Modern Research in Catalysis
Vol.2 No.2A(2013), Article ID:32934,6 pages DOI:10.4236/mrc.2013.22A004
One-Pot Synthesis of Dimethyl Carbonate over Basic Zeolite Catalysts
Key Laboratory of New Fiber Materials and Modern Textile, School of Chemistry, Chemical Engineering and Environments, Qingdao University, Qingdao, China
Email: *jianqyu@qdu.edu.cn
Copyright © 2013 Wenshuai Xu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 1, 2013; revised May 2, 2013; accepted May 26, 2013
Keywords: Dimethyl Carbonate; Methanol; Zeolite; Basic Catalysts; One-Pot Synthesis
ABSTRACT
One-pot synthesis of dimethyl carbonate (DMC) from methanol, propylene oxide (PO) and carbon dioxide has been investigated using the basic zeolites as catalysts. Among the zeolites studied, Beta showed the best catalytic performance for DMC production. That the desilication of zeolite structure resulted in a hierarchical porosity of Beta, leading to more amount of KOH can be loaded on the surface of zeolite and therefore enhancing the base strength of the catalyst was proposed to be the reason for improved catalytic performance.
1. Introduction
Dimethyl carbonate (DMC) has attracted great interests as an important green chemical. Due to the molecular structure of DMC contains several active groups, e.g. methyl, carbonyl and methoxyl, it can be used to replace phosgene, dimethyl sulfate and methyl chloroformate in many chemical reactions, such as methylation, carbonylation and ester interchange reaction [1,2]. Moreover, DMC can be used in industry as food additive, electronic chemical and green solvent due to its low toxicity, good solubility and environmental benign property. DMC has high oxygen content (53%) and may improve the octane number of gasoline, so it is also regarded as a potential additive for gasoline to replace methyl tert-butyl ether (MTBE) as an octane value promotion agent [3-5].
So far, various routes, such as the phosgenation of methanol, the oxidative carbonylation of methanol, alcoholysis of urea or the transesterification between methanol and cyclic carbonate, and so on had been explored to synthesize DMC. The phosgenation of methanol is a traditional route for DMC synthesis, however, this process takes use of phosgene, an intense toxic substance as the main reactant, and also coproduces hydrochloric acid, which results in a serious erosion of the equipments and environment demolishment [1]. The route of oxidative carbonylation of methanol with carbon monoxide and oxygen may be a better route for the synthesis of DMC, owing to its low cost of raw materials. However, it is dangerous and potentially explosive due to the utilizations of carbon monoxide and oxygen mixture as starting materials [6]. Synthesis of DMC by alcoholysis of urea has many advantages such as low price of feedstock and mild conditions. However, this process is limited by the chemical equilibrium [7]. The transesterification method can be divided into two-step route and one-step route. The former two-step route, which has been industrialized, is a friendly process to environment, and the yield of DMC is more skillful than that of others process [8,9]. For one-step route, the methanol, carbon dioxide and epoxide were introduced simultaneously into a reactor, by the catalysis of a base catalyst under a certain condition, DMC will be synthesized. This one-pot synthesis of DMC has received more and more attentions, because it is an environmental-friendly process, in which the carbon dioxide, the chief offender of greenhouse effect is utilized [10].
Up to now, many kinds of catalysts, such as KOH/4A [11], KI/K2CO3 [12], MgO [13], n-Bu4NBr/n-Bu3N [14] and Ionic liquids [15], had been explored for the one-pot synthesis of DMC. Zeolites are a group of special minerals with a well-defined microporous crystalline structure and a three dimensional four-connected aluminosilicate anionic framework. The basic structural building units of a zeolite framework are comprised of TO4 (T = Si, Al, or P) tetrahedra through corner sharing. The substitution of Al3+ for Si4+ in the framework results in a residual negative charge on the framework. The space in the cages and channels are filled with water molecules. A wide variety of cations, such as Na+, K+, Ca2+, Mg2+ and others are float in the aqueous environment to balance the negative charge of the aluminosilicate framework. These positive ions are rather loosely held and can be readily exchanged for others in a contact solution. Zeolites can be either acidic or basic depending on the exchangeable cations that are used to balance the charges. A proton-substituted zeolite will be acidic, while an alkali metal ion exchanged zeolite will be basic.
