Pharmacology & Pharmacy
Vol.3 No.1(2012), Article ID:16959,9 pages DOI:10.4236/pp.2012.31004
Radio Frequency-Microchannels for Transdermal Delivery: Characterization of Skin Recovery and Delivery Window
![]()
TransPharma Medical, Lod, Israel.
Email: *Yossi.kam@mail.huji.ac.il
Received October 17th, 2011; revised November 14th, 2011; accepted December 20th, 2011
Keywords: Microporation; Microchannels; RF Ablation; Testosterone; Transdermal Delivery
ABSTRACT
Transdermal delivery through Radio-Frequency-MicroChannels (RF-MCs) was proven to be a promising delivery method for hydrophilic drugs and macromolecules that must be injected. An important issue in assessing this technology is the life span of the microchannels (MCs). The time window in which the MCs remain open affects the delivery rate and determine the effective delivery duration. The present work focused on the characterization of the ViaDor-MCs recovery and closure process by measurements of transepidermal water loss (TEWL) before and after the formation of MCs, evaluation of the delivery window, and assessment of skin histology. Testosterone-cyclodextrin complex was used as the model drug for evaluation of the transdermal delivery. In-vitro permeation system and in-vivo guinea pig animal model were used in the delivery studies. Our findings demonstrate the recovery process of MCs created by the RF ablation technology. The observed gradual skin recovery affected the transdermal delivery rate. A significant transdermal delivery was shown up to 24 hrs post device application suggesting that an extended delivery of water soluble drugs, including macromolecules, is possible. The histology assessments demonstrated repair and healing of the induced MCs indicating that the RF micro-channeling technology is minimally invasive, transient in nature with no resulting skin trauma.
1. Introduction
Transdermal drug delivery (TDD) offers an attractive alternative to oral or parenteral routes. It has the potential to enable prolonged drug delivery, avoid gastrointestinal drug degradation or adverse effects, first-pass metabolism, hepatotoxicity, pain on injection, safety issues related to the use of needles and improve bioavailability and patient compliance [1]. Current commercial transdermal technologies are limited to the delivery of small potent and lipophilic drugs molecules. Permeation of therapeutic levels of water soluble drugs, charged compounds, and macromoleculs such as proteins and peptides, into the systemic circulation is restricted [2,3]. Various enhancement techniques such as chemical enhancers, iontophoresis, electroporation and sonophoresis were developed and their ability to transfer drugs across the skin barrier was reported [4,5]. Microporation technologies such as laser ablation, thermal ablation and mechanical microneedles create transient micro sized aqueous transport pathways across the skin [6-8]. Transdermal delivery of various hydrophilic compounds including macromolecules such as plasmid DNA, FSH and interferon was demonstrated using these techniques [9-12].
One of these minimally invasive transdermal delivery technologies in development is based on radio-frequency (RF) electrical cell ablation, a well-known medical technology used in electro-surgery for laparoscopic medical procedures [13,14]. The technology is based on an electronic device, named ViaDorTM (previously named ViaDermTM), which generates an alternating electrical current at a high frequency (100 - 500 kHz). This electrical current is transferred, via an array of microelectrodes placed on the skin, to the upper skin layer. Exposure of skin cells in the vicinity of each electrode to the high frequency electrical current causes ionic vibrations within these cells, which in turn lead to localized heating and cell ablation without significantly heating or damaging the deeper skin layers. This procedure creates an array of small-size microchannels in the skin [15].
We reported previously that RF-generated MCs reside in the epidermis or the superficial dermis were suitable for the effective transdermal delivery of water soluble small molecules [16] or proteins [17] into the systemic circulation as well as gene vectors [9] into viable skin layers. Unlike classic transdermal delivery, formation of MCs in the skin, which are filled with interstitial fluid, enables and increases dramatically the delivery of highly water soluble molecules. Enhancement of transdermal delivery of hydrophobic drugs depends on specific formulation that increases the drug solubility in water. One possibility is complexation of the drug with cyclodextrin [18-21].
An important issue in assessing skin microporation delivery techniques is the length of time in which the MCs remains open, however, this issue was not extensively study [22]. This time window determines the effective delivery duration. Thus, a slow closure process is desirable for prolonged delivery of high doses. On the other hand, a quick and natural closure process is desirable due to safety aspects.
The present work focused on the characterization of the ViaDorTM-MCs closure process by measurements of transepidermal water loss (TEWL) post device application, assessment of skin histology and evaluation of delivery rate at various time points after MCs formation. The effect of MCs closure on transdermal delivery was measured using testosterone-cyclodextrin complex as the model drug.
2. Materials and Methods
2.1. ViaDorTM Device
The device principles were previously described in details [16]. Briefly, the ViaDorTM device is made of two primary components: a reusable electronic controller that generates high frequency alternating electrical current, and a disposable electrode array that snaps onto the end of the controller. The electrodes are small stainless-steel wires that project out of the plastic surface of the array, touch the skin and enable the passage of the electrical current from the device to skin upper layer.
2.2. Animals
Study protocols were approved by the Institutional Animal Care and Use Committee of Assaf Harofeh Medical Center (Zriffin, Israel), and all procedures were conducted according to the Principles of Labotarory Animal Care (NIH Publication No. 85 - 23, revised 1985). Male hairless guinea pigs (GPs’), 600 - 750 g (Crl:IAF(HA)- hrBR, Charles River Laboratories Inc, Wilmington, Massachusetts), and domestic pigs (a mix breed of landrace and large white pigs), 10 - 12 kg (Lahav Research Institute, Kibutz-Lahav, Israel), were used. They were kept at a constant temperature (~24˚C) with a 12 hour light-dark cycle. Water and pelleted food (Teklad, Harlan Ltd, Rehovot, Israel) for guinea pigs and food mixture (Matmor Ltd, Ashdod, Israel) for pigs were provided ad-libitum.
2.3. Materials
Ketamine hydrochloride (Ketaset, Fort Dodge, IA, USA), xylazine (Xyl-M2 veterinary, VMD, Arendonk, Belgium), Isoflurane and halothane gases (Rhodia Bristol, UK) were used for anesthesia. Euthanasia was performed using pentobarbitone sodium (CTS Chemical Industries, Hod Hasharon, Israel). Acetonitrile was purchased from J.T Baker, Deventer,Holland. Buffered formaldehyde solution (4%) was obtained from Sigma Chemical, IL. WEBCOLTM (isopropyl alcohol pads) for skin cleaning were purchased from Kendall, MA, USA. For in-vitro permeation studies, testosterone (Fluka, Seelze, Germany), beta-cyclodexterin (Captisol, CyDex, Kansas, USA) and PEG 400 (Ineos Oxide, Zwiindrecht, Belgium) were used.
2.4. Equipment
Evaluation of skin barrier function was performed using transepidermal water loss (TEWL) measurements using DermaLab instrument (Cortex Technology, Hadsund, Denmark).
