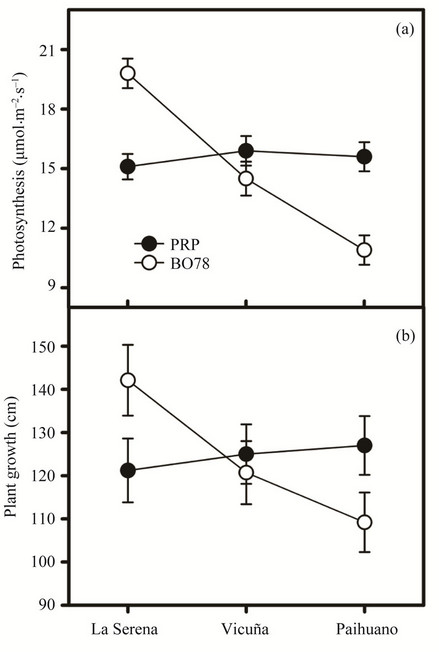
Figure 2. Photosynthetic performance (a) and plant growth (b) of BO78 and PRP varieties of Quinoa growing in 3 sites along East-West gradient in a semi-arid zone of Coquimbo region are shown mean ± SD.
has been demonstrated that abiotic conditions can affect many ecophysiological processes, which makes its production highly dependent upon environmental variation [19]. Despite the limitations for cultivation the demand is growing constantly, thus many studies have aimed to evaluate the effects of climate variability on physiology and production [20].
In this study we found that different varieties of Quinoa subjected to high thermal amplitude conditions decreased their photosynthetic parameters, thus affecting the accumulation of fresh biomass and therefore performance. Similar results have been found in other studies. For instance, [21] demonstrated negative impacts of climate variability on production in many crops, where the temperature variability increased the risks to yield, as shown via computer simulation and experimental studies. On the other hand, it has been reported that thermal oscillation may reduce both photosynthesis parameters and survival percentage, affecting the final performance of a determined crop [22]. Although both varieties decreased their performance in both field and greenhouse conditions when they were exposed to higher thermal amplitudes, they did so in different ways. Those individuals belonging to variety BO78 showed higher performance at lower thermal amplitude than PRP, but the latter showed higher performance under a higher thermal amplitude scenario.
These results suggest that the varieties respond in different ways to thermal amplitude; while BO78 showed an ecotypic response, those individuals belonging to PRP showed a plastic response. Similar responses have been shown previously for others varieties of Quinoa under abiotic stress [20,23]. For example, a recent study showed that PRP and BO78 displayed different strategies in order to cope with salt stress; both proved to be tolerant varieties, with plastic and ecotypic mechanisms, respectively [2].
It has been shown that plants can respond to challenges imposed by environmental conditions by means of phenotypic plasticity and/or ecotypic differentiation [24]. Ecological theory predicts that phenotypic plasticity should be the main adaptive mechanism in heterogeneous or changing environments, whereas relatively stable environments should select for locally adapted ecotypes [25]. Thus, PRP and BO78 may be successful varieties in zones with lower and higher thermal amplitudes, respectively, determining that each variety shows better performance in certain areas of the environmental conditions prevailing in the semi-arid region of Coquimbo.
Patterns of precipitation and temperature are changing globally [26]. In a global change scenario, semi-arid areas would be available for stress tolerant crops, showing different yields according to the variety used. Although it is important to understand how different varieties of crops respond to temporally and spatially changing environments, we are still far from understand the optimal “variety-site” interaction underlying the undisputable success of Quinoa crop. This study has identified promising avenues of research to accomplish such a goal.
5. Acknowledgements
This work was partially supported by CONICYT, Project FONDEF D05I10038 and the International Exchange Project BMBF-CONICYT 066-2007. Authors thank SCC (Steinbuch Centre for Computing, Karl Institut für Technologie (KIT), Germany) for the supply of computing time on NEC SX8 in the context of Campos Grid Project. Also, we thank to I. Bischoff-Gauss and N. Kalthoff for useful discussion.