In this paper, solid base catalysts were prepared by loading strong base KOH onto zeolites with various structures. The one-pot catalytic conversion of DMC over these basic zeolites was investigated. It was found that the base strength is the main factors to influence the yield of DMC and the conversion of propane epoxide.
2. Experiments
2.1. Preparation of Catalyst
All the basic zeolites catalysts were prepared by the reaction of KOH with the protonic form of zeolites. The protonic form of zeolites, e.g. H-Beta, was obtained by ionexchange method from Na-Beta (purchased comercially) as following: Na-Beta was added into a 1.0 mol·L−1 of NH4NO3 aqueous solution and stirred at 80˚C for 3 h. This process was repeated for three times. The products were obtained by filtration, washed with distilled water and dried at 100˚C for 3 h. Finally, the products were calcined at 550˚C for 5h and used as supports.
For the catalysts preparation, KOH was used as active ingredient, while H-Beta, 4A, SAPO-18, ZSM-5 and NaX were used as supports. Measured amount of KOH was dissolved into distilled water and the support was added under stirring. The slurry was kept in static state for 24 h and dried at 100˚C for 12 h. The dried samples were calcined at 600˚C for 5h under N2 atmospheric condition.
2.2. The Catalytic Performances
The one-pot synthesis of DMC from methanol, carbon dioxide and PO was carried out in a 50 ml stainless steel autoclave equipped with a temperature controlling system and a magnetic stirrer. For each reaction, methanol, PO and catalyst were charged into the drying reactor. The oxygen in the reactor was eliminated by filling nitrogen for 3 times. Then, carbon dioxide was filled into the reactor to a certain pressure. The reactor was heated to the reaction temperature under stirring. After the reaction was finished, the reactor was quenched to room temperature by tap water and the products were recovered with centrifugation. Then reaction products were analyzed by GC-5890A (Nanjing, China) equipped with an FFAP capillary column and an FID detector. The selectivity of products was calculated on the basis of propane epoxide.
3. Results and Discussions
3.1. Effect of the Base Strength of Catalysts on the Catalytic Performance
For many catalytic reactions over supported catalysts, the activity, selectivity and lifetime are strongly influenced by the carrier type, preparation approach, and so on. Therefore, first of all, the effects of zeolite structure on the catalytic performances were investigated. Zeolites with various silica/alumina (Si/Al) molar ratio and porous structure were selected as supports, and KOH was selective as active ingredient, because the alkalinity of KOH is higher than NaOH and other alkalines. The catalytic results for one-pot synthesis of dimethyl carbonate from epoxide, methanol and carbon dioxide are listed in Table 1. From the results, it can be found that all the five catalysts with different zeolite support show good reaction activity for the one-pot synthesis of DMC. However, great differences in the conversion of PO and selectivity of DMC can be observed. When zeolite Beta was used as support, the conversion of PO reaches to a high level of 91.3%, which is much better than that over other supports. When zeolites ZSM-5 and 4A were selected as carrier, the conversions of PO are dramatically decreased, while the selectivity of DMC over KOH/4A and KOH/ ZSM-5 is higher than that of others, reaching to 28%.
