American Journal of Plant Sciences
Vol.3 No.7A(2012), Article ID:21057,4 pages DOI:10.4236/ajps.2012.327121
Hepatoprotection: A Hallmark of Citrullus colocynthis L. against Paracetamol Induced Hepatotoxicity in Swiss Albino Rats
![]()
Pest Control & Ayurvedic Drug Research Lab. S.S.L Jain P.G College, Vidisha, India.
Email: *drarshediqbal@gmail.com
Received May 10th, 2012; revised June 11th, 2012; accepted June 21st, 2012
Keywords: Citrullus colocynthis L.; Hepatoprotective; Paracetamol; Silymarin
ABSTRACT
Objective: To demonstrate the in-vivo hepatoprotective effect of the ethanolic extracts of Citrullus colocynthis (Linn.) against paracetamol induced hepatotoxicity in albino rats. Animal Model: Swiss Albino rats of either sex were used, divided into six groups with six in each group. Group 1-Normal control: The animals were maintained under normal control, which were given distilled water only. Group 2-Induction of hepatotoxicity: The animals received paracetamol 500 mg/kg b.w. (p.o) every 72 h for 10 Days. Groups 3 to 5: Animals received ethanolic extract of Citrullus colocynthis L. at 50, 100 & 200 mg/kg bw/day for 7 days (p.o). Group 6: The animals were treated with Silymarin (100 mg/kg p.o) which served as standard. Groups 3 to 6 were intoxicated with paracetamol (500 mg/kg bw) 1 h before the administration of extract or Silymarin for 10 days. Histopathological findings, different hepatic biochemical parameters viz. AST, ALT, ALP, Total bilirubin, Total cholesterol, Triglycerides, & the body weight before & after treatment were evaluated to investigate the hepatoprotective activity. Results: Paracetamol induced a significant rise in AST, ALT, ALP, Total Bilirubin, Total Cholesterol, Triglycerides. Administration of 200 mg/kg bw of ethanolic extract of Citrullus colocynthis L. effectively reduced these pathological damages caused by paracetamol intoxication. In addition to serum parameters treatment of 200 mg/kg bw of ethanolic extract of Citrulus colocynthis L. also promotes the body weight in albino rats as shown in Figure 1. Histopathological changes of the liver samples were compared with the normal control as shown in Figures 2-5 respectively. Conclusion: From our results we may infer that the mode of action of 90% ethanolic extract of Citrullus colocynthis L. (200 mg/kg bw) in affording the in-vivo hepatoprotective activity against paracetamol may be due to the cell membrane stabilization, hepatic cell regeneration & normalizing the serum parameters.
1. Introduction
Liver is a major organ system involved in the metabolism of various drugs, xenobiotics & toxins. During the metabolism, excessive free radicals are generated & may cause liver damage. In spite of tremendous strides in modern medicine, there are hardly any drugs that stimulate liver function, offer protection to the liver from damage or help regeneration of hepatic cell [1]. There are, however, a number of drugs employed in traditional system of medicine for liver afflictions [2]. Therefore drugs from natural source are being adopted to treat hepatitis/ liver diseases. It has great capacity to detoxicate toxic substances & synthesize useful principles. Therefore, damage to the liver inflicted by hepatotoxic agents is of grave consequences [3]. Citrullus colocynthis L. (Family Cucurbitaceae) commonly known as bitter apple, just because of its bitter principle is a tropical plant that grows widely in the Persian, Arabian & Sahara desert & was introduced by Arabs first, in Spain & Cyprus in the middle Ages [4,5]. Refining & washing with citric acid removes its bitter taste [6]. It is annual or perennial (in wild), herbaceous, flowers monoecious, fruit pepo & seed numerous. Its fruit, which is lemon sized, yellowish, green mottled, spongy, & extremely bitter, is a powerful hepatic stimulant & hydragogue cathartic. It is used as a strong laxative. The dried pulp of Citrullus colocynthis L. has been used for constipation, edema, bacterial infections, cancer & diabetes [7]. From the traditional knowledge it is very clear that the fruits of Citrullus colocynthis L. have the hepatoprotective activity. But still no scientific & methodical investigations have so far been reported in the literature regarding its in-vivo hepatoprotective activity against paracetamol induced hepatotoxicity in albino rats. This is the reason why the present study was undertaken to evaluate the in-vivo hepatoprotective activity of ethanolic extract of the complete fruit of Citrullus colocynthis L. against paracetamol induced hepatotoxicity in rat model.
