Crystal Structure Theory and Applications
Vol. 2 No. 3 (2013) , Article ID: 37256 , 4 pages DOI:10.4236/csta.2013.23016
Crystal Structure of 3-Amino-5,6-Dimethyl-2-Nitrobiphenyl-4-Carbonitrile
School of Chemistry and Chemical Engineering, Xuchang University, Xuchang, China
Email: namijin@126.com
Copyright © 2013 Wanqiang Zhang. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 3, 2013; revised June 7, 2013; accepted July 2, 2013
Keywords: polysubstituted benzene; Crystal Structure; hydrogen bonds
ABSTRACT
A kind of polysubstituted benzene, 3-amino-5,6-dimethyl-2-nitrobiphenyl-4-carbonitrile(C15H13N3O2), was synthesized and its crystal structure was determined. The molecule is composed of two phenyl moieties, two methyl groups, a cyano group, an amino group and a nitro group. The methyl groups, cyano group and amino group are nearly coplanar with the connected benzene ring. Because of the large volume, the nitro group and the connected benzene ring are twisted. The dihedral angle between the two benzene rings is 83.51˚. In the crystal, molecules are linked by N—H···N and C—H···O hydrogen bonds.
1. Introduction
Polysubstituted benzenes and aromatic compounds are widely used in the chemical industry as well in the laboratory [1]. Many liquid crystals belong to the group of polysubstituted benzenes and their derivatives [2-6]. Such compounds can be used for Organic Light Emitting Diode [7-10]. Heteroaromatic components and their derivatives play important parts in pharmaceutical and agrochemical chemicals [11-13]. Hence, more and more researchers have concerned about these compounds. The determination of crystal structure is helpful for its application and realization of property. In this paper, we report about the synthesis and structure of a kind of polysubstituted benzene, 3-amino-5,6-dimethyl-2-nitrobiphenyl-4-carbonitrile.
2. Experimental
2.1. Synthesis of the Compound
A 25 mL round bottom flask was charged with 120 mg 2- sec-Butylidene-malononitrile, 149 mg (2-nitrovinyl) benzene and 68 mg EtONa. The mixture was refluxed with vigorous stirring for 50 hours. The solvent was removed under reduced pressure. The yellow needle product was purified by column chromatography (EtOAc/ hexanes, 1:8). The product was recrystallized from n-hexane and single crystals suitable for X-ray diffraction were obtained.
2.2. Determination of the Crystal Structure
The crystal Structure was characterized by X-ray diffraction (XRD) using Bruker Smart-1000 X-ray single crystal Diffractometer equipped with graphite monochromatized MoKa radiation (λ = 0.71073 Å). The diffraction data were obtained at the temperature of 296 K and analyzed by the software of SHELXTL-97 [14]. The details were shown in Table 1.
3. Results and Discussions
3.1. Refinement Details
All reflections were defined based on F2. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ (F2) is used only for calculating R-factors (gt) etc., and is not relevant to the choice of reflections for refinement. Rfactors based on F2 are statistically about twice as large as those based on F, and R-factors based on all data will be even larger.
3.2. Geometry Details
All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion
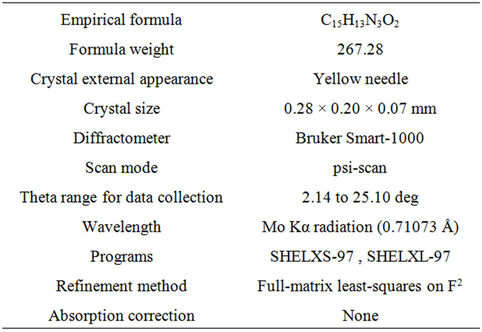
Table 1. Data collection and handling.
angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes.
3.3. Structure Details
The structure was solved by direct methods [14], then refined by full-matrix least-squares technique, the position and anisotropic parameters of all the non-hydrogen atoms were obtained. Table 2 showed some crystal and refinement parameters. The crystal system of the title compound belongs to Triclinic and the space group is P-1, with cell parameters a = 8.607(3)Å, b = 8.616(3)Å, c = 10.540(3)Å. Atomic coordinates and displacement parameters of the compound were shown in Table 3, from these data the structure was gained, which was shown in Figure 1. This compound is composed of two phenyl moieties, two methyl groups, a cyano group, an amino group and a nitro group. In the crystal, the methyl groups, cyano group and amino group are nearly coplanar with the connected benzene ring (C7-C12; A). Because of the large volume, the nitro group and the benzene ring A are twisted, the torsion angles are −80.2(6)˚ (O1-N3-C8-C7) and 99.3(5)˚ (O2-N3-C8-C7). To avoid steric conflicts, the two benzene rings are not coplanar, the dihedral angle between them is 83.51 (0.13)˚. Figure 2 showed packing diagram of the title compound. Hydrogen bonds are shown as dashed line.There are two kinds of hydrogen bonds in the crystal, N—H···N and C—H···O. In the crystal, Crosslinks among the molecules are provided by the hydrogen bonds (Figure 2), leading to one-dimensional supermolecular structure.