For the reaction of DMC synthesized directly from methanol and carbon dioxide, it has been found that both the conversion of carbon dioxide and the yield of DMC are very low. However, the introduction of PO may enhance the activity of carbon dioxide. Scheme 1 presents the proposed reaction routes and the formatted products for this one-pot synthesis of DMC. The target product dimethyl carbonate can be synthesized via Route I. This is a sequential reaction of PO with carbon dioxide to form propenyl carbonate (PC), and transesterification to
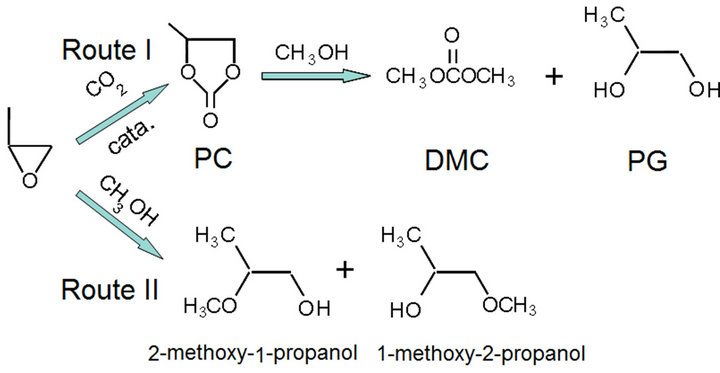
Scheme 1. The proposed reaction routes and the formatted products for this one-pot synthesis of DMC.
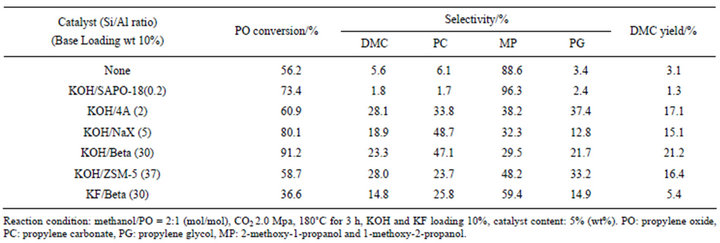
Table 1. Synthesis of DMC from methanol, PO and CO2 by KOH loaded on various molecular sieves.
dimethyl carbonate. Route II exhibits the formation of byproducts, 2-methoxy-1-propanol and 1-methoxy-2-propanol, which were generated by the direct alcoholysis of propylene epoxide. For Route I, the alkaline catalysts and metal compounds may be effective for the reaction to PC and the further transesterification reaction to DMC [16]. However, the above catalytic results over the investigated basic zeolite catalysts demonstrated that the strong base is of beneficial for the reaction of propylene epoxide with CO2 to PC, while it is not necessary to have strong basic site for the transesterification from PC to DMC. From Table 1 it can be observed that the selectivities to the byproducts over KOH/4A, KOH/NaX and KOH/Beta are relatively low, implying that more CO2 can be adsorbed and activated on the catalysts, which resulting in more propylene oxide is converted via Route I. As a comparison, the catalytic property over KF/Beta, which is a weak base catalyst due to the relative weak basicity of KF, was investigated. It can be found that the conversion of PO and the selectivity toward DMC are relative low. On the contrary, the formation of byproducts via Route II is the dominant reaction. These observations confirm once again the above mechanism on the other hand.
Zeolite molecular sieves are crystalline, highly porous aluminosilicates materials, which are characterized by a three-dimensional pore system [17]. The tetrahedras of [AlO4] and [SiO4] are the basic building blocks and form the framework of various zeolite structures. In terms of the framework stability of zeolites, ZSM-5, 4A, X and so on are stable and relatively difficult to be destroyed by strong base or acid, because the pores of these zeolites are much regular with precisely defined diameter. However, Beta molecular sieve is a highly faulted intergrowth of two distinct but closely related structures and both have fully three-dimensional pore system with 12-rings channel ((6.6 Ǻ × 6.7 Ǻ) × (5.6 Ǻ × 5.6 Ǻ)). Such kind of structure is easier to be changed by desilication or dealumination.
The textural properties of H-Beta, 10% KOH/Beta, 10% KOH/4A, 10% KOH/NaX and 10% KOH/ZSM-5 are summarized in Table 2. It can be found that the BET surface area and pore size of KOH loaded Beta zeolite have a great deal variation with comparison to its precursor H-Beta after the loading of KOH. The above data of BET surface area and average pore diameter demonstrated that the framework of Beta zeolite has been partly destroyed by the loading of KOH. However, just due to the collapse of its framework, more amount of base may be loaded on the Beta zeolite, which thus offered a beneficial effect on the conversion of PO. Moreover, by loading KOH, the desilication of the framework Si might result in the generation of a hierarchical porosity of zeolite Beta. The superior activity and selectivity exhibited by this sample has been related to the presence of the hierarchical porosity, which decreases the steric and diffusional hindrances, favouring the accessibility to the active sites and allowing the occurrence of the tranesterification reaction.
