Modern Research in Catalysis
Vol.02 No.04(2013), Article ID:38768,9 pages
10.4236/mrc.2013.24020
An Efficient Activated Carbon for the Wastewater Treatment, Prepared from Peanut Shell
Mohammad Sadiq, Sajid Hussian
Department of Chemistry,
Email: mohammad_sadiq26@yahoo.com, sadiq@uom.edu.pk
Copyright © 2013 Mohammad Sadiq, Sajid Hussian. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received December 26, 2012; revised March 8, 2013; accepted May 5, 2013
ABSTRACT
Peanut shell was converted into activated carbon and its surface was then modified through impregnation with KOH. The activated carbon was characterized by BET surface area with pore size analyzer, EDX, Tap and apparent density, pH, PZC and titratable surface functional groups. The activated carbon was used for the removal of pyridine from arti- ficially contaminated aqueous solutions. Adsorption studies were carried out in a batch system by considering the effect of various parameters like contact time, initial concentration of adsorbate, pH of solution and temperature. Langmuir and Freundlich models were applied to experimental data. The results show that the Langmuir adsorption isotherm model gives better fit as compared to Freundlich model.
Keywords:
Peanut Shell; Activated Carbon; Adsorption; Pyridine
1. Introduction
The increasing contamination of environment by waste materials as a result of human activities poses a continu- ously growing and serious problem. If these waste mate- rials are used for preparing adsorbents, it will be used in overcoming the contamination of environment and waste materials disposal. Materials having high carbon and low inorganic contents such as wood, peanut shells, rice husk, wheat straw, corn cob, waste cellulosic material, etc., can be used as raw materials for the preparation of activated carbon [1-11]. The raw material can be converted into activated carbon by physical and/or chemical activation. In chemical activation, an activating agent such as KOH, ZnCl2 and H3PO4, etc., is used. The raw material can be treated with activating agent before or after pyrolysis.
Pyridine is a colorless liquid with unpleasant odor and is toxic. It is either derived from coal tar or manufactured by chemical synthesis [12] and is widely used as a sol- vent and an intermediate in the production of piperidine, agricultural chemicals, drugs, dyestuffs and paints, rub- ber products, polycarbonate resins and textile water-re- pellents, and in laboratories [13]. It is a major component in the basic fraction of oil shale retort waters. Pharma- ceuticals agents such as isoniazid, ceptylpyridinium bro- mide, analgesic dermal and cephalexin are manufactured using pyridine as a catalyst. Therefore, increasing amounts
of pyridine containing wastewater are let as effluent by various industries. Many of the pyridine compounds are hazardous and persist for long periods in the environment. The removal of pyridine from wastewater is therefore of great importance. The methods used for the removal of pyridine from water/wastewater include biodegradation [14-17], adsorption [18-20], adsorption and electro-sorp- tion [12], ozonation [21], and ion exchange [18]. Among these adsorption systems, those using activated carbons prepared from agricultural waste material are rapidly gaining importance due to their economic feasibility [22- 24].
The present work deals with the preparation of acti- vated carbon from peanut shell with KOH activation, un- der oxidative atmosphere in air, and non-oxidative at- mosphere (under nitrogen), at moderate temperature and compares the efficiency of the prepared samples for the removal of pyridine from aqueous solution. The influ- ence of several operating parameters, such as pH, contact time, temperature and initial concentration of the adsor- bent on the adsorption capacity was investigated.
2. Experimental
2.1. Raw Material, Chemicals and Impregnating Agent
Peanut shells were collected from the local market. All other chemicals used were AR-grade chemicals and were used without further purification. The impregnating agent used for the chemical activation of peanut shells was KOH.
2.2. Procedure
Peanut shells were washed, dried, ground and mixed with 10 weight % KOH solution (6:100). The sample was then transferred to a series of 100 mL conical flasks and kept in ultrasonic cleaner for ten minutes at 30˚C. The sample was then shaken on a shaker bath for six hours at 50˚C. At the end of impregnation process, the sample was im- mediately filtered, washed with hot double distilled water for removal of its alkalinity and water soluble compo- nents, and dried.