Flow through Franz diffusion cell system (Laboratory Glass Apparatus, Berkeley, CA) with a delivery area of 3.1 cm2, receptor volume of 5.5 ml and a fraction collector (Retriever IV, ISCO Inc., NE) were used in order to assess testosterone permeability in vitro.
HPLC system (ProStar modules, Varian Inc, Netherlands) equipped with an autosampler and a UV detector (Varian’s ProStar model 310) and a pre-packed C18 column (Phenomenex LUNATM, 5 mm, 150 × 4 mm) were used.
2.5. Anesthesia Procedure and Skin Preparation
Prior to any procedure guinea pigs were anesthetized by intraperitoneal (i.p.) injection and domestic pigs by intramuscular (i.m) injection of a combination of ketamine hydrochloride/xylazine. The anesthesia was maintained using a mixture of oxygen and isoflurane or halothane gas. Hair on skin treatment sites was clipped using an Oster A5 clipper (McMinnville, TN, USA) using a no. 40 blade following by a Braun 3615 shaver (NY, USA), and a gentle wiping with an isopropyl alcohol pad. Thirty minutes later transepidermal water loss (TEWL) measurements were performed, in order to verify skin integrity. Then, ViaDorTM treatment was performed followed by a second TEWL measurement. Only skin sites with initial TEWL levels lower than 8.5 g/m2/h that increased after ViaDorTM application by more than 20 g/m2/h were included in the studies. Skin harvesting was performed following euthanasia using intracardiac administration of pentobarbitone sodium, at the proper time after ViaDorTM treatment.
2.6. Assessment of Skin Barrier Function by TEWL Measurements and Visual Assessment
The barrier integrity of pig skin following ViaDorTM device poration was examined using TEWL measurements. Two pigs were anesthetized and skin sites (n = 6) were prepared as described above. TEWL measurements were performed before and immediately after ViaDorTM application (150 MCs/cm2). Additional TEWL measurements were performed 24h following application in order to examine the barrier recovery with time. MCs visualization was performed as previously described [17]. Briefly, skin samples were stained with 1% methylene blue solution (Pioneer Research Chemicals, UK) followed by removal of the excessive stain using isopropyl alcohol pads. Skin sites were than photographed using a digital camera (Canon Inc., USA).
2.7. In Vitro Transdermal Testosterone Complex Delivery using ViaDorTM-Evaluation of Delivery Time Window
2.7.1. Principle of Experiment
Testosterone-cyclodextrin complex was selected as the model drug due to its molecular size (~2500 dalton). A complex at such size cannot penetrate the micropores that can be formed due to skin handling during the experiment. Thus, all the delivery was done through the MCs formed by ViaDorTM.
For evaluation of MCs delivery window, a two parts study was performed: an in-vivo part in which transdermal applications were performed on live GPs. At various time points following MCs formation, the animals were sacrificed and skin samples containing the device application sites were harvested. An in-vitro delivery study was then conducted. In this part the delivery rate of testosterone complex via the harvested skin samples was tested using Franz diffusion cells.
2.7.2. Preparation of Testosterone-Cyclodextrin Complex Solution and Dry Patches
In order to increase testosterone solubility in water, 0.1 g testosterone powder was added to 0.1197 M β-cyclodextrin (Captisol) solution to a final volume of 5 ml, and vigorously mixed until complete wetting of the testosterone in the Captisol solution. The solution was shaken over night to yield a clear 2% testosterone-cyclodextrin soluble complex. The concentration was confirmed using high performance liquid chromatography (see HPLC analysis below). This solution was used in the in vitro delivery study.
For the in vivo delivery study, 2 mg dry patches of testosterone complex were prepared by air drying the suitable amount of testosterone complex solution in a frame similar in size to the ViaDor application area (1.4 cm2).
2.7.2.1. Part I: In Vivo Study
GPs (n = 24) were anaesthetized as described above and ViaDorTM was applied on their abdomen, followed by skin site occlusion. Animals were sacrificed immediately (time 0) and 4, 8, 10, 12, 24 and 36 hrs following device application. Full thickness skin samples (4 × 4 cm size) containing the device application sites were harvested and used immediately for the in vitro study. Six skin samples were tested for each time point.
2.7.2.2. Part II: Diffusion Cells
Testosterone-cyclodextrin permeability through the ViaDorTM treated hairless guinea pigs skin was measured in vitro using flow through Franz diffusion cell system as described above. The skin samples were placed on the receiver chambers with the stratum corneum facing upwards, than the donor chambers were clamped in place. Testosterone-cyclodextrin (testosterone concentration: 2% w/v) solution (300 µl) was added to the donor. Phosphate buffered saline (PBS, pH 7.4) containing 10% PEG 400 at a flow rate of 1 ml/hr was used as the receptor phase and was collected into test tubes using a fraction collector, at 3 hr fractions for a 15 hr total duration. The samples were kept at 4˚C until analyzed by HPLC.
2.7.2.3. HPLC Analysis of Samples from Receiver Solutions
Aliquots of 20 µl from each sample were injected into the HPLC system. Samples were assayed using an isocratic mobile phase consisting of acetonitrile-water (1:1) at a flow rate of 0.9 ml/min. The quantification of testosterone was carried out at 242 nm. Data was expressed as the cumulative substance permeation per unit of skin surface area. The steady-state fluxes (Jss) were calculated by linear regression interpolation of the experimental data.
2.8. In Vivo Transdermal Testosterone Complex Delivery through ViaDorTM Treated GP Skin-Evaluation of the MCs Healing Effect
The delivery of testosterone-cyclodextrin complex through ViaDorTM treated hairless guinea pigs skin was measured in vivo. Animals were anaesthetized and ViaDorTM was applied on GPs’ (n = 16) abdomen. A 2 mg patch of testosterone dry complex was applied to the following three tested groups:
1) Immediate application group (n = 4): Testosterone complex patch was placed on the ViaDorTM treated site immediately following ViaDorTM application.
2) Delayed patch application group (n = 4): after ViaDorTM treatment the treated skin area was covered with an occluded patch. Eight hours later, the placebo patch was removed and testosterone-cyclodextrin complex patch was placed on the treated site.
3) Patch replacement group (n = 8): immediately following ViaDorTM application, a testosterone-cyclodextrin complex patch was applied. After eight hrs, the first patch was replaced with a second one for additional 16 hrs.
2.9. Histology Study
Skin histology assessment of ViaDorTM application on pigs’ dorsal skin was performed in order to assess histological skin changes following device applications and to evaluate site recovery process. Two pigs (10 - 12 kg) were prepared and anaesthetized as previously described, followed by ViaDorTM device application. In order to increase the probability for MC detection in the histological sections, MC density used was increased to 450 MCs/cm2 by applying the device three times on the same site. Skin harvesting was performed using biopsy punch (0.8 cm diameter) immediately (time 0), 4, 8 and 24 hrs following device application. Tested sites were occluded until harvesting to mimic the occlusive effect of a patch.