3.2. Effect of Alkali Content of Catalysts on the Catalytic Performance
To find out the effect of the base strength on the catalytic properties of the synthesis reaction, various amount of KOH loaded Beta zeolite catalysts were prepared. The results of the catalytic reaction are listed in Table 3. It shows that 82.1% of PO is converted, while 26.1% of which was selective transformed to DMC over 5% KOH/Beta. When the percentage of KOH loading becomes to 10%, a superior catalytic activity were observed. This result may also be interpreted due to the enhanced textural properties of 10% KOH/Beta. In this case, the bulky nature of both substrate and products may cause the existence of diffusional problems inside the zeolitic channels, which are attenuated in this Beta catalyst due to the presence of the hierarchical porosity. A further improvement in the loading amount of KOH also in-
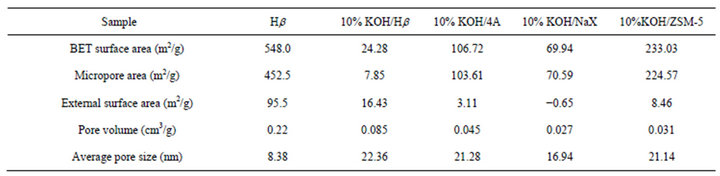
Table 2. The textural properties of the prepared basic zeolite catalysts.
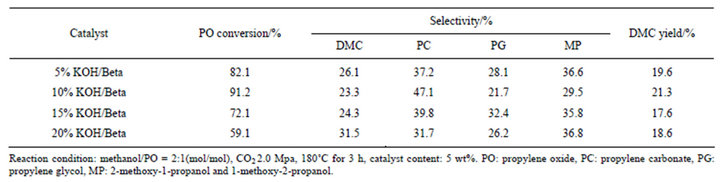
Table 3. Synthesis of DMC from methanol, PO and CO2 over various KOH percentage of KOH/Beta as catalysts.
creases the selectivity of DMC. However, the activity of PO decreased on the contrary, suggesting that even though the base content is of beneficial for the improveement of DMC selectivity, an excessive addition of KOH may resulted in the damage of zeolite framework.
Figure 1 compares the XRD spectra of H-Beta and KOH/Beta catalysts with various contents of KOH. It can be found that the typical diffraction peaks of H-Beta are intensive, showing the high crystallinity of the precursor zeolite. When 5 wt% of KOH was loaded on Beta, the peaks intensities were not reduced greatly, suggesting that the framework was not destroyed by lower content of strong base. Further improved the content of KOH to 10 wt%, the peaks become less intense and broader compared to those corresponding to the former two zeolite samples, demonstrating that the 10% KOH/Beta presents smaller crystalline domains.
When the zeolite H-Beta is treated by alkali liquor, the desilication process may appear in its framework. As a result, the defects are introduced into the framework and lead to the formation of hierarchical porosity in the framework of zeolite Beta. An extension of this phenomenon is the decrease of crystallinity. As the concentration of KOH reaches a certain extent, the desilication was much more serious, even resulted in the framework collapse of Beta zeolite. When the amount of KOH increases to 15%, the framework of KOH-Beta catalyst has collapsed absolutely to an amorphous phase. Therefore, the alkali contents of the basic zeolite catalysts should be controlled in an optimum value.