2.3. Carbonization
The impregnated sample was heated at 170˚C (±5˚C) in a tube furnace in quartz reactor for one hour under nitrogen flow (flow rate 100 mL/minute). The temperature was then increased to 450˚C (±5˚C) in the same atmosphere for one hour and then lowered down to room temperature in nitrogen flow/breathing grade air. The cooled sample was washed in modified Soxhlet apparatus, dried and sieved by
2.4. Characterization of Sample
The pH of the prepared activated carbon was determined by standard reported method [24]. To measure PZC of the carbon samples,
2.5. Sorption Procedure
Stock solutions of the test reagents were made by dis- solving pyridine in double distilled water. The pH of the test solutions was adjusted using either dilute HCl (
 (1)
(1)
Where C0 and Ce are the initial and equilibrium con- centrations (mg/L) of pyridine in solution, V is the vol- ume and W is the weight adsorbent in grams. Same pro- cedure was adopted for other temperatures.
For kinetic studies known amounts of sample were taken in a number of flasks (100 mL capacities), each containing 40 mL of solution of pyridine, and were pla- ced on a shaker. A known amount of activated carbon was added to each flask and adjusted to the desired pH and temperature. The solutions were then agitated. At pre-determined intervals of time, the solutions of speci- fied flasks were separated from the activated carbon and analyzed for the uptake of pyridine at the corresponding λmax. The same procedure was adopted for different con- centrations. The pyridine concentration retained in the adsorbent phase was calculated by using Equation (1).
3. Results and Discussion
3.1. pH, PZC, Tap Density and Apparent Density
Table 1 shows the pH values of different carbon samples. The pH value of oxidized carbon was greater than the un- oxidized carbon sample. This means that oxidized carbon was more basic than the un-oxidized carbon which may be due to the presence of more basic oxygen surface functional groups on the oxidized carbon.
Table 1 shows the PZC of carbons (oxidized and un-oxidized) respectively, determined by salt addition method. The PZC value of oxidized and un-oxidized car- bon was determined to be 8.9 and 8.3, respectively, which shows that PZC value of oxidized carbon was higher than that of the un-oxidized carbon. Table 1
shows the tap and apparent densities of both the oxidized and un-oxidized activated carbons. The tap densities of KOH treated carbons (oxidized and un-oxidized) were smaller than their apparent densities. The volume meas- ured by tapping of both un-oxidized and oxidized car- bons includes the void/packing volume while the appar- ent volume did not include the void/packing volume. The tap density of un-oxidized carbon was greater than that of the oxidized carbon while the apparent densities of un- oxidized carbon were smaller than that of the oxidized carbon. The decrease in the tap density of oxidized car- bon might be due to an increase in void packing volume, and an increase in the apparent density might be due to the enlargement of pores and/or sintering of particles.
3.2. Titratable Surface Functional Groups, BET Surface Area and EDS
The surface oxides on a carbon can have acidic as well as basic properties [28-31]. The acidity of a given func- tional group depends on its chemical environment, i.e. the size and shape of the polyaromatic layers, the pre- sence and position of other constituents, and the charge of neighboring dissociated groups. Table 2 shows the content of functional groups on activated carbon samples as determined by Bohem’s titration. NaOH is a strong base in aqueous medium and has the ability to neutralize acids with strong or weak ionization potential. Therefore NaOH titration is a true acid-base reaction and measures the surface acidity attributed to carboxylic acids, phenols, lactones and other groups with acidic or ionizable hy- drogen, present on the surface of activated carbons. It is evident from (Table 2) that the KOH treated un-oxidized carbon has higher NaOH, NaHCO3, Na2CO3, C2H5ONa titratable surface groups than that of the oxidized carbon. It may be concluded that the air activation of the un-oxi- dized carbon decreases the number of acidic groups on the surface of the carbon.