Skin samples were preserved in 4% buffered formaldehyde solution, embedded in paraffin wax, cut to a thickness of 5 mm, stained with hematoxyline and eosin (H & E) and examined microscopically.
3. Results
3.1. Assessment of Barrier Perturbation by TEWL Measurements
As shown in Table 1, an increase in TEWL value was measured immediately following treatment with the ViaDorTM device. A measurable difference from the baseline is indicative of disruption of the Stratum Corneum. A significant decrease was observed after 24 hrs indicating MC recovery and closure. The measured values after 24 hrs post application were slightly higher than baseline. The images of application sites (see Table 1) are also indicative of the healing process that takes place after the MCs are formed. Immediately after device application the MCs are fresh and open, and thus are intensely colored by methylene blue. After 24 hrs, lower intensity of the dye’s blue color and a decrease of MC’s number were seen due to MCs closure.
3.2. In Vitro Transdermal Testosterone Complex Delivery Using ViaDorTM-Evaluation of Delivery Time Window
Delivery rates of testosterone-cyclodextrin complex are presented in Figure 1. As expected, skin harvested immediately following ViaDorTM application (time 0) resulted in the highest cumulative testosterone permeation (473 μg/cm2). As the healing process progressed, the testosterone permeability gradually decreased, resulting in the lowest permeability rate (14.9 µg/cm2) using skin removed 36 hrs post ViaDorTM application which was close to that of intact skin (8.39 µg/cm2).
Figure 2 presents a rapid decrease in the pseudo steady state flux rates during the first 10 hrs followed by a moderate decrease during the next 26 hrs. A pseudo
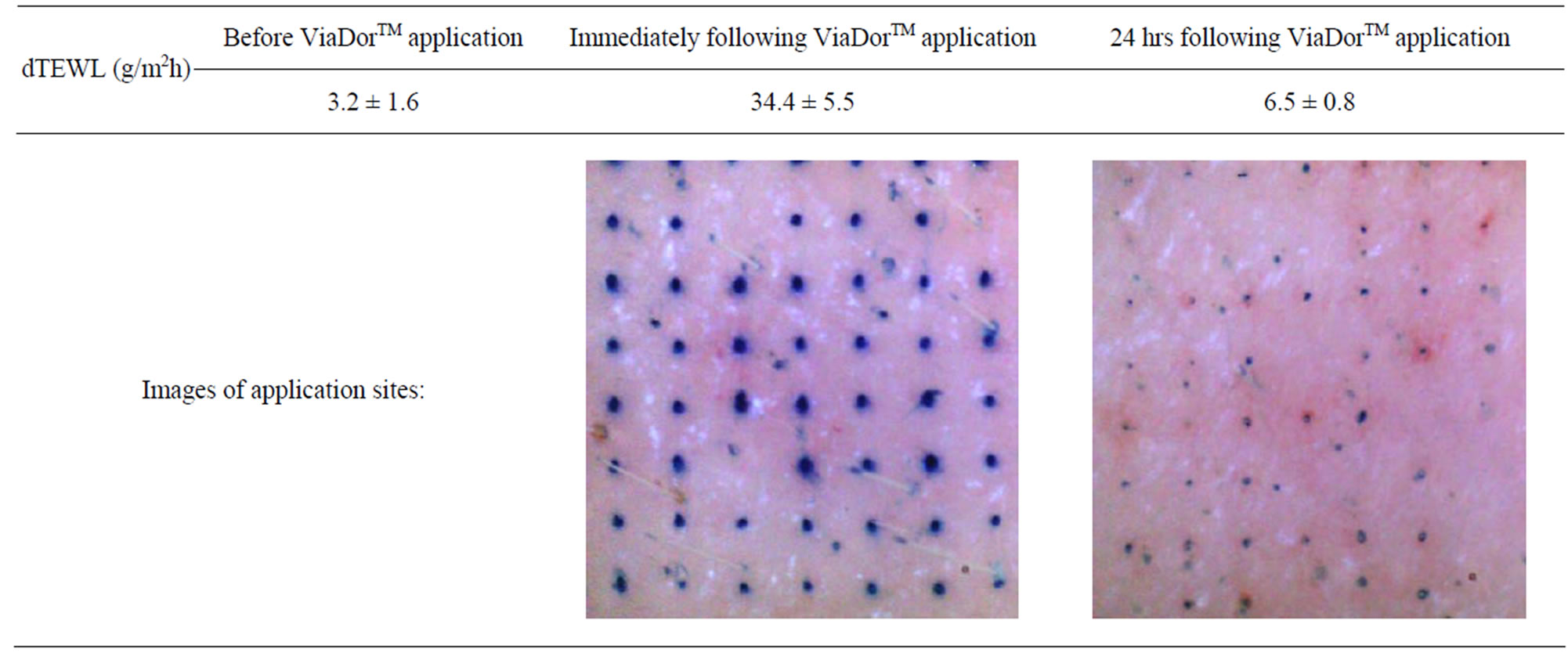
Table 1. TEWL values (g/m2h), before, immediately and 24 hrs following ViaDorTM poration. Mean ± SD, N = 6.