2. Materials and methods
2.1. Plant Materials
The fruits of Citrullus colocynthis L. used for the present study were collected from the catchment areas of Vidisha district of MP in India, during the months of September. The plant was acknowledged by a senior Botanist Dr. P. N. Srivastav, Professor of the Department of Botany, S.S.L Jain P.G College Vidisha (MP). After identification of the fruit, its herbarium specimen was kept in the Herbarium of Pest Control & Ayurvedic Drug Research Lab. S.S.L Jain P.G College Vidisha, (MP). The fruits of Citrullus colocynthisi L. were shade dried, pulverized & stored in an air-tight, light resistant container for further use.
2.2. Preparation of Extract
The extraction of shade dried powder material of the complete fruit was carried out by soxhlet apparatus using different solvents according to increasing order of polarity viz. n-hexane, Pet-ether, Chloroform, Ethyl acetate, Ethanol & distilled water. The extracts thus obtained were dried under reduced pressure yielding 4.52%, 0.29%, 0.9%, 0.2%, 1.82%, and 3.93% respectively.
2.3. Phytochemical Analysis
The 90% ethanolic extract of Citrullus colocynthis was subjected to identify the presence of various phytoconstituents viz. Flavonoids (Shinoda test), Alkaloids (Harbone phytochemical methods [8]), steroids and terpenoids (Leibermann Burchard test), tannin and phenolic compounds (ferric chloride test), amino acids (Ninhydrin test), etc. The 90% ethanolic extract showed the +ve results for alkaloids, flavonoids & glycosides.
2.4. Experimental Animals
Albino rats weighing between 150 - 200 g, bred in Animal House of Pest Control & Ayurvedic Drug Research Laboratory, were used for the study. The animals were procured & housed in the animal house maintained under standard hygienic conditions, at a temperature of 25˚C ± 1˚C with 50% ± 10% relative humidity & with a 12:12 hr light/dark cycle. Food pellets (Hindustan lever Ltd. Mumbai, India) & tap water were provided ad libitum. Studies were performed in accordance with the CPCSEA guidelines.
2.5. Acute Oral Toxicity Studies
Healthy albino rats of either sex weighing 150 - 200 g maintained under standard laboratory conditions were used for acute oral toxicity test according to Organization for Economic Co-operation and Development guidelines 423 (OECD, 1996). A total of three animals were used which received a single oral dose of (2000 mg/kg) of 90% ethanolic extract. After administration of extract the food was withheld for further 3 - 4 h. Animals were observed individually at least once during the first 30 min after dosing, periodically during first 24 h (with special attention during the first 4 h) and daily thereafter for period of 3 days. All the animals were observed for lethal or toxic signs up to 2000 mg/kg.
2.6. Experimental Design
The animals were divided into six groups of six animals in each group. Group 1 served as normal control which received distilled water only. Group 2 served as paracetamol control & received paracetamol at a dose of 500 mg/kg bw (p.o) at every 72 h for 10 Days. Groups 3 to 5 received ethanolic extract of the Citrullus colocynthis at 50, 100 & 200 mg/kg bw/day for 10 days (p.o). Group 6 served as standard control & received Silymarin (100 mg/kg p.o). Groups 3 to 6 were intoxicated with paracetamol (500 mg/kg bw) 1 h before the administration of extract or Silymarin for 10 days.
2.7. Biochemical Investigation
Rats of all groups were anaesthetized using anesthetic ether, & blood collected by retro orbital puncture & biochemical parameters like ALT, AST, ALP, Total Bilirubin, Total Cholesterol, & Triglycerides were estimated. The animals were sacrificed by overdose of ether & autopsied [9]. Livers from all animals were removed, washed with ice cold saline, weighed. Small piece of liver tissue was collected & preserved in 10% formalin solution for histopathological studies. Liver tissues were homogenized with ice-chilled 10% KCl solution & centrifuged at 2000 rpm for 10 min.
2.8. Histopathological Investigation
Liver slices fixed for 48 h in 10% formosaline were processed for paraffin embedding & sectioned at 5 µm following the standard microtechnique. Sections were stained with Haematoxylin & Eosin & were mounted in Canada balsam. Light microscopic examination of the sections was then carried out & micrographs were produced using Olympus BX-60 photographic microscope at Jawaharlal Nehru Cancer Hospital & Research Centre. Bhopal.
2.9. Statistical Analysis
The data are expressed as mean value ± standard error of mean. Statistical significance was determined by one way ANOVA followed by Dunnet’s test. Differences among the control & treatment groups were also determined by using statistical package (Graph Pad Instant). A probability level of less than 5% (p < 0.05) was considered significant.
3. Observations & Results
When the plasma membrane of a hepatocyte is damaged, a variety of enzymes that are normally located in the cytosol are released into the bloodstream. Numerical analysis of these enzymes in the serum serves as a useful quantitative marker for the proper evaluation of liver damage. A number of serum enzymes involved are evaluated to distinguish and assess the hepatocellular injury.