Table 2 also shows the HCl titratable surface func- tional groups for the KOH treated carbons (oxidized and un-oxidized). HCl is a strong acid in aqueous medium
and has the ability to neutralize basic groups on the sur- face of activated carbon. It is clear from Table 2 that the KOH treated oxidized carbon has higher HCl titratable surface groups than that of the un-oxidized carbon which may be due to the air activation process. Table 3, sum- marizes the BET surface area SDR, SBJH, micro pore vol- ume and average pore width of KOH treated carbons (oxidized and un-oxidized). The BET surface area and pore volume of the oxidized carbons were greater than that of the un-oxidized carbons while the average pore width and pore diameter of the oxidized activated car- bons were smaller than that of the un-oxidized carbons. The SBJH and SDR were also larger for oxidized carbon than the un-oxidized carbon. It thus may be concluded that the air activation of un-oxidized carbon increases the BET surface area and pore volume by facilitating the evolution of volatile matter from the precursor.
Table 1 shows the EDS elemental analysis of different carbon samples. The major elements present in activated carbons samples were carbon and oxygen, and a small amount of potassium, which may be due to the process of activation with KOH. It is evident from Table 1, that the percentage of carbon in the oxidized carbon was greater than that in the un-oxidized carbon while the percentage of oxygen in the un-oxidized carbon was greater than that in the oxidized carbon. The elemental analysis can be compared with oxygen surface functional groups present on the surface of activated carbons.
3.3. Effect of Various Parameters on Adsorption
Pyridine behaves as a base (Pka = 5.23) due to the lone pair of electron on nitrogen atom which is readily avail- able for protonation. Pyridine removal was studied on KOH treated carbons as a function of pH at the initial
concentration of 50 mg/L and 80 mg/L, respectively, and the results are shown in Figure 1. It is clear from Figure 1 that the percent adsorption of pyridine on oxidized (KOH treated) sample increases with an increase in pHin from 2 to 4. After that there is a decrease in adsorption reaching a minimum at pH 7, and at pHin > 7 it becomes nearly constant. Therefore, in case of oxidized carbon, all the adsorption studies were carried out at the pH of 5. In case of the un-oxidized carbon, the removal of pyridine was found to increase from pH 2 to 5 and then decrease at pH ≥ 6. Hence all the subsequent studies were carried out at pH 6. Activated carbons are species with ampho- teric character, thus depending on the pH of the solution, their surface may be either positively or negatively charged [32]. The cell wall of activated carbon contains a large number of surface functional groups.
The pH dependence of pyridine adsorption can largely be related to the type and ionic state of these functional groups and to the adsorbate chemistry in the solution. At lower pH [pH ≤ (pka = 5.2)] the pyridine molecule is mostly converted to PyH+, resulting in the low adsorption of protonated pyridine on the positively charged carbon surface, while at higher pH [pH ≤ (Pka = 5.2)] pyridine molecule is un-protonated, resulting in the higher uptake on the charged activated carbons due to dispersion inter- action. [23]. These observations suggest that a significant amount of pyridine is adsorbed through van der Waals forces. It is also possible that small amount is adsorbed through the formation of protonated pyridine on the sur- face at lower pH. Thus it may be concluded that at higher pH electrostatic interactions also take place as pointed out by Radovic et al. [33].
The amount of pyridine adsorbed on KOH treated (oxidized and un-oxidized) carbons were studied as a function of shaking time at different initial concentra- tions of pyridine at 25˚C. Other parameters such as dose of adsorbent and pH of solution were kept constant. The
Figure 1. Effect of pHi on % removal of pyridine from aqueous solution at 25˚C (KOH treated carbon).