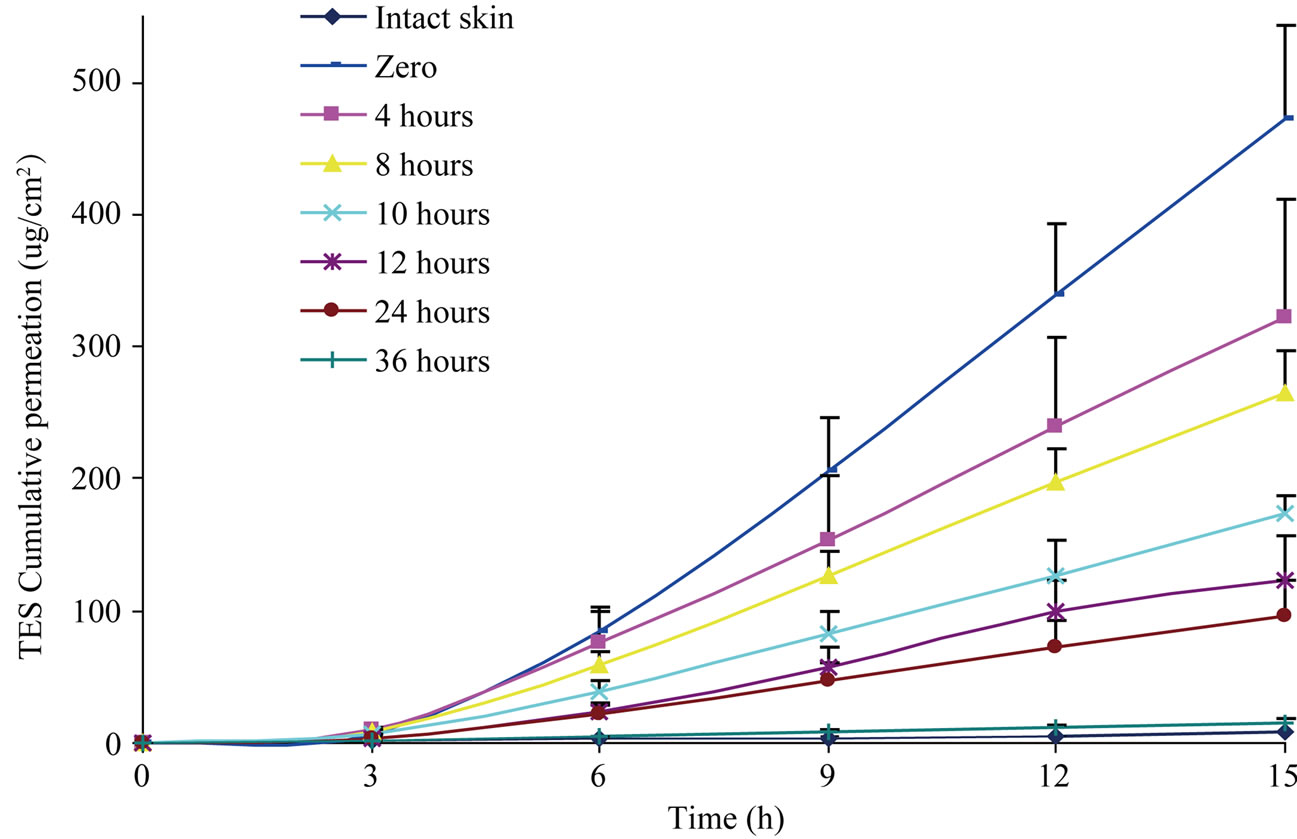
Figure 1. Testosterone-cyclodextrin complex cumulative permeation in vitro using hairless guinea pig skin. Skin harvesting was performed 0, 4, 8, 10, 12, 24, and 36 hrs following ViaDorTM application. The control group presents the passive diffusion through untreated skin. Mean values + SE. Six samples per time point.
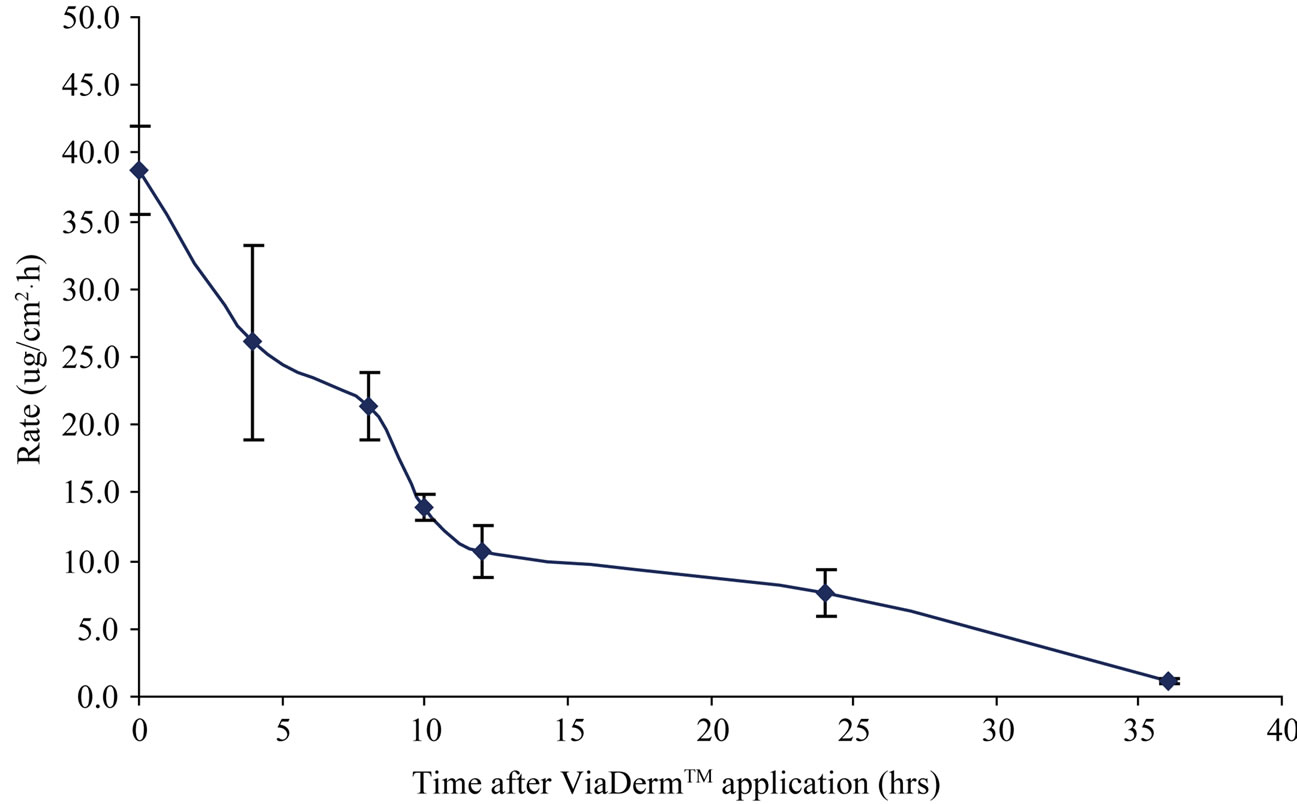
Figure 2. Testosterone-cyclodextrin complex permeation rates following ViaDorTM application. The steady state fluxes (Jss) were calculated by linear regression interpolation of the experimental data. Jss of intact skin was 0.56 μg/cm2×h. Presented are Mean ± SE, N = 6.
steady state flux of 39.6 μg/cm2×h was observed at time 0, compared to fluxes of 1.08 μg/cm2×h after 36 hrs. Only minor differences were observed between skin harvested at 36 hr and the control intact skin/passive delivery (Jss = 0.56 μg/cm2×h).