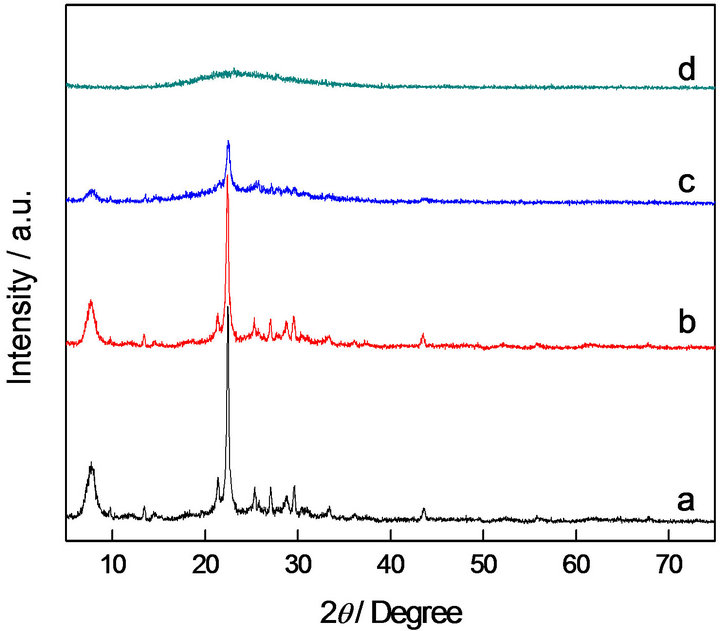
Figure 1. XRD patterns of H-Beta and KOH/Beta catalysts with different contents of KOH. (a) H-Beta; (b) 5% KOH/ Beta; (c) 10% KOH/Beta; (d) 15% KOH/Beta.
3.3. The Dependence of Catalytic Properties on the Reaction Conditions
Figure 2 shows the dependence of conversion of PO and selectivity to DMC and PC on the reaction temperature over 10% KOH/Beta catalyst. It can be seen from the results that both the conversion of PO and the yield of DMC and PC increased as the reaction temperature improved from 413 K to 453 K. However, the yield of PC and DMC decreases as the temperature improved further from 453 K to 473 K. These observations suggested that the optimum temperature for the one-pot synthesis of DMC over 10% KOH/Beta catalyst is around 453 K. As we have known, temperature plays an extremely important role for a catalytic reaction. For one-pot synthesis of DMC, it can be assumed that the reaction proceed as two sequential reactions: cycloaddition and transesterification. The former cycloaddition reaction is an exothermic reaction and low temperature favors for its proceeding, while the latter transesterification reaction is an endothermic reaction and high temperature is favourable. Therefore, allowing the reaction to proceed at an optimum temperature, the best results of the conversions of the reactants and the selectivity to products will be obtained.
Figure 3 shows the dependences of conversion of PO, selectivity to DMC and PC on methanol/PO molar ratio. It can be observed that the conversion of PO and selectivity of DMC increases as the molar ratio of methanol/ PO varied from 0.5 to 2.0, while that of PC decreases.
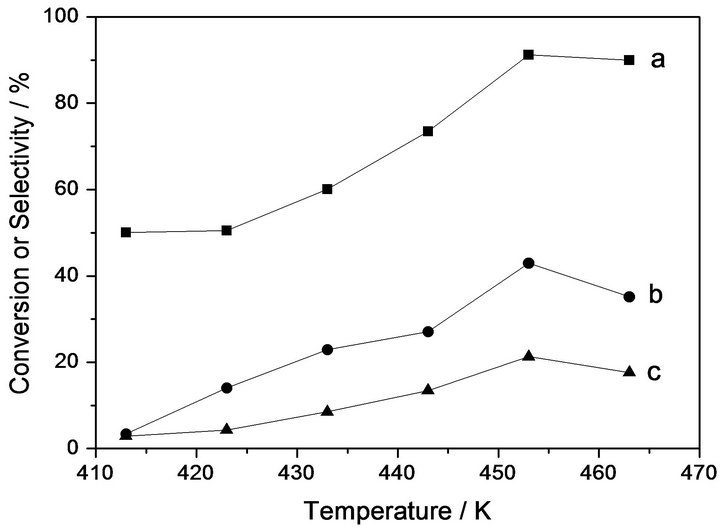
Figure 2. Dependence of the catalytic properties on the reaction temperature over 10%KOH/Beta catalyst. Reaction condition: CO2 2.0 Mpa, methanol/PO molar ratio = 2.0, catalyst content 5 wt%. (a) Conversion of PO; (b) Selectivity of PC; (c) Selectivity of DMC.