REFERENCES
- T. J. Flowers, “Improving Crop Salt Tolerance,” Journal of Experimental Botany, Vol. 55, No. 396, 2004, pp. 307- 319. doi:10.1093/jxb/erh003
- K. Ruiz-Carrasco, F. Antognoni, A. Konotie-Coulibaly, S. Lizardi, A. Covarrubias, E. A. Martínez, M. A. MolinaMontenegro, S. Biondi and A. Zurita-Silva, “Variation in Salinity Tolerance of Four Lowland Varieties of Quinoa (Chenopodium quinoa Willd.) as Assessed by Growth, Physiological Traits, and Sodium Transporter Gene Expression,” Plant Physiology and Biochemistry, Vol. 49, No. 11, 2011, pp. 1333-1341. doi:10.1016/j.plaphy.2011.08.005
- B. Kruk, P. Insausti, A. Razul and R. Benech-Arnold, “Light and Thermal Environments as Modified by a Wheat Crop: Effects on Weed Seed Germination,” Journal of Applied Ecology, Vol. 43, No. 2, 2006, pp. 227-236. doi:10.1111/j.1365-2664.2006.01140.x
- P. W. Barnes, S. D. Flint and M. M. Caldwell, “Morphological Responses of Crop and Weed Species of Different Growth Forms to Ultraviolet-B Radiation,” American Journal of Botany, Vol. 77, No. 10, 1990, pp. 1355-1360. doi:10.2307/2444596
- H. W. Koyro and S. S. Eisa, “Effect of Salinity on Composition, Viability and Germination of Seeds of Chenopodium quinoa Willd,” Plant and Soil, Vol. 302, 2008, pp. 79-90. doi:10.1007/s11104-007-9457-4
- N. S. Mattson and W. R. Leatherwood, “Potassium Silicate Drenches Increase Leaf Silicon Content and Affect Morphological Traits of Several Floriculture Crops Grown in a Peat-Based Substrate,” HortScience, Vol. 45, No. 1, 2010, pp. 43-47.
- M. S. Heschel, S. E. Sultan, S. Glover and D. Sloan, “Population Differentiation and Plastic Responses to Drought Stress in the Generalist Annual Polygonum persicaria,” International Journal of Plant Sciences, Vol. 165, No. 5, 2004, pp. 817-824. doi:10.1086/421477
- M. A. Molina-Montenegro, A. Zurita-Silva and R. Oses, “Effect of Water Availability on Physiological Performance and Lettuce Crop Yield (Lactuca sativa),” Ciencia e Investigación Agraria, Vol. 38, No. 1, 2011, pp. 65-74.
- M. Rosa, M. Hilal, J. A. González and F. E. Prado, “Changes in Soluble Carbohydrates and Related Enzymes Induced by Low Temperature during Early Developmental Stages of Quinoa (Chenopodium quinoa) Seedlings,” Journal of Plant Physiology, Vol. 161, No. 6, 2004, pp. 683-689. doi:10.1078/0176-1617-01257
- J. F. Bois, T. Winkel, J. P. Lhomme, J. P. Raffaillac and A. Rocheteau, “Response of Some Andean Cultivars of Quinoa (Chenopodium quinoa Willd.) to Temperature: Effects on Germination, Phenology, Growth and Freezing,” European Journal of Agronomy, Vol. 25, No. 4, 2006, pp. 299-308. doi:10.1016/j.eja.2006.06.007
- G. Adrian and F. Fiedler, “Simulation of Unstationary Wind and Temperature Fields over Complex Terrain and Comparison with Observations,” Contribution to Atmospheric Physics, Vol. 64, 1991, pp. 27-48.
- S. Montecinos, W. Boersch-Supan, V. Favier, O. Astudillo and Y. Tracol, “Impacts of Agricultural Activities on the Regional Climate in an Arid Zone in Chile,” Die Erde, Vol. 139, 2008, pp. 77-95.