effects of contact time for the four different concentra- tions of pyridine (30, 40, 50, 60 mg/L) for KOH treated carbon were studied. For KOH treated carbon, the ad- sorption equilibrium was established with in 24 hours in case of oxidized carbon, and at 30 hrs in case of un-oxi- dized carbon. The removal of pyridine was found to be dependent on the initial concentration. The amount ad- sorbed increased with an increase in initial concentration. Furthermore, the adsorption is rapid in the early stages and then gradually decreases and finally becomes almost constant after the equilibrium point. At low concentra- tions the ratio of the available surface to the initial pyri- dine concentration is larger, so the removal is independ- ent of initial concentrations. However, in the case of higher concentrations this ratio is low; the percent re- moval thus depends upon the initial concentration. The curves also indicate that the adsorption leads to satura- tion, suggesting the possible monolayer coverage of pyridine on the surface of adsorbent [34]. In case of oxi- dized carbon the pyridine removal was decreased from 45.94% to 36.458% as the pyridine concentration was increased from 30 to 60 mg/L and the amount of pyridine adsorbed increased from 5.513 to 8.75 mg/g. while in case of un-oxidized carbon, the pyridine removal was decreased from 29.241% to 24.833% as the pyridine con- centration was increased from 30 to 60 mg/L and the amount of pyridine adsorbed increased from 3.509 to 5.06 mg/g.
3.4. Kinetics of Adsorption
Lagergren equation was applied to the experimental data (Figure 2) and first order kinetics was proposed for pyri- dine adsorption process [35-37].
Table 1. pH, PZC, densities and elemental analysis of KOH treated (oxidized and un-oxidized) carbons.
Table 2. Titratable surface functional groups of un-oxidized and oxidized activated carbons.
Table 3. Surface area and pore volume of activated carbon.
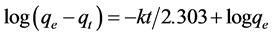 (2)
(2)
Figure 2. Lagergren plot for pyridine adsorption on un-oxi- dized/oxidized carbon (KOH treated) at 25˚C and different concentration of pyridine.
Where qe and qt are the amounts of pyridine adsorbed (mg/g) at equilibrium and at time t, respectively, and k is the rate constant of first order adsorption (h−1) given in Table 4.
Effect of Temperature
The adsorption of Pyridine on KOH treated carbons (oxi- dized and un-oxidized) was studied at different temperatures, and the results are shown by Figures 3 and 4 respectively. It is clear from these figures that the amount of pyridine adsorbed increases with a rise in temperature, which means that pyridine adsorption from aqueous so- lutions on both oxidized and un-oxidized carbons is an endothermic process. The enhancement of adsorption ca- pacity may be due to a chemical interaction between ad- sorbate and adsorbent, creation of some new adsorption sites, or an increased rate of intra particle diffusion into the pores of the adsorbate, at higher temperature [38,39]. To examine the relationship between sorbed (qe) and aqueous concentration (Ce) at equilibrium, sorption iso-
Table 4. Reaction rate constant (kh−1) values for pyridine adsorption on KOH treated carbons (oxidized and un-oxi- dized).
Figure 3. Isotherm study of pyridine adsorption on oxidized carbon at different temperatures.
Figure 4. Isotherm study of pyridine adsorption on un-oxi- dized carbon at different temperatures.
therm models are widely employed for fitting the data, of which Langmuir and Freundlich equations are most widely used. The Langmuir model assumes that uptake of adsorbate molecules occurs on a homogenous surface by monolayer adsorption without any interaction be- tween the adsorbed molecules. Frenundich model as- sumes heterogeneous adsorption due to the diversity of sorption sites or the diverse nature of adsorbate mole- cules adsorbed [34]. To get the equilibrium data, initial pyridine concentrations were varied while the adsorbent mass for each sample was kept constant. To ensure equi- librium conditions, the linear form of the Langmuir equa- tion was used.