3.1. Serum Glutamate Oxaloacetate Transaminase (SGOT)
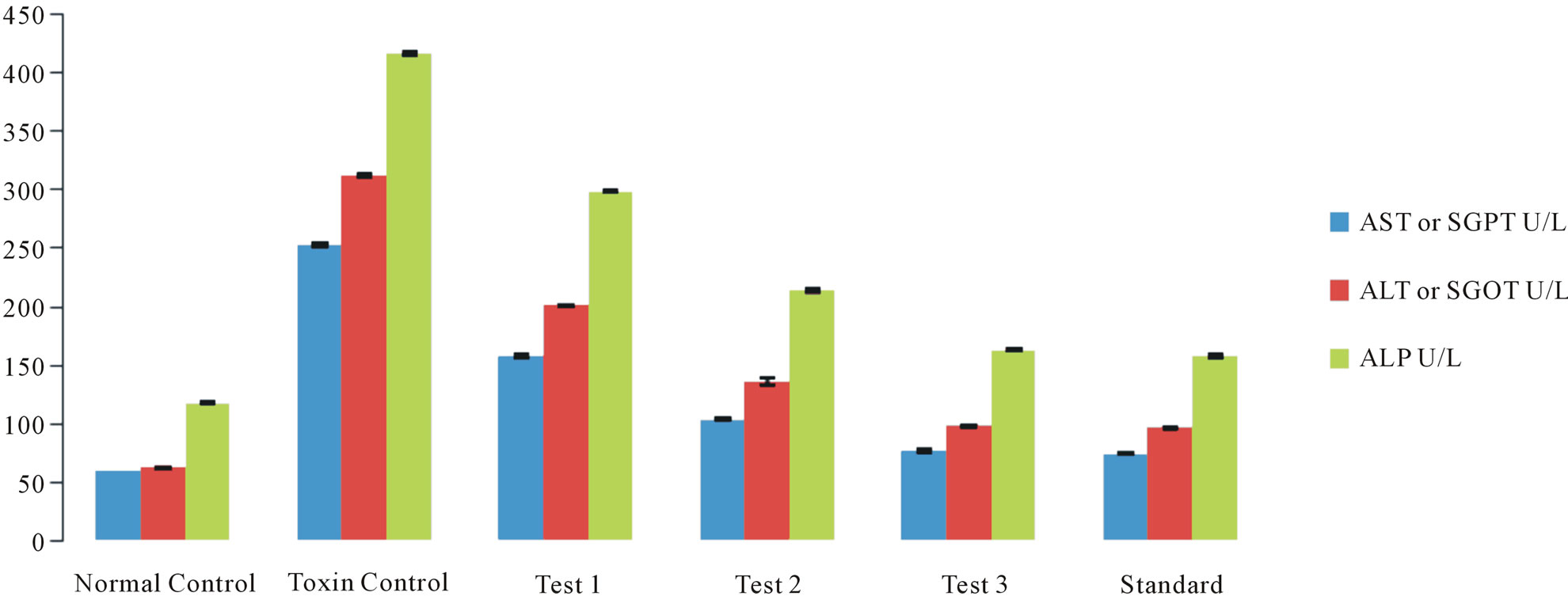
Analysis of the enzyme serum glutamate oxaloacetate transaminase—also known as aspartate aminotransferase (AST)—in the serum of a patient provides an indication of hepatic damage. The release of these enzymes from damaged liver cells causes a significant increase in the level of SGOT in the serum (Figure 1(a)).
3.2. Serum Glutamate Pyruvate Transaminase (SGPT)
The level of the enzyme serum glutamate pyruvate transaminase—also known as alanine aminotransferase (ALT)—in liver is lower in comparison with SGOT. Any increase in the level of this enzyme in the serum is a specific indication for liver damage (Figure 1(a)).
3.3. Serum Alkaline Phosphatase (ALP)
This microsomal enzyme is present in a higher concentration in the liver. Any disorder related to bile flow increases the synthesis of this enzyme and is a specific marker for cholestasis and to lesser extent for hepatocyte damage (Figure 1(a)).
3.4. Serum Bilirubin
Bilirubin is an end product of heme but is excreted in bile. In the case of obstructive jaundice, excretion of bilirubin in the bile is impeded, which causes increased levels of bilirubin in the blood; this increase is thus indicative of liver damage (Figure 1(b)).
Histological examination of the liver sections reveals that the normal liver architecture as shown in Figure 2 was disturbed by hepatotoxins intoxication which shows apoptotic hepatocytes & conjested central veins (Figure 3). Treatment of rat with 90% ethanolic extract of Citrullus colocynthis L. exhibit regenerative changes like normal appearance of hepatic cells with nucleus, less vacuolization & fatty change supplements the protective effect of the extract (Figure 4). Standard drug Silymarin treatment improved the functional integrity of the hepatocytes and normalizing the serum parameters as shown in Figure 5. Figure 6 showed the promotion of body weights after the treatment of 90% ethanolic extract of Citrullus colocynthis L.in albino rats.