3.3. In Vivo Transdermal Testosterone Complex Delivery to ViaDorTM Treated GP Skin-Evaluation of the MCs Healing Effect
The effect of MCs healing progress on in-vivo transdermal delivery was evaluated in guinea pigs ViaDorTM treated skin. Figure 3 shows pharmacokinetic profiles of testosterone-cyclodextrin complex delivery in guinea pigs for up to 24 hrs. Testosterone plasma levels in guinea pigs pretreated with ViaDorTM followed by immediate application of testosterone-complex patch reached a peak plasma levels (Cmax) of 31.8 ± 12.1 ng/ml at T = 4 hrs. A 3.4 fold decrease in testosterone peak level (Cmax of 9.3 ± 6.6 ng/ml) was observed when the patch was applied 8 hrs post MCs formation (i.e. 8 hrs closure period; delayed patch group). A similar phenomenon was observed when a first patch was applied immediately after MCs formation and then replaced by a second patch after 8 hrs. This second patch resulted in a 1.8 fold decreased peak levels (Cmax of 17.1 ± 14.8 ng/ml; replacement group).
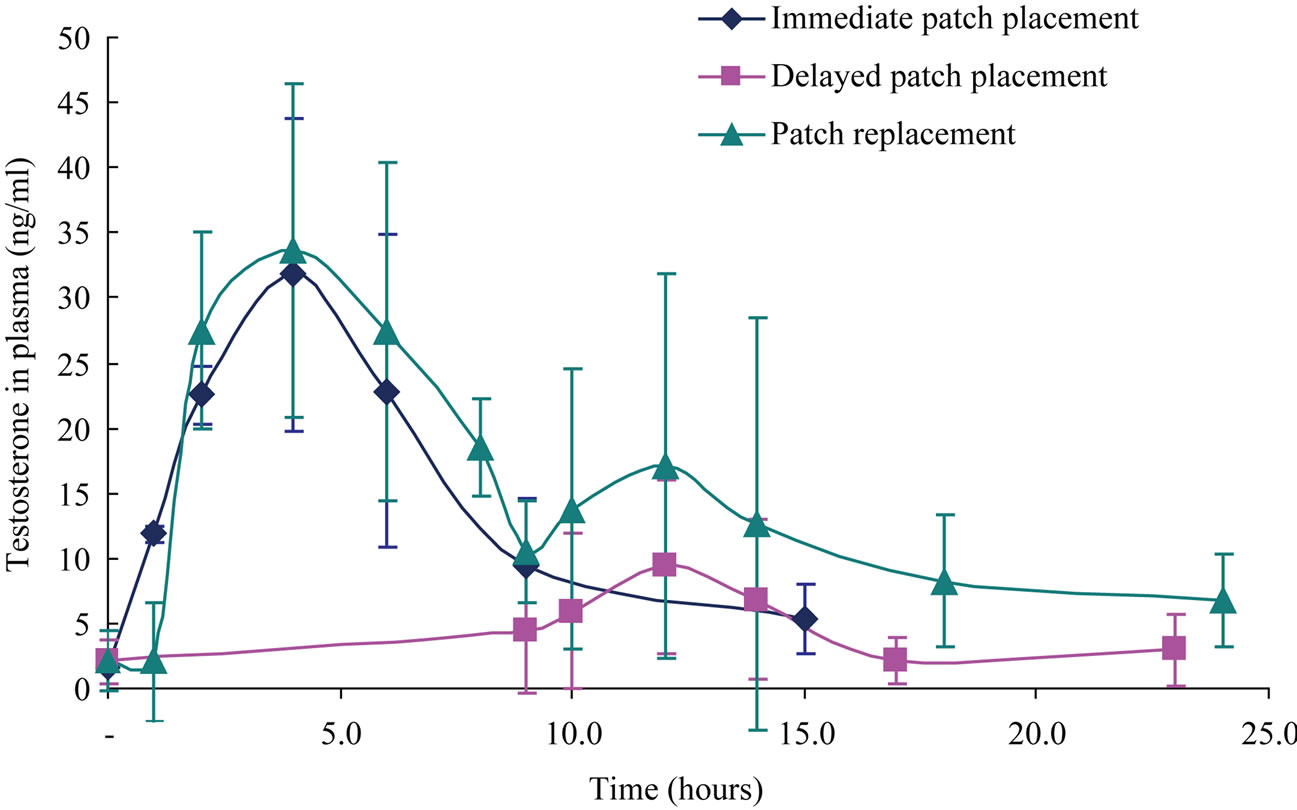
Figure 3. Testosterone-cyclodextrin complex transdermal permeation in Guinea pigs following ViaDorTM treatment and patch placement in three different time points: Immediate (time 0, N = 4), 8hrs following ViaDorTM application (delayed patch placement, N = 4) and replacement of an immediate patch (time 0) in a new patch after 8 hrs (patch replacement group, N = 8). Mean ± SD.
3.4. Histology
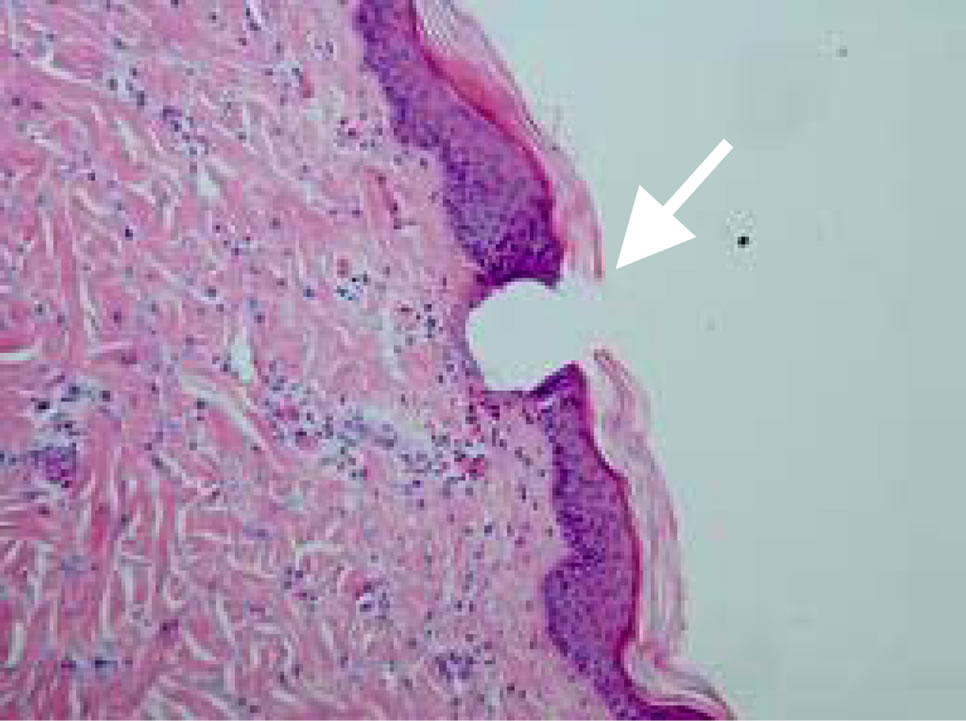
Figures 4(a)-(d) presents MCs at various time points post ViaDorTM application.