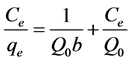 (3)
(3)
Where qe is the solid-phase adsorbate concentration at equilibrium (mg/g), Ce is the concentration of pyridine solution (mg/dm3) at equilibrium. The constant Q0 gives the theoretical monolayer adsorption capacity (mg/g). And b is related to the energy of adsorption (dm−3/mg). Straight lines were obtained by plotting Ce/qe against Ce as shown in Figures 5 and 6 for KOH treated carbons. The linear plot of Ce/qe against Ce indicates the applica- bility of Langmuir adsorption isotherm. Consequently suggesting the formation of monolayer coverage of the adsorbate on the surface of the adsorbent, Langmuir con- stants Q0 and b were calculated from the slopes and in- tercepts of plots of Ce/qe against Ce respectively and are given in Table 5.
It is observed from the Table 5 that the Q0 and b val- ues are larger for higher temperatures. The essential cha- racteristics of the Langmuir isotherm can be expressed by a dimensionless constant called equilibrium parameter RL [37]
Figure 5. Langmuir adsorption isotherms for pyridine adsorption on oxidized carbon at different temperatures.
Figure 6. Langmuir adsorption isotherms for pyridine ad- sorption on un-oxidized carbon at different temperatures.
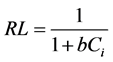 (4)
(4)
Where b is the Langmuir constant and Ci is the initial concentration (mg/g) and RL values indicate the type of isotherm. The values of RL at different temperatures and concentrations were found to be less than 1.0, and greater than 0 indicate the favorable adsorption of pyridine on KOH treated carbons (oxidized and unoxidized). The linear form of Freundlich isotherm as expressed by Equa- tion (5) was also applied to the adsorption data of pyri- dine.
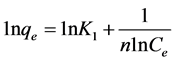 (5)
(5)
Where Kl (mg/g) and 1/n (g/L) are Freundlich con- stants, indicating the adsorption capacity and adsorption intensity, respectively. Straight lines were obtained by plotting lnqe against lnCe for KOH treated carbons which shows that adsorption of pyridine fitted into Freundlich
Table 5.Various Langmuir and thermodynamic parameters from Ce/q vs Ce plot of pyridine adsorption on KOH treated car- bon (un-oxidized/oxidized).
isotherm reasonably. The thermodynamic parameters such as ∆H˚ (enthalpy change) and ∆S˚ (entropy change) were calculated from the slopes and intercepts of the plots of lnb versus 1/T as shown in (Figure 7) for KOH treated carbons (oxidized and un-oxidized) respectively by using the following relation.
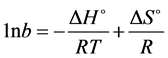 (6)
(6)
Where lnb was obtained from the Langmuir plots of Figures 5 and 6. The ∆G˚ (free energy change) was cal- culated from the following relation.
 (7)
(7)
The values of ∆H˚, ∆S˚, and ∆G˚ are given in Table 5. The value of ∆H˚ is positive in case of pyridine adsorp- tion on both oxidized and un-oxidized carbons, indicat- ing endothermic process.
The value of ∆G˚ is negative for pyridine adsorption on both oxidized and un-oxidized carbons, indicating spontaneous process of adsorption. The values of ∆S˚ are
Figure 7. Plot of lnb versus 1/T for pyridine adsorption on oxidized/un-oxidized KOH treated activated carbon.
positive in case of both oxidized and un-oxidized carbons which confirms the possibility of favorable pyridine ad- sorption [40,41].
4. Conclusion
New alternative adsorbents for pyridine removal have been explored by converting peanut shell into activated carbon with surface modification through KOH. The adsorption of pyridine was found to be highly dependent on the pH value of the system, with the best results being obtained at pH 5 for oxidized (KOH treated) sample, at pH 6 for un-oxidized carbon. At lower concentrations, removal is independent of initial concentrations. How- ever, at higher concentrations, removal depends upon the initial concentration. The kinetic data were well fitted by a first order kinetic model. The Langmuir adsorption iso- therm model gives better fit as compared to Freundlich model. Thermodynamic parameters suggest that pyridine adsorption on both oxidized and an un-oxidized carbon is endothermic, spontaneous and favorable.
5. Acknowledgements
The author gratefully acknowledge NCE in Physical Chemistry, PCSIR Labs for sample analysis and Pakistan Science Foundation for financial support (Research Pro- ject No: PSF/RES/F-UM/Chem(434).