4. Discussions
The aim of this study is to assess the hepatoprotective activity of the fruits of Citrullus colocynthis L. in Swiss albino rats against paracetamol as hepatotoxin to prove its claims in folklore practice against liver disorders. The extent of hepatic damage is assessed by histological evaluation & the level of various biochemical parameters in circulation. Paracetamol (N-acetyl-p-aminophenol) is a widely used analgesic & antipyretic drug & is safe when used in therapeutic doses. However, over dosage of paracetamol is known to be hepatotoxic & nephrotoxic in man & in experimental animals [9]. Overdose of paracetamol causes a potentially fatal, hepatic centrilobular necrosis. The hepatotoxicity of paracetamol has been attributed to the formation of a toxic metabolite, N acetylp-benzoquinoneimine (NAPQI) by the action of cytochrome P4502E1 [10]. When liver plasma membrane gets damaged, a variety of enzymes normally located in the cytosol are released into the circulation [11]. In the present study the damage of liver due to paracetamol over dosage was confirmed by elevated levels of biochemical parameters like AST, ALT, ALP, Serum bilirubin, total cholesterol, & triglycerides. This is due to the fact that hepatic cells possess a variety of metabolic activities & contain a host of enzymes. SGPT and SGOT were found in higher concentration in cytoplasm & SGPT particularly in mitochondria. In liver injury the transport function of hepatocytes is disturbed, resulting in the leakage of plasma membrane [12], thereby causing leakage of such enzymes leading to the increased serum levels of them. Oral administration of various doses of Citrullus colocynthis L. ethanolic extract to paracetamol intoxicated rats resulted in gradual normalization of the activities of AST, ALT and ALP Figure 1(a). This evi-
 (a)
(a)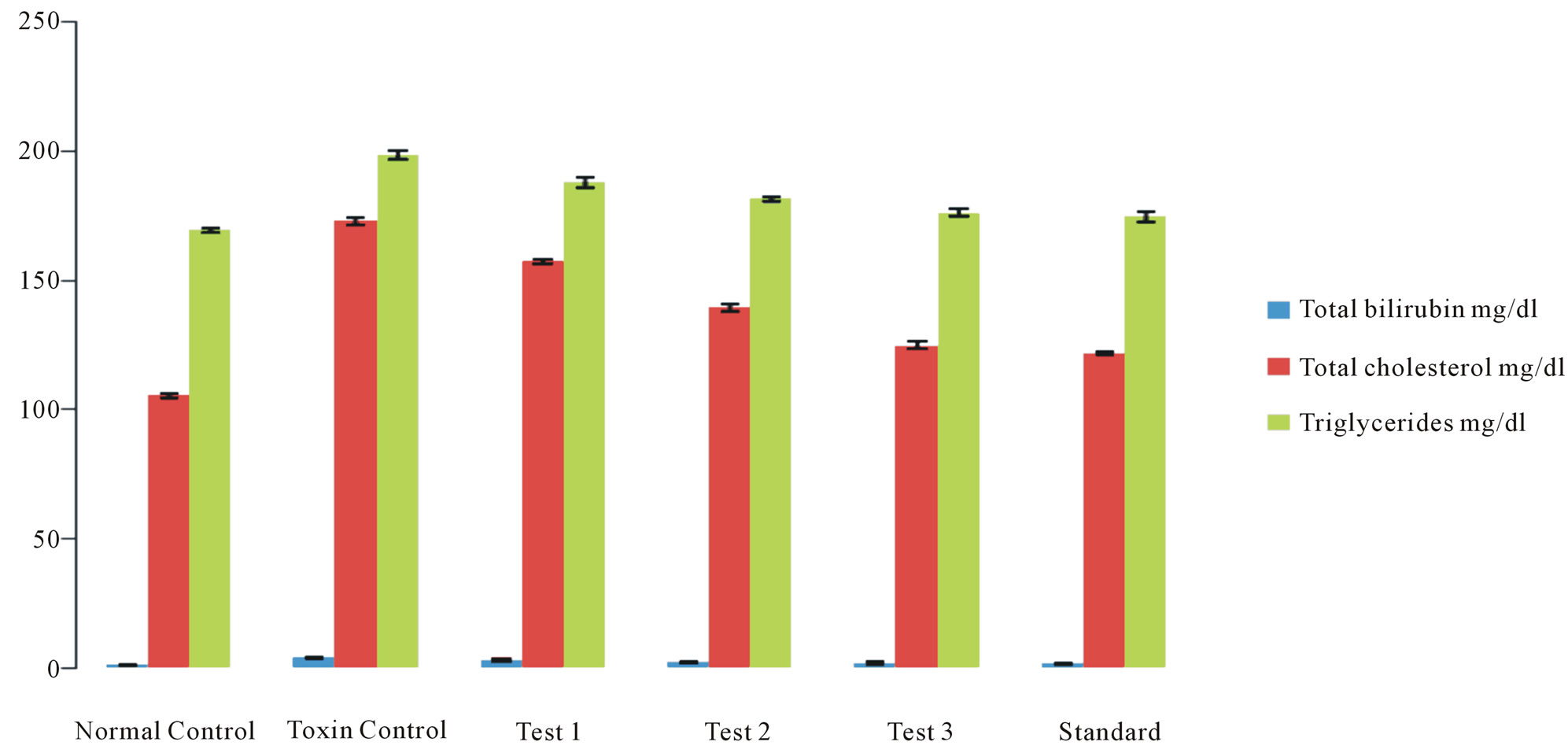 (b)
(b)
Figure 1. Effects of 90% ethanolic extract of fruits of Citrullus colocynthis L. on serum parameters against paracetamol induced hepatotoxicity in swiss albino rats.