Immediately following the device application (Figure 4(a)) the MCs crossed the stratum corneum, the entire epidermis and penetrated the papillary dermis. Four hrs following ViaDorTM treatment (Figure 4(b)), skin reaction to the MCs formation was already evident. Cells associated with the acute response to injury (polymorphonuclear leukocytes, PMN) were present in the MC cavity and around its borders.
A more advanced healing process was observed eight hours following ViaDorTM treatment (Figure 4(c)). This was evident in early granulation tissue formation in the immediate border of the MC, with early regenerating epithelial cells. PMNs were present inside the cavity and around the MC. The superficial dermis adjacent to the MC already showed increased capillary presence.
The MCs seems completely sealed 24 hrs following ViaDorTM application on the skin (Figure 4(d)). In general, no abnormalities in the skin tissue were observed. The cavity of the MC was filled with PMNs and debris and the remnants of the MC composed mostly of granulation tissue. A newly formed layer of epidermis was clearly visible. The presence of mononuclear cells, which indicating a later stages of healing, was already present.
4. Discussion
The use of microporation techniques to induce transient skin structure changes for transdermal delivery is extensively studied in the recent years [6,7]. Significant increase in the transdermal flux of drugs was demonstrated probably due to creation of transient aqueous “pores” in the lipid bilayers of the SC [8,12,16,17,23,24]. The RFMCs technology has already been shown effective for transdermal delivery of water soluble small molecules [16], proteins [17], macromolecules and gene therapy vectors [9] into the systemic circulation or inner skin layers. Our innovative study presents the recovery process of the skin following RF ablation in terms of changes in delivery rate and skin function and morphology.
One of the methods to assess the skin integrity is by measuring transepidermal water loss [25-28]. TEWL measurements allow the discovery of changes in the stratum corneum barrier of the skin in an early stage, even before they are noticeable. In general, normal skin allows very little water loss whereas the water loss is much higher for a compromised skin [6]. The TEWL measurements were used to study the changes in barrier nature of the skin that occurs due to RF ablation. Significant TEWL elevation was observed in the pig skin sites treated with the ViaDorTM indicating skin barrier disruption. Twenty four hrs following ViaDorTM device application the TEWL values decreased dramatically and were slightly higher than the baseline values, suggesting that a healing process occurred.
Currently, there are no ideal animal models for wounds or burns healing processes. Therefore multiple animal models are typically used to assess wound healing process [29]. In our study we used guinea pigs and domestic pigs as animal models for studying the MCs healing. The testosterone complex in vitro permeation study demonstrated that application of a drug on freshly formed microchannels resulted in a delivery rate increase of 70 fold compared to the control, untreated skin (See Figure 2). A