REFERENCES
[1] E. Sayan, “Ultrasound-Assisted Preparation of Activated Carbon from Alkaline Impregnated Hazelnut Shell: An Optimization Study on Removal of Cu2+ from Aqueous Solution,” Chemical Engineering Journal, Vol. 115, No. 3, 2006, pp. 213-218. http://dx.doi.org/10.1016/j.cej.2005.09.024
[2] K. Mohanty, M. Jha, B. C. Meikap and M. N. Biswas, “Removal of Chromium (VI) from Dilute Aqueous Solutions by Activated Carbon Developed from Terminalia Arjuna Nuts Activated with Zinc Chloride,” Chemical Engineering Science, Vol. 60, No. 11, 2005, pp. 3049- 3059. http://dx.doi.org/10.1016/j.ces.2004.12.049
[3] S. B. Lyubchik, I. I. Perepichka, O. L. Galushko, A. I. Lyubchik, E. S. Lygina and I. M. Fonseca, “Optimization of the Conditions for the Cr(III) Adsorption on Activated Carbon,” Adsorption, Vol. 11, No. 5-6, 2005, pp. 581- 593. http://dx.doi.org/10.1007/s10450-005-5616-1
[4] E. Demirbas, M. Kobya, S. Oncel and S. Sencan, “Re- moval of Ni(II) from Aqueous Solution by Adsorption onto Hazelnut Shell Activated Carbon: Equilibrium Stud- ies,” Bioresource Technology, Vol. 84, No. 3, 2002, pp. 291-293. http://dx.doi.org/10.1016/S0960-8524(02)00052-4
[5] M. Kobya,
[6] C. A. Toles, W. E. Marshal and M. M. Johns, “Surface Functional Groups on Acid-Activated Nutshell Carbons,” Carbon, Vol. 37, No. 8, 1999, pp. 1207-1214. http://dx.doi.org/10.1016/S0008-6223(98)00315-7
[7] D. Mohan, K. P. Singh, S. Sinha and D. Gosh, “Removal of Pyridine from Aqueous Solution Using Low Cost Ac- tivated Carbons Derived from Agricultural Waste Materi- als,” Carbon, Vol. 42, No. 12-13, 2004, pp. 2409-2421. http://dx.doi.org/10.1016/j.carbon.2004.04.026
[8] A. Namane, A. Mekarzia, K. Benrachedi, N. Belhaneche- Bensemra and A. Hellal, “Determination of the Adsorption Capacity of Activated Carbon Made from Coffee Grounds by Chemical Activation with ZnCl2 and H3PO4,” Journal of Hazardous Materials, Vol. 119, No. 1-3, 2005, pp. 189-194. http://dx.doi.org/10.1016/j.jhazmat.2004.12.006
[9] K. Okada, N. Yamamoto, Y. Kameshma and A. Yasu- mori, “Adsorption Properties of Activated Carbon from Waste Newspaper Prepared by Chemical and Physical Activation,” Journal of Colloid and Interface Science, Vol. 262, No. 1, 2003, pp. 194-199. http://dx.doi.org/10.1016/S0021-9797(03)00108-5
[10] J. Pastor-Vilegas and C. J. Duran-Valle, “Pore Structure of Activated Carbons Prepared by Carbon Dioxide and Steam Activation at Different Temperatures from Ex- tracted Rockrose,” Carbon, Vol. 40, No. 3, 2002, pp. 397- 402. http://dx.doi.org/10.1016/S0008-6223(01)00118-X
[11] W. T. Tasi, C. Y. Chang, S. Y. Wang, C. F. Chang, S. F. Chien and H. F. Sun, “Preparation of Activated Carbons from Corn Cob Catalyzed by Potassium Salts and Subse- quent Gasification with CO2,” Bioresource Technology, Vol. 78, No. 2, 2001, pp. 203-208. http://dx.doi.org/10.1016/S0960-8524(00)00111-5
[12] J. Niu and B.