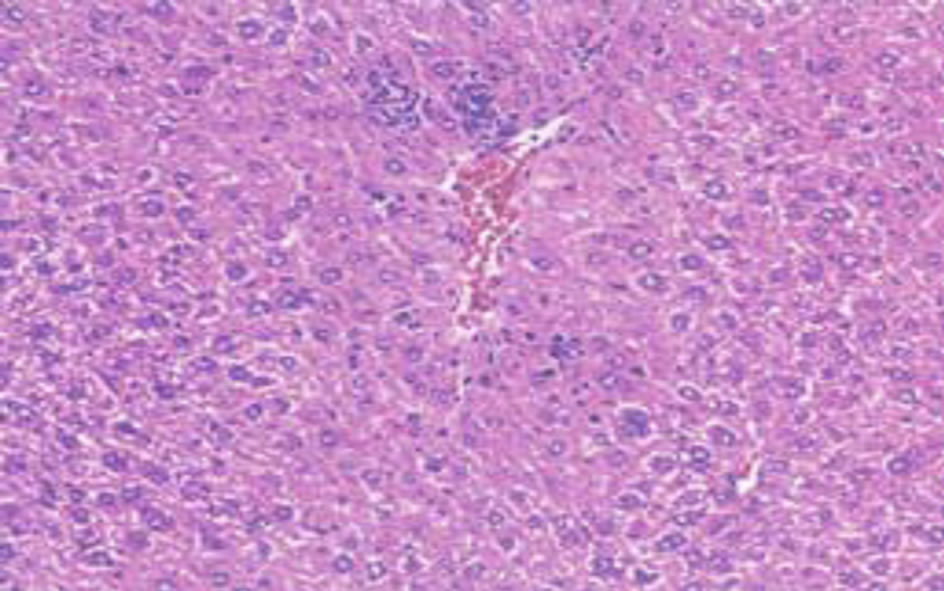
Figure 2. Normal control.
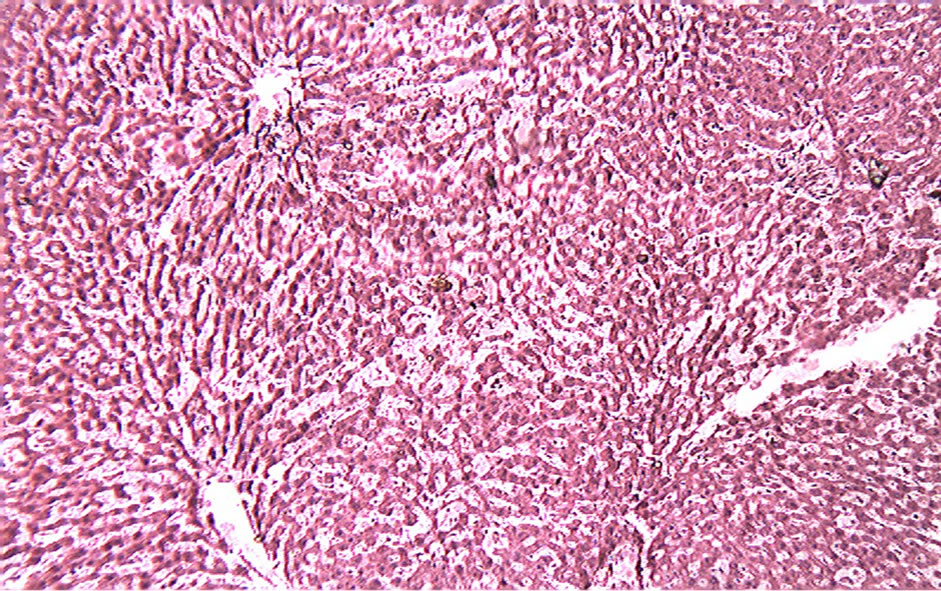
Figure 3. Toxin (PCM) control.