 (a)
(a)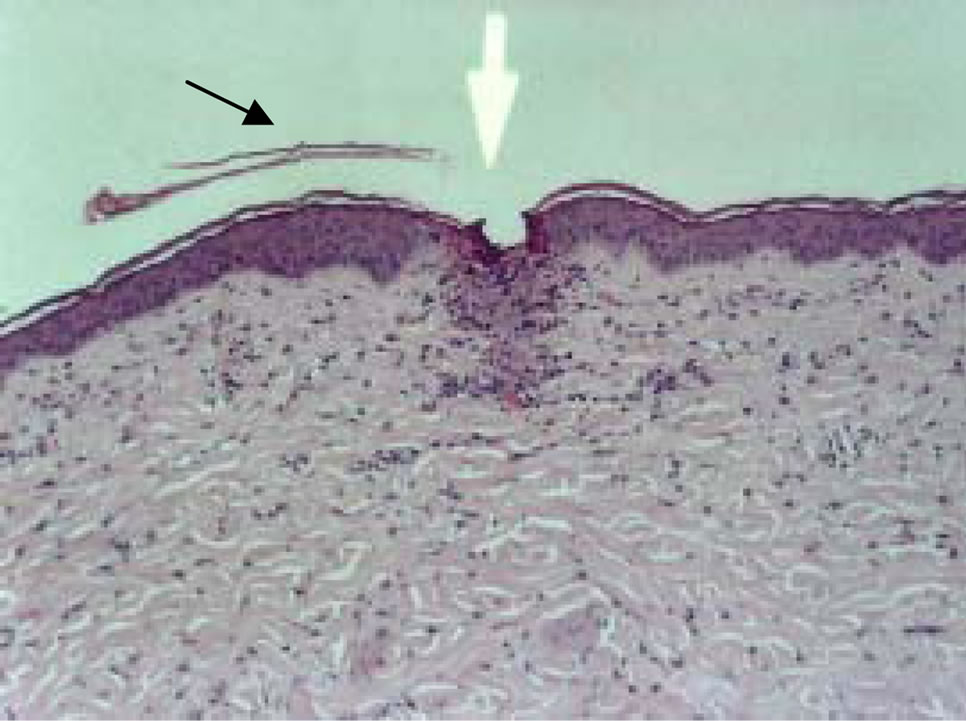 (b)
(b)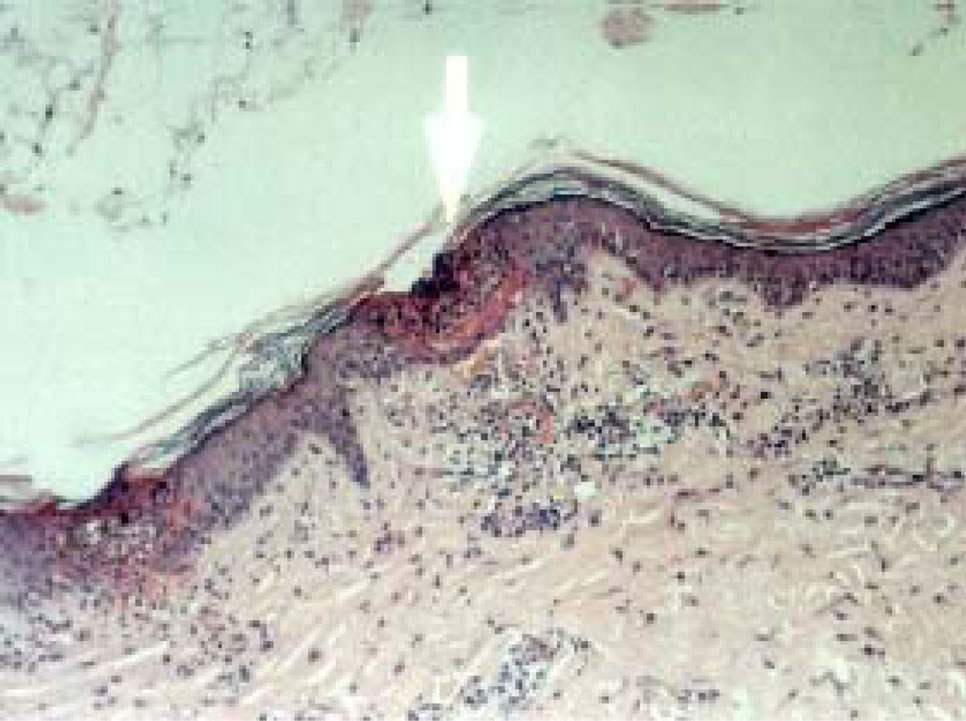 (c)
(c)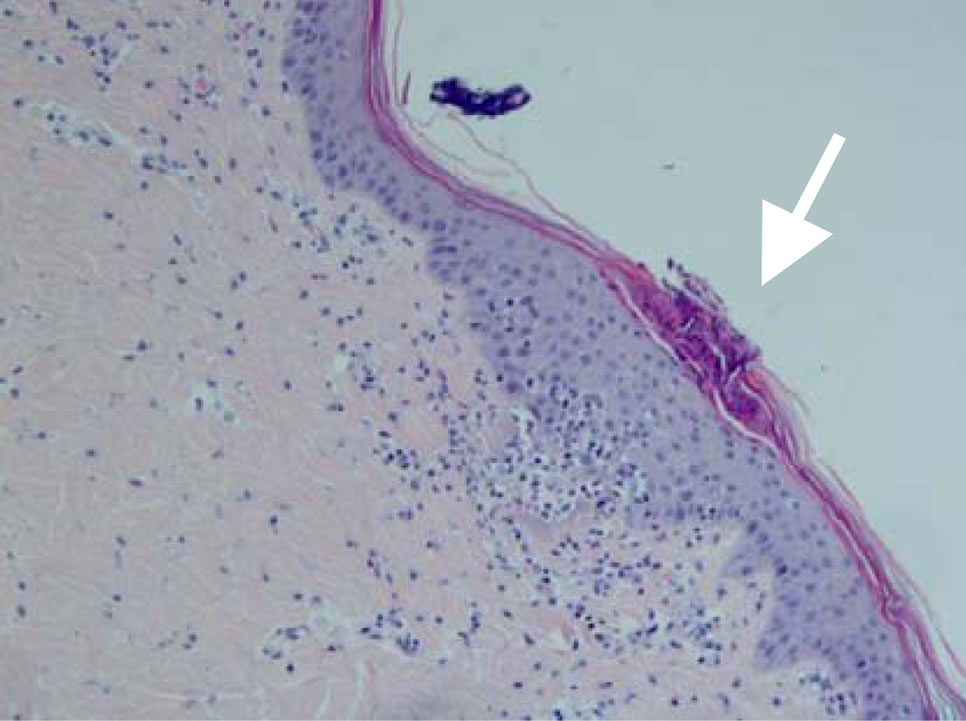 (d)
(d)
Figure 4. Visualization of MCs at various time points post ViaDorTM application on pig’s skin (×100). (a) Immediately post ViaDorTM poration (T = 0); (b) Four hrs; (c) Eight hrs; (d) 24 hrs.
significant and constant decrease in testosterone complex permeation was observed in later time points, probably due to the MCs’ recovery. While at 36 hrs post application the permeation rate was similar to that of intact skin, delivery rate was still higher than that of intact skin even 24 hrs post device application. This show a clear potential for 24 hrs extended delivery. Moreover, it may be expected that with smaller molecules the delivery window will be longer.
The in vivo experiment results (Figure 3) indicates that there is a clear MCs healing process. This is demonstrated by the lower testosterone plasma levels in the delayed patch application group and the second patch of the patch replacement group. Assuming that most of the drug delivered through the induced MCs, the decrease observed in testosterone permeability 8 hrs following ViaDorTM poration, indicates skin barrier recovery process.
Representative pictures of pig skin histological sections during 24 hrs post device applications are shown in Figure 4. The histological assessments were performed on pig’s skin due to its many similarities to human skin [30]. Application of RF-ablation technology to the pig’s skin appears to be minimally invasive and of a transient nature. The MCs crossed the stratum corneum, the entire epidermis and penetrated the papillary dermis. The histological findings demonstrate that healing takes place immediately after the MCs formation and that the healing process of the epidermis is gradual. In a time window of 24 hrs almost complete and natural recovery is observed. Since the skin serves as a protective barrier from the surrounding environment, any damage of this multilayered protective organ must be rapidly repaired. A temporary repair is achieved in the form of fibrin clot. This fibrin net plugs the compromised site while skin regeneration of the breached skin is initiated. Our histological findings (Figure 4) support a mechanism of classic wound healing [31-33].
5. To Conclude
Our findings demonstrate the recovery process of MCs created by the RF ablation technology. The observed gradual skin recovery affected the transdermal delivery rate. A significant transdermal delivery was shown up to 24 hrs post device application suggesting that an extended delivery of water soluble drugs, including macromolecules, is possible. The histology assessments demonstrated repair and healing of the induced MCs indicating that the RF micro-channeling technology is minimally invasive, transient in nature with no resulting skin trauma.