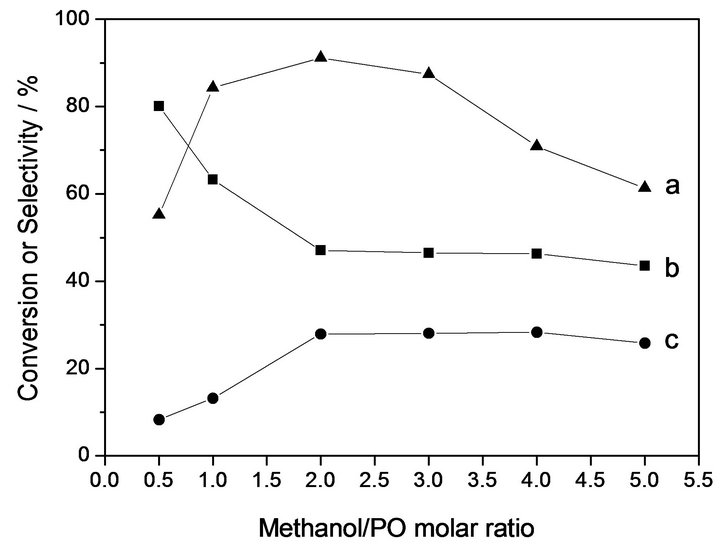
Figure 3. Dependence of the catalytic properties on the methanol/PO molar ratio over 10% KOH/Beta catalyst. Reaction condition: CO2 2.0 Mpa, 453 K for 3h, catalyst content 5 wt%. (a) Conversion of PO; (b) Selectivity of DMC; (c) Selectivity of PC.
When the molar ratio of methanol/PO becomes higher than 2.0, the selectivity of PC and DMC have no great deal of variations. These observations demonstrated that the appropriate methanol/PO molar ratio is around 2.0.
4. Conclusions
For the one-pot synthesis of DMC, the basic zeolite catalysts prepared by loading KOH on zeolite molecular sieves showed good reaction properties. Both the base strength and the alkali content of molecular sieve have great impact on the catalytic performance of catalysts. Stronger base strength and higher alkali content are of beneficial for the yield improvement of DMC. Zeolite Beta has been explored to be an optimum support for the catalyst preparation for one-pot synthesis of DMC, because the highly faulted intergrowth of framework can be modified to a catalyst with hierarchical porosity.
REFERENCES
- Y. Ono, “Catalysis in the Production and Reactions of Dimethyl Carbonate, an Environmentally Benign Building Block,” Applied Catalysis A: General, Vol. 155, No. 2, 1997, pp. 133-166. doi:10.1016/S0926-860X(96)00402-4
- N. Keller, G. Rembmann and V. Keller, “Catalysts, Mechanisms and Industrial Processes for the Dimethyl Carbonate Synthesis,” Journal of Molecular Catalysis A: Chemical, Vol. 317, No. 1-2, 2010, pp. 1-18. doi:10.1016/j.molcata.2009.10.027
- A. G. Shaikh and S. Sivaram, “Organic Carbonates,” Chemical Reviews, Vol. 96, No. 3, 1996, pp. 951-976. doi:10.1021/cr950067i
- M. A. Pacheco and C. L. Marshall, “Review of Dimethyl Carbonate (DMC) Manufacture and Its Characteristics as a Fuel Additive,” Energy Fuels, Vol. 11, No. 1, 1997, pp. 2-29. doi:10.1021/ef9600974
- T. Wei, M. H. Wang, W. Wei, Y. H. Sun and B. Zhong, “Synthesis of Dimethyl Carbonate by Transesterification over CaO/Carbon Composites,” Green Chemistry, Vol. 5, No. 3, 2003, pp. 343-346. doi:10.1039/b210716n
- U. Romano, “Dimethyl Carbonate and Its Production Technology,” Chimica e l’Industria, Vol. 75, No. 4, 1993, pp. 303-306.