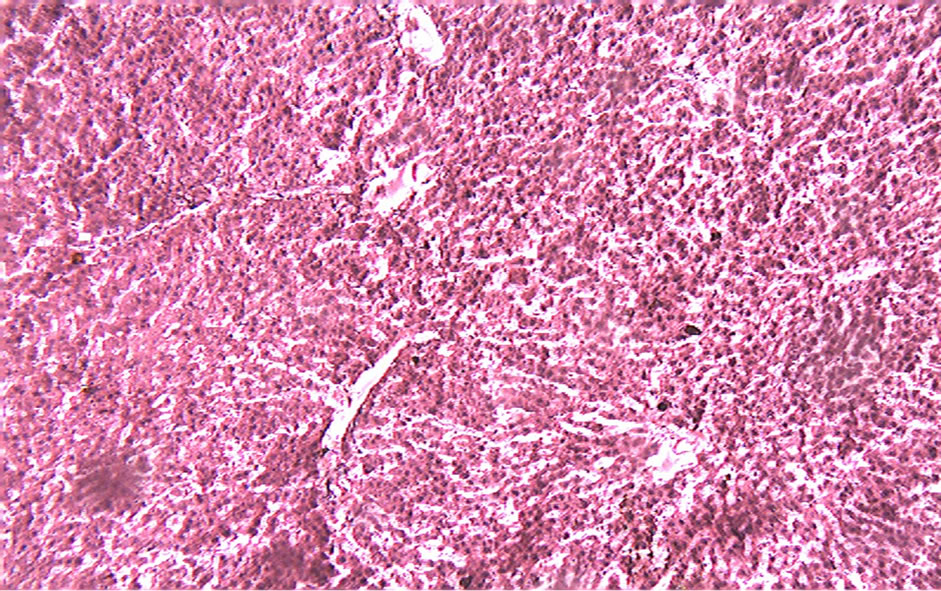
Figure 4. Treated with 90% ethanolic extract of the fruits of Citrullus colocynthis L.
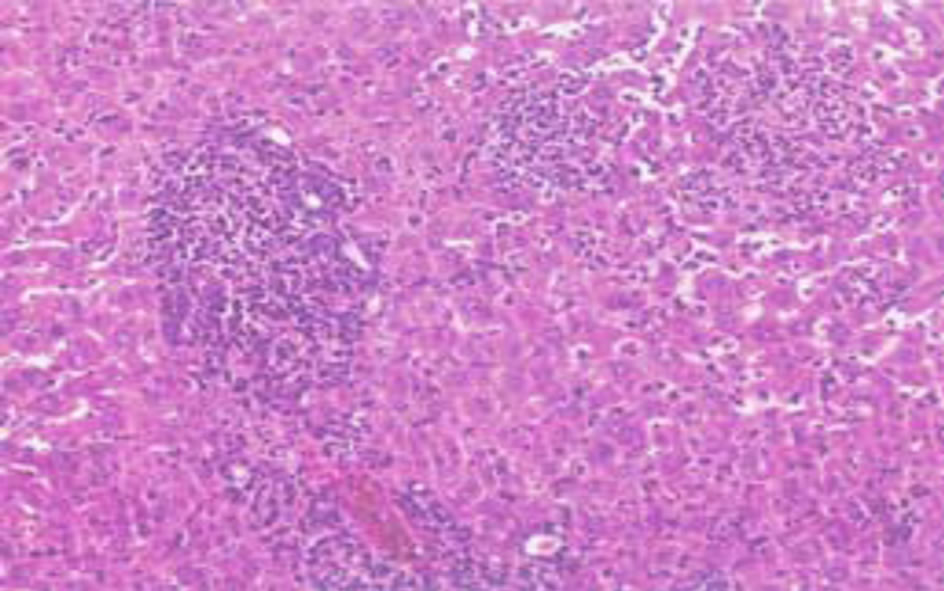
Figure 5. Treated with Silymarin.
dently suggests the protective effect of the extract in improving the functional integrity of liver cells. Bilirubin is considered as an index for the assessment of hepatic function and any abnormal increase indicates hepatobiliary disease and severe disturbance of hepatocellular architecture [13]. Paracetamol administration resulted in increased bilirubin, total cholesterol & triglyceride levels (Figure 1(a)) thereby suggesting severe hepatic injury and confirming the hepatotoxic nature of paracetamol.