4. Conclusion
A kind of polysubstituted benzene, C15H13N3O2, was synthesized and its crystal structure was determined. In the crystal, methyl groups, cyano group and amino group are nearly coplanar with the connected benzene ring.
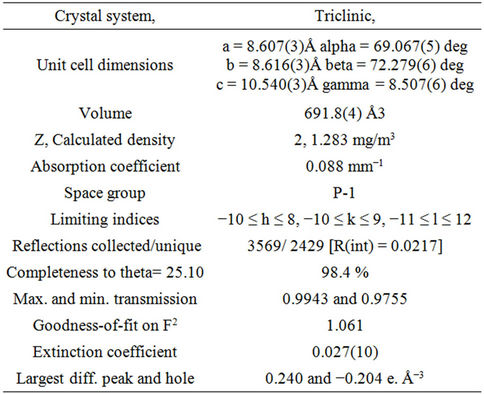
Table 2. Crystal and refinement parameters.
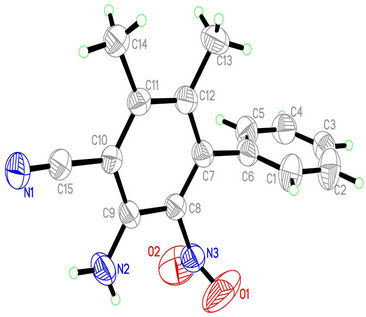
Figure 1. The molecular structure of the compound.
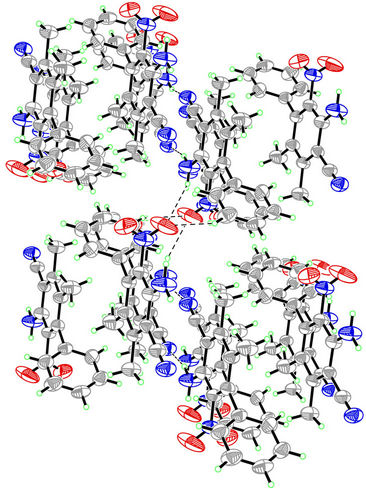
Figure 2. The packing diagram of the compound. Intermolecular hydrogen bonds are shown as dashed line.
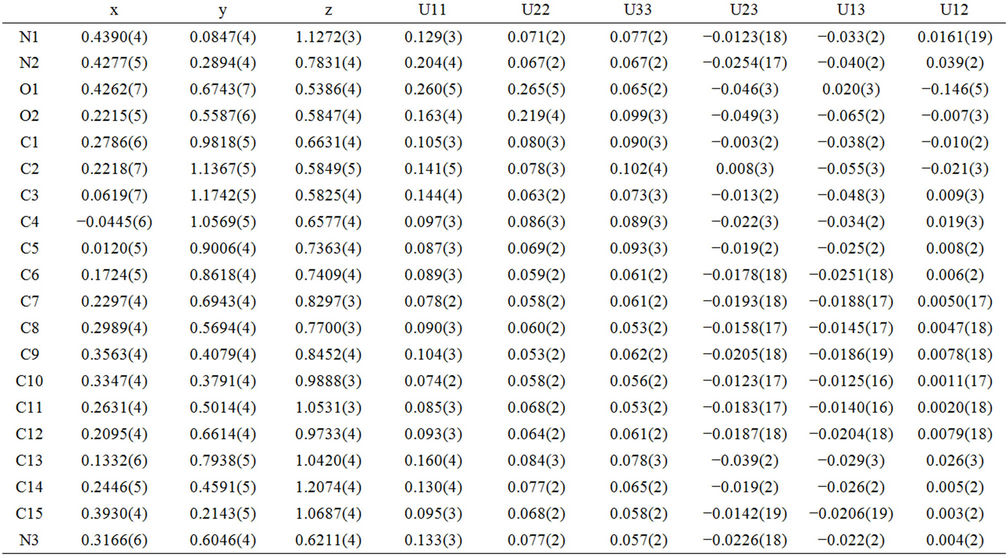
Table 3. Atomic coordinates and displacement parameters (Å2).
Because of the large volume, the nitro group and the connected benzene ring are twisted. The dihedral angle between the two benzene rings is 83.51˚. In the crystal, molecules are linked by N—H···N and C—H···O hydrogen bonds.