[13] G. L. Goe, “Kirk-Othmer Encyclopedia of Chemical Te- chnology,” 3rd Edition, Wiley Interscience,
[14] M. Dinesh, S. P. Kunwar and G. Deblina “Removal of α- Picoline, β-Picoline, and γ-Picoline from Synthetic Waste- water Using Low Cost Activated Carbons Derived from Coconut Shell Fibers,” Environmental Science & Tech- nology, Vol. 39, No. 13, 2005, pp. 5076-5086. http://dx.doi.org/10.1021/es048282g
[15] S. K. Rhee, G. M. Lee and S. T. Lee, “Influence of a Supplementary Carbon Source on Biodegradation of Pyridine by Freely Suspended and Immobilized Pimelobacter sp,” Applied Microbiology and Biotechnology, Vol. 44, No. 6, 1996, pp. 816-822.
[16] S. T. Lee, S. K. Rhee and G. M. Lee, “Biodegradation of Pyridine by Freely Suspended and Immobilized Pimelo- bacter sp,” Applied Microbiology and Biotechnology, Vol. 41, No. 6, 1994, pp. 652-657. http://dx.doi.org/10.1007/BF00167280
[17] G. K. Sims and L. E. Sommers, “Biodegradation of Pyri- dine Derivatives in Soil Suspensions,” Environmental Toxicology and Chemistry, Vol. 5, No. 6, 1986, pp. 503- 509. http://dx.doi.org/10.1002/etc.5620050601
[18]
[19] E. Sabah and M. S. Ceilk, “Interaction of Pyridine De- rivatives with Sepiolite,” Journal of Colloid and Interface Science, Vol. 251, No. 1, 2002, pp. 33-38. http://dx.doi.org/10.1006/jcis.2002.8394
[20] Y. Yokoi, G. Yelken, Y. Oumi, Y. Kobayashi, M. Kubo, A. Miyamoto and M. Komiyama, “Monte Carlo Simulation of Pyridine Base Adsorption on Heulandite (0 1 0),” Applied Surface Science, Vol. 188, No. 3-4, 2002, pp. 377-380. http://dx.doi.org/10.1016/S0169-4332(01)00961-8
[21] M. Stern, H. Elmar, O. M. Kut and K. Hungerbuhler, “Removal of Substituted Pyridines by Combined Ozona- tion/Fluidized Bed Biofilm Treatment,” Water Science and Technology, Vol. 35, No. 4, 1997, pp. 329-335. http://dx.doi.org/10.1016/S0273-1223(97)00042-5
[22] C. A. Toles, W. E. Marshal and M. M. Johns, “Surface Functional Groups on Acid-Activated Nutshell Carbons,” Carbon, Vol. 37, No. 8, 1999, pp. 1207-1214. http://dx.doi.org/10.1016/S0008-6223(98)00315-7
[23] D. Mohan, K. P. Singh, S. Sinha and D. Gosh, “Removal of Pyridine from Aqueous Solution Using Low Cost Ac- tivated Carbons Derived from Agricultural Waste Materi- als,” Carbon, Vol. 42, No. 12-13, 2004, pp. 2409-2421. http://dx.doi.org/10.1016/j.carbon.2004.04.026
[24] O. Hernandez-Ramirez and S. M. Holmes, “Novel and Modified Materials for Wastewater Treatment Applica- tions,” Journal of Materials Chemistry, Vol. 18 No. 24 2008, pp. 2751-2761. http://dx.doi.org/10.1039/b716941h
[25] H. P. Boehm,
[26]
[27] K. Katsumi, “Determination of Pore Size and Pore Size Distribution: 1. Adsorbents and Catalysts,” Journal of Membrane Science, Vol. 96, No. 1-2, 1994, pp. 59-89. http://dx.doi.org/10.1016/0376-7388(94)00126-X
[28] F. Rodriguez-Reinoso, M. Molina-Sabio and M. T. Gon- zalez, “The Use of Steam and CO2 as Activating Agents in the Preparation of Activated Carbons,” Carbon, Vol. 33, No. 1, 1995, pp. 15-23. http://dx.doi.org/10.1016/0008-6223(94)00100-E
[29] M. I. Kandah, R. Shawabkeh and M. A. Al-Zboon, “Syn- thesis and Characterization of Activated Carbon from Asphalt,” Applied Surface Science, Vol. 253, No. 2, 2006, pp. 821-826.