Treatment with 90% ethanolic extract of fruits of Citrullus colocynthis L. normalizes the bilirubin, cholesterol & triglyceride levels indicating its hepatoprotective efficacy (Figure 1(b)). Paracetamol gets metabolically activated to a reactive metabolite NAPQI by cytochrome P4502E1. NAPQI, in turn, is detoxified by conjugating with glutathione (GSH). Thus, GSH constitute the first line of defence against paracetamol induced generation of free radicals [14]. In paracetamol toxicity, total hepatic GSH was found to be depleted due to the damage caused to hepatic cells. As a result, formation of NAPQIglutathione conjugate is diminished. Administration of Citrullus colocynthis L. ethanolic extract effectively replenished the paracetamol induced depletion of hepatic GSH presumably due to diminished production of toxic metabolite, NAPQI through the inhibition of cytoP450 enzyme system. Histological examination of the liver sections reveals that the normal liver architecture is disturbed by hepatotoxins intoxication which shows apoptotic hepatocytes & conjested central veins (Figure 3). Treatment of rat with 90% ethanolic extract of Citrullus colocynthis L. exhibit regenerative changes like normal appearance of hepatic cells with nucleus, less vacuolization & fatty change supplements the protective effect of the extract (Figure 4). However the results strongly suggest an initiation of the process of liver regeneration, which is also evident from the various biochemical parameter results. (Figure 6) showed the promotion of body weights after the treatment of 90% ethanolic extract of Citrullus colocynthis L. in albino rats. Some agents exert their hepatotoxic effects through the metabolic pathways that are responsible for drug detoxification. For example, the toxicity of paracetamol frequently used drug is believed to be caused by the formation of a free radical intermediate N-acetylimidoquinone during detoxification processes that inactivate many enzymes and proteins by binding irreversibly to their sulfhydryl groups. Overdose of paracetamol increases the concentration of free radi-
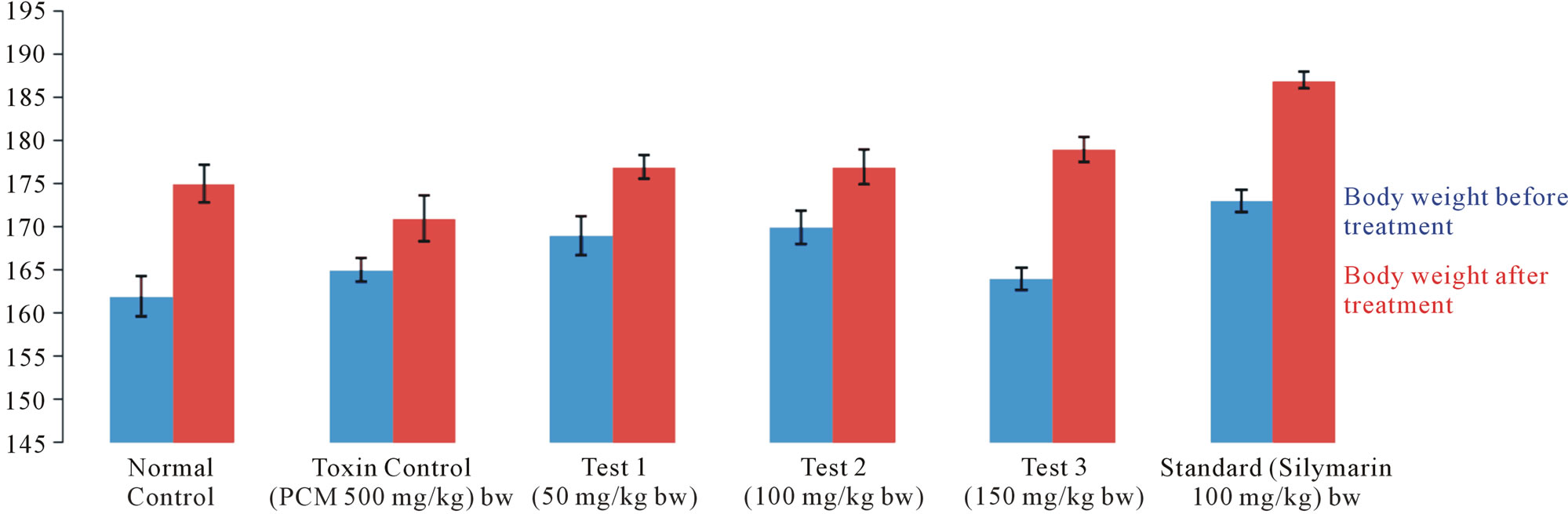
Figure 6. Effect of 90% ethanolic extracts of Citrullus colocynthis L. on the body weight (before & after treatment) in albino rats.
cals, which deplete the glutathione levels of the hepatocytes and thereby lead to inactivation of cellular proteins and widespread hepatocellular necrosis [15]. In the present study, the toxic effect that arises from overdose of paracetamol has been prevented by early administration of 90% ethanolic extract of fruits of Citrullus colocynthis L. (200 mg/kg bw) in Swiss albino rats. Thus in future this herbal extract may be used as strong constituent for the formulation of hepatoprotective herbal drug.