- M. H. Wang, H. Wang, N. Zhao, W. Wei and Y. H. Sun, “High-Yield Synthesis of Dimethyl Carbonate from Urea and Methanol Using a Catalytic Distillation Process,” Industrial & Engineering Chemistry Research, Vol. 46, No. 9, 2007, pp. 2683-2687. doi:10.1021/ie061101u
- M. Mikkelsen, M. Jorgensen and F. C. Krebs, “The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide,” Energy & Environmental Science, Vol. 3, No. 1, 2010, pp. 43-81. doi:10.1039/b912904a
- T. Sakakura, J. C. Choi and H. Yasuda, “Transformation of Carbon Dioxide,” Chemical Reviews, Vol. 107, No. 6, 2007, pp. 2365-2387. doi:10.1021/cr068357u
- S. Inoue and H. Koinuma, “Catalytic Fixation of Carbon Dioxide by Metal Complexes,” Reviews in Inorganic Chemistry, Vol. 6, No. 4, 1984, pp. 291-355.
- Y. Li, X. Q. Zhao and Y. J. Wang, “Synthesis of Dimethyl Carbonate from Methanol, Propylene Oxide and Carbon Dioxide over KOH/4A Molecular Sieve Catalyst,” Applied Catalysis A: General, Vol. 279, No. 1-2, 2005, pp. 205-208. doi:10.1016/j.apcata.2004.10.030
- H. Y. Cui, T. Wang, F. J. Wang, C. R. Gu and P. L. Wang, “One-Pot Synthesis of Dimethyl Carbonate Using Ethylene Oxide, Methanol, and Carbon Dioxide under Supercritical Conditions,” Industrial & Engineering Chemistry Research, Vol. 42, No. 17, 2003, pp. 3865-3870. doi:10.1021/ie021014b
- B. M. Bhanage, S. Fujita, Y. Ikushima, K. Torii and M. Arai, “Synthesis of Dimethyl Carbonate and Glycols from Carbon Dioxide, Epoxides and Methanol Using Heterogeneous Mg Containing Smectite Catalysts: Effect of Reaction Variables on Activity and Selectivity Performance,” Green Chemistry, Vol. 5, No. 1, 2003, pp. 71-75. doi:10.1039/b207750g
- J. S. Tian, J. Q. Wang, J. Y. Chen, J. G. Fan, F. Cai and L. N. He, “One-Pot Synthesis of Dimethyl Carbonate Catalyzed by n-Bu4NBr/n-Bu3N from Methanol, Epoxides, and Supercritical CO2,” Applied Catalysis A: General, Vol. 301, No. 2, 2006, pp. 215-221. doi:10.1016/j.apcata.2005.12.002
- J. Li, L. G. Wang, F. Shi, S. M. Liu, Y. D. He, L. J. Lu and X. Y. Ma, “Quaternary Ammonium Ionic Liquids as Bifunctional Catalysts for One-Step Synthesis of Dimethyl Carbonate from Ethylene Oxide, Carbon Dioxide and Methanol,” Catalysis Letters, Vol. 141, No. 2, 2011, pp. 339-346. doi:10.1007/s10562-010-0498-6
- T. Sakakura, J. C. Choi, Y. Saito and T. Sako, “Synthesis of Dimethyl Carbonate from Carbon Dioxide: Catalysis and Mechanism,” Polyhedron, Vol. 19, No. 5, 2000, pp. 573-576. doi:10.1016/S0277-5387(99)00411-8
- R. Xu, W. Pang, et al., “Chemistry—Zeolite and Porous Materials (21st Century SP’s Series in Chemistry),” Science Press, Beijing, 2004.
NOTES
*Corresponding author.