- M. R. Anderberg, “Cluster Analysis for Applications,” Academic Press, New York, 1973.
- J. H. Ward, “Hierarchical Grouping to Optimize an Objective Function,” Journal of the American Statistical Association, Vol. 58, No. 301, 1963, pp. 236-244. doi:10.1080/01621459.1963.10500845
- E. Kalnay, M. Kanamitsu, R. Kistler, W. Collins, D. Deaven, L. Gandin, M. Iredell, S. Saha, G. White, J. Woollen, Y. Zhu, M. Chelliah, W. Ebisuzaki, W. Higgins, J. Janowiak, K. C. Mo, C. Ropelewski, J. Wang, A. Leetmaa, R. Reynolds, R. Jenne and D. Joseph, “The NCEP/NCAR 40-Year Reanalysis Project,” Bulletin of the American Meteorological Society, Vol. 77, No. 3, 1996, pp. 437-471. doi:10.1175/1520-0477(1996)077<0437:TNYRP>2.0.CO;2
- CONAMA, “Catastro y Evaluación de Recursos Vegetacionales nativos de Chile,” Universidad Austral de Chile, Pontificia Universidad Católica de Chile, Universidad Católica de Temuco, Chile, 1999.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 43, 1962, pp. 473-479. doi:10.1111/j.1399-3054.1962.tb08052.x
- E. A. Martínez, E. Veas, C. Jorquera, R. San Martín and P. Jara, “Re-Introduction of Chenopodium quinoa Willd. into arid Chile: Cultivation of Two Lowland Races under Extremely Low Irrigation,” Journal of Agronomy and Crop Science, Vol. 195, No. 1, 2009, pp. 1-10. doi:10.1111/j.1439-037X.2008.00332.x
- S. Geerts, D. Raes, M. Garcia, C. Taboada, R. Miranda, J. Cusicanqui, T. Mhizha and J. Vacher, “Modeling the Potential for Closing Quinoa Yield Gaps under Varying Water Availability in the Bolivian Altiplano,” Agricultural Water Management, Vol. 96, 2009, pp. 1652-1658. doi:10.1016/j.agwat.2009.06.020
- J. A. González, M. Gallardo, M. Hilal, M. Rosa and F. E. Prado, “Physiological Responses of Quinoa (Chenopodium quinoa Willd.) to Drought and Waterlogging Stresses: Dry Matter Partitioning,” Botanical Studies, Vol. 50, No. 1, 2009, pp. 35-42.
- J. R. Porter and M. A. Semenov, “Crop Responses to Climatic Variation,” Philosophical Transaction of the Royal Society B, Vol. 360, No. 1463, 2005, pp. 2021- 2035. doi:10.1098/rstb.2005.1752
- H. R. Huarte and R. L. Benech-Arnold, “Understanding Mechanisms of Reduced Annual Weed Emergence in Alfalfa,” Weed Science, Vol. 51, No. 6, 2003, pp. 876-885. doi:10.1614/P2002-140
- S. E. Jacobsen, C.Monteros, J. L. Christiansen, L. A. Bravo, L. J. Corcuera and A. Mujica, “Plant Responses of Quinoa (Chenopodium quinoa Willd.) to Frost at Various Phenological Stages,” European Journal of Agronomy, Vol. 22, No. 2, 2005, pp. 131-139. doi:10.1016/j.eja.2004.01.003
- C. D. Schlichting and M. Pigliucci, “Phenotypic Evolution: A Reaction Norm Perspective,” Sunderland, Sinauer, 1998.
- M. A. Molina-Montenegro, C. Atala and E. Gianoli, “Phenotypic Plasticity and Performance of Taraxacum officinale (Dandelion) in Habitats of Contrasting Environmental Heterogeneity,” Biological Invasions, Vol. 12, No. 7, 2010, pp. 2277-2284. doi:10.1007/s10530-009-9638-6
- IPCC, “Intergovernmental Panel of Climate Change,” 2007. http://www.ipcc.ch
NOTES
*Corresponding author.