[30] H. P. Boehm, “Surface Oxides on Carbon and Their Ana- lysis: A Critical Assessment,” Carbon, Vol. 40, No. 2, 2002, pp. 145-149. http://dx.doi.org/10.1016/S0008-6223(01)00165-8
[31] J. B. Weber, “Molecular Structure and pH Effects on the Adsorption of 13 s-Triazine Compounds on Montmoril- lonnite Clay,” American Mineralogist, Vol. 51, No. 11- 12, 1996, pp. 1657-1670.
[32] T. Y. Kim, S. J. Kim and S. Y. Cho, “Effect of pH on Adsorption of 2,4-Dinitrophenol onto an Activated Carbon,” Korean Journal of Chemical Engineering, Vol. 18, No. 5, 2001, pp. 755-760. http://dx.doi.org/10.1007/BF02706396
[33] L. R. Radovic, L. R. Silva, J. I. Ume J. A. Menendez, L. Y. Leon and A. W. Scaroni, “Irreversible and Reversible Adsorption of Some Heavy Transition Metals on Graph- itic Carbons From Dilute Aqueous Solutions,” Carbon, Vol. 35, No. 9, 1997, pp. 1337-1339.
[34] C. Namasivayam and D. Sangeetha, “Removal and Re- covery of Vanadium(V) by Adsorption onto ZnCl2 Acti- vated Carbon: Kinetics and Isotherms,” Adsorption, Vol. 12, No. 2, 2006, pp. 103-117. http://dx.doi.org/10.1007/s10450-006-0373-3
[35] Y. S. HO, “Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reactions,” Scientometrics, Vol. 59, No. 1, 2004, pp. 171-177. http://dx.doi.org/10.1023/B:SCIE.0000013305.99473.cf
[36] T. Karthieyan,
[37] Y. S. Ho and G. McKay, “Pseudo-Second Order Model for Sorption Processes,” Process Biochemistry, Vol. 34, No. 5, 1999, pp. 451-465. http://dx.doi.org/10.1016/S0032-9592(98)00112-5
[38] R. Dhodapkar, P. Borde and T. Nandy, “Super Absorbent Polymers in Environmental Remediation,” Global NEST Journal, Vol. 11, No. 2, 2009, pp. 223-234.
[39] N. A. Klimenko, L. A. Savchina, I. P. Kozyatnik, Y. V. Topkin and T. V. Polyakova, “The Impact of Surface Chemistry of Activated Carbon and Its Structure on Ad- sorption of Fulvic Acids from Aqueous Solutions,” Jour- nal of Water Chemistry and Technology, Vol. 30, No. 6, 2008, pp. 344-350. http://dx.doi.org/10.3103/S1063455X08060039
[40] G. Z. Memon, M. I. Bhanger and M. Akhtar, “Peach-Nut Shells―An Effective and Low Cost Adsorbent for the Removal of Endosulfan from Aqueous Solutions,” Paki- stan Journal of Analytical & Environmental Chemistry, Vol. 10, No. 1-2, 2009, pp. 14-18.
[41] A. Ramesh, D. J. Lee and J. W. C. Wong, “Thermody- namic Parameters for Adsorption Equilibrium of Heavy Metals and Dyes from Wastewater with Low-Cost Ad- sorbents,” Journal of Colloid and Interface Science, Vol. 291, No. 2, 2005, pp. 588-592. http://dx.doi.org/10.1016/j.jcis.2005.04.084