REFERENCES
- D. Astruc, “Modern Arene Chemistry,” Wiley-VCH., Weinheim, 2002. doi:10.1002/3527601767
- G. Yeap, S. Balamurugan and S. Rakesh, “Synthesis and Mesomorphic Properties of Chiral Liquid Crystal Dimers Derived from Azobenzene and Substituted Naphthol,” Liquid Crystals, Vol. 40, No. 4, 2013, pp. 555-563. doi:10.1080/02678292.2013.765608
- G. Mao, J. Wang, R. S. Clingman and C. K. Ober, “Molecular Design, Synthesis, and Characterization of Liquid Crystal-Coil Diblock Copolymers with Azobenzene Side Groups,” Macromolecules, Vol. 30, No. 9, 1997, pp. 2556-2567. doi:10.1021/ma9617835
- S. Luo, J. Xiong and Z. Wang, “Design and Synthesis of 2(5H)-Furanone Liquid-Crystal Compounds Based on Natural Molecules and Biphenyl Derivatives,” Research on Chemical Intermediates, Vol. 39, No. 4, 2013, pp. 1865-1876. doi:10.1007/s11164-012-0721-8
- D. Pauluth and K. Tarumi, “Advanced Liquid Crystals for Television,” Journal of Materials Chemistry, Vol. 14, No. 8, 2004, pp. 1219-1227. doi:10.1039/b400135b
- H. S. Bhatt, P. D. Patel and J. S. Dave, “Synthesis of Liquid Crystals with Lateral Substituents in Central and Terminal Benzene Cores and Their Mesomorphic Characterization,” Molecular Crystals and Liquid Crystals, Vol. 575, 2013, pp. 104-11. doi:10.1080/15421406.2013.768497
- O. Lavastre, I. Illitchev, G. Jegou and P. H. Dixneuf, “Discovery of New Fluorescent Materials from Fast Synthesis and Screening of Conjugated Polymers,” Journal of American Chemistry Society, Vol. 124, No. 19, 2002, pp. 5278-5279. doi:10.1021/ja025764o
- C. W. Lee, K. S. Yook and J. Y. Lee, “Synthesis and Device Application of Hybrid Host Materials of Carbazole and Benzofuran for High Efficiency Solution Processed Blue Phosphorescent Organic Light-Emitting Diodes,” Organic Electronics, Vol. 14, No. 3, 2013, pp. 1009-1014. doi:10.1016/j.orgel.2013.01.025
- X. Q. Lin, B. J. Chen, X. H. Zhang, C. S. Lee, H. L. Kwong, et al., “A Novel Yellow Fluorescent Dopant for High-Performance Organic Electroluminescent Devices,” Chemistry of Materials, Vol. 13, No. 2, 2001, pp. 456- 458. doi:10.1021/cm0004679
- J. Sun, H. Jiang and J. Zhang, Y. Tao and R. Chen, “Synthesis and Characterization of Heteroatom Substituted Carbazole Derivatives: Potential Host Materials for Phosphorescent Organic Light-Emitting Diodes,” New Journal of Chemistry, Vol. 37, No. 4, 2013, pp. 977-985. doi:10.1039/c2nj40900c
- T. Verdelet, G. Mercey, N. Correa, L. Jean and P. Y. Renard, “Straightforward and Efficient Synthesis of 3-Benzyloxy-4-bromopicolinate ester and 3-Benzyloxy-5- bromopicolinate Ester, Common Building Blocks for Pharmaceuticals and Agrochemicals,” Tetrahedron, Vol. 67, No. 45, 2011, pp. 8757-8762. doi:10.1016/j.tet.2011.09.024
- C. J. C. Connolly, J. M. Hamby, M. C. Schroeder, M. Barviana, G. H. Lub, et al., “Discovery and Structure-activity Studies of a Novel Series of Pyrido [2,3-d] Pyrimidine Tyrosine Kinase Inhibitors,” Bioorganic & Medicinal Chemistry Letters, Vol. 7, No. 18, 1997, pp. 2415- 2420. doi:10.1016/S0960-894X(97)00445-9
- X. Zhang, G. C. G Paisa, E. S. Svarovskaiab, C. Marchandc, A. A. Johnson, et al., “Azido-Containing Aryl β-Diketo Acid HIV-1 Integrase Inhibitors,” Bioorganic & Medicinal Chemistry Letters, Vol. 13, No. 6, 2003, pp. 1215-1219. doi:10.1016/S0960-894X(03)00059-3
- G. M. Sheldrick, “SHELXL97. Program for the Refinement of Crystal Structures,” University of Göttingen, Germany, 1997.