5. Conclusion
It appears from our results that the mode of action of 90% ethanolic extract of Citrullus colocynthis L. (200 mg/kg bw) in affording the hepatoprotective activity against paracetamol may be due to the cell membrane stabilization, hepatic cell regeneration, & normalizing the serum parameters. Hence the present study justified the traditional use of Citrullus colocynthis L. in the treatment of liver diseases.
6. Acknowledgements
The Authors are gratefully acknowledged to the staff of Pest Control & Ayurvedic Drug Research Lab. S.S.L Jain P.G College Vidisha, SAIF CDRI Lucknow, Krishna Pathological Lab, and Jawaharlal Nehru Cancer Hospital & Research Center Bhopal, India for providing all support during the study period.
REFERENCES
- T. K. Chaterjee, “Medicinal plants with hepatoprotective properties. Herbal Options,” Books & Allied (P) Ltd., Calcutta, 2000.
- R. R. Chattopadhyay, “Possible mechanism of Hepatoprotective Activity of Azadirachta indica Leaf Extract, Part II,” Ethnopharmacol, Vol. 89, No. 2-3, 2003, pp. 217-219. doi:10.1016/j.jep.2003.08.006
- S. Shahani, “Evaluation of Hepatoprotective Efficacy of APCL-A Polyherbal Formulation in-vivo in rats,” Indian Drugs, Vol. 13, 1999, p. 79.
- G. Trease and W. Evans, “Text Book of Pharmacognosy,” Tindal & Cassell, London, 1970.
- USDA, ARS, National Genetic Resources Program, “Germplasm Resources Information Network (GRIN) [Online Database],” National Germplasm Resources Laboratory, Beltsville, 2006.
- G. Ramakrishna, G. Azeemoddin and T. Lakshminarayana, “Processing of Tuba Seeds & oil,” Oil Technologists Association Of India, Vol. 25, 1993, p. 3.
- F. Al-Ghaithi, M. R. El-Ridi, E. Adeghate and M. H. Amiri, “Biochemical effects of Citrullus colocynthis in Normal & Diabetic Rats,” Molecular and Cellular Biochemistry, Vol. 261, No. 1, 2004, pp. 143-149. doi:10.1023/B:MCBI.0000028749.63101.cc
- J. B. Harbone, “Phytochemical methods,” Chapman & Hall, London, 1973.
- K. K. Senthil and K. K. L. Senthil, Der Pharmacia Lettre, Vol. 2, No. 1, 2010, pp. 261-264.
- V. Dipak, Parmar, A. Gazala, A. Milind, A. Khandkar and S. K. Surendra, “Mitochondrial ATPase, a target for Paracetamol Induced Hepatotoxicity,” European Journal of Pharmacology: Environmental Toxicology and Pharmacology, Vol. 293, No. 3, 1995, pp. 225-229. doi:10.1016/0926-6917(95)00021-6
- S. S. T. Lee, J. T. M. Buters, T. Pineau, P. FernandezSalguero and F. L. Gonzales, “Role of CYP2E1 in hepatotoxiciy of acetaminophen,” Journal of Biological Chemistry, Vol. 271, 1996, pp. 12063-12067. doi:10.1074/jbc.271.20.12063
- S. K. Mitra, M. V. Venkataranganna, R. Sundaram and S. Gopumadhavan, “Protective effect of HD-03, a herbal formulation, against Various Hepatotoxic Agents in rats,” Journal of Ethnopharmacology, Vol. 63, No. 3, 1998, pp. 181-186. doi:10.1016/S0378-8741(98)00088-9
- M. G. Rajesh and M. S. Latha, “Preliminary evaluation of the Antihepatotoxic Activity of Kamilari, a polyherbal formulation,” Journal of Ethnopharmacology, Vol. 91, No. 1, 2004, pp. 99-104. doi:10.1016/j.jep.2003.12.011
- P. Martin and L. S. Friedman, “Assessment of Liver Function and Diagnostic Studies,” In: L. S. Freidman and E. B. Keefe, Eds., Hand Book of Liver Disease, Churchill Livingstone, Philadelphia, 1992, p. 1.
- L. P. James, P. R. Mayeux and J. A. Hinson, “Acetaminophen—induced hepatotoxicity,” Drug Metabolism & Disposition, Vol. 31, No. 12, 2003, pp. 1499-1506. doi:10.1124/dmd.31.12.1499
NOTES
*Corresponding author.