REFERENCES
- M. R. Prausnitz and R. Langer, “Transdermal Drug Delivery,” Nature Biotechnology, Vol. 26, No. 11, 2008, pp. 1261-1268. doi:10.1038/nbt.1504
- M. R. Prausnitz, S. Mitragotri and R. Langer, “Current Status and Future Potential of Transdermal Drug Delivery,” Nature Reviews Drug Discovery, Vol. 3, No. 2, 2004, pp. 115-124. doi:10.1038/nrd1304
- M. B. Brown, et al., “Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects,” Drug Delivery, Vol. 13, No. 3, 2006, pp. 175-187. doi:10.1080/10717540500455975
- A. Samad, et al., “Transdermal Drug Delivery System: Patent Reviews,” Recent Patents on Drug Delivery & Formulation, Vol. 3, No. 2, 2009, pp. 143-152. doi:10.2174/187221109788452294
- R. K. Subedi, et al., “Recent Advances in Transdermal Drug Delivery,” Archives of Pharmacal Research, Vol. 33, No. 3, 2010, pp. 339-351. doi:10.1007/s12272-010-0301-7
- A. K. Banga, “Microporation Applications for Enhancing Drug Delivery,” Expert Opinion on Drug Delivery, Vol. 6, No. 4, 2009, pp. 343-354. doi:10.1517/17425240902841935
- T. R. Singh, et al., “Microporation Techniques for Enhanced Delivery of Therapeutic Agents,” Recent Patents on Drug Delivery & Formulation, Vol. 4, No. 1, 2010, pp. 1-17. doi:10.2174/187221110789957174
- V. Sachdeva and A. K. Banga, “Microneedles and Their Applications,” Recent Patents on Drug Delivery & Formulation, Vol. 5, No. 2, 2011, pp. 95-132. doi:10.2174/187221111795471445
- J. Birchall, et al., “Cutaneous Gene Expression of Plasmid DNA in Excised Human Skin Following Delivery via Microchannels Created by Radio Frequency Ablation,” International Journal of Pharmaceutics, Vol. 312, No. 1-2, 2006, pp. 15-23. doi:10.1016/j.ijpharm.2005.12.036
- N. H. Zech, M. Murtinger and P. Uher, “Pregnancy after Ovarian Superovulation by Transdermal Delivery of Follicle-Stimulating Hormone,” Fertility and Sterility, Vol. 95, No. 8, 2011, pp. 2784-2785. doi: 10.1016/j.fertnstert.2011.03.073
- A. V. Badkar, et al., “Transdermal Delivery of Interferon Alpha-2B Using Microporation and Iontophoresis in Hairless Rats,” Pharmaceutical Research, Vol. 24, No. 7, 2007, pp. 1389-1395. doi:10.1007/s11095-007-9308-2
- H. Kalluri and A. K. Banga, “Transdermal Delivery of Proteins,” AAPS PharmSciTech, Vol. 12, No. 1, 2011, pp. 431-441. doi:10.1208/s12249-011-9601-6
- F. J. McGovern, et al., “Radio Frequency Ablation of Renal Cell Carcinoma via Image Guided Needle Electrodes,” Journal of Urology, Vol. 161, No. 2, 1999, pp. 599-600. doi:10.1016/S0022-5347(01)61961-X
- S. N. Goldberg, “Radiofrequency Tumor Ablation: Principles and Techniques,” European Journal of Ultrasound, Vol. 13, No. 2, 2001, pp. 129-147. doi:10.1016/S0929-8266(01)00126-4
- J. H. Park, et al., “The Effect of Heat on Skin Permeability,” International Journal of Pharmaceutics, Vol. 359, No. 1-2, 2008, pp. 94-103. doi:10.1016/j.ijpharm.2008.03.032
- A. C. Sintov, et al., “Radiofrequency-Driven Skin Microchanneling as a New Way for Electrically Assisted Transdermal Delivery of Hydrophilic Drugs,” Journal of Controlled Release, Vol. 89, No. 2, 2003, pp. 311-320. doi:10.1016/S0168-3659(03)00123-8
- G. Levin, et al., “Transdermal Delivery of Human Growth Hormone through RF-Microchannels,” Pharmaceutical Research, Vol. 22, No. 4, 2005, pp. 550-555. doi:10.1007/s11095-005-2498-6
- K. Okimoto, R. A. Rajewski and V. J. Stella, “Release of Testosterone from an Osmotic Pump Tablet Utilizing (SBE)7m-Beta-cyclodextrin as Both a Solubilizing and an Osmotic Pump Agent,” Journal of Controlled Release, Vol. 58, No. 1, 1999, pp. 29-38. doi:10.1016/S0168-3659(98)00142-4
- J. Pitha, S. M. Harman and M. E. Michel, “Hydrophilic Cyclodextrin Derivatives Enable Effective Oral Administration of Steroidal Hormones,” Journal of Pharmaceutical Sciences, Vol. 75, No. 2, 1986, pp. 165-167. doi:10.1002/jps.2600750213
- T. Loftsson, et al., “Effects of Cyclodextrins on Drug Delivery through Biological Membranes,” Journal of Pharmaceutical Sciences, Vol. 96, No. 10, 2007, pp. 2532- 2546. doi:10.1002/jps.20992
- M. E. Brewster and T. Loftsson, “Cyclodextrins as Pharmaceutical Solubilizers,” Advanced Drug Delivery Reviews, Vol. 59, No. 7, 2007, pp. 645-666. doi:10.1016/j.addr.2007.05.012
- H. Kalluri and A. K. Banga, “Formation and Closure of Microchannels in Skin Following Microporation,” Pharmaceutical Research, Vol. 28, No. 1, 2011, pp. 82-94. doi: 10.1007/s11095-010-0122-x
- I. Lavon and J. Kost, “Ultrasound and Transdermal Drug Delivery,” Drug Discovery Today, Vol. 9, No. 15, 2004, pp. 670-676. doi:10.1016/S1359-6446(04)03170-8
- B. E. Polat, D. Blankschtein and R. Langer, “Low-Frequency Sonophoresis: Application to the Transdermal Delivery of Macromolecules and Hydrophilic Drugs,” Expert Opinion on Drug Delivery, Vol. 7, No. 12, 2010, pp. 1415-1432. doi:10.1517/17425247.2010.538679
- K. V. Roskos and R. H. Guy, “Assessment of Skin Barrier Function Using Transepidermal Water Loss: Effect of Age,” Pharmaceutical Research, Vol. 6, No. 11, 1989, pp. 949-953. doi:10.1023/A:1015941412620
- G. Grubauer, P. M. Elias and K. R. Feingold, “Transepidermal Water Loss: The Signal for Recovery of Barrier Structure and Function,” Journal of Lipid Research, Vol. 30, No. 3, 1989, pp. 323-333.
- A. Anigbogu, et al., “An in Vivo Investigation of the Rabbit Skin Responses to Transdermal Iontophoresis,” International Journal of Pharmaceutics, Vol. 200, No. 2, 2000, pp. 195-206. doi:10.1016/S0378-5173(00)00371-9
- F. W. Benech-Kieffer and H. Schaefer, “Transepidermal Water Loss as an Integrity Test for Skin Barrier Function in Vitro: Assay Standardization,” In: K. R. Brain, V. J. James and K. A. Walters, Eds., Perspectives in Percutaneous Penetration, STS Publishing, Cardiff, 1977, p. 56.
- FDA Wound Healing Clinical Focus Group, “Guidance for Industry: Chronic Cutaneous Ulcer and Burn WoundsDeveloping Products for Treatment,” Wound Repair and Regeneration, Vol. 9, No. 4, 2001, pp. 258-268. doi:10.1046/j.1524-475X.2001.00258.x
- R. Panchagnula, K. Stemmer and W. A. Ritschel, “Animal Models for Transdermal Drug Delivery,” Methods & Findings in Experimental & Clinical Pharmacology, Vol. 19, No. 5, 1997, pp. 335-341.
- R. F. Diegelmann and M. C. Evans, “Wound Healing: An Overview of Acute, Fibrotic and Delayed Healing,” Frontiers in Bioscience, Vol. 9, 2004, pp. 283-289. doi:10.2741/1184
- D. Montandon, G. D’Andiran and G. Gabbiani, “The Mechanism of Wound Contraction and Epithelialization: Clinical and Experimental Studies,” Clinics in Plastic Surgery, Vol. 4, No. 3, 1977, pp. 325-346.
- J. Gupta, et al., “Kinetics of Skin Resealing after Insertion of Microneedles in Human Subjects,” Journal of Controlled Release, Vol. 154, No. 2, 2011, pp. 148-155. doi:10.1016/j.jconrel.2011.05.021
NOTES
*Corresponding author.

