Open Journal of Applied Biosensor
Vol.1 No.3(2012), Article ID:24556,9 pages DOI:10.4236/ojab.2012.13006
Sensitive Colorimetric and Fluorescent Detection of Mercury Using Fluorescein Derivations
1Key Laboratory of Chemical Biology and Molecular, Engineering of Ministry of Education, Institute of Molecular Science (IMS), Shanxi University, Taiyuan, China
2Research Institute of Applied Chemistry (RIAC), Shanxi University, Taiyuan, China
Email: #yincx@sxu.edu.cn
Received September 23, 2012; revised October 24, 2012; accepted November 1, 2012
Keywords: Fluorescein Hydrazide; Hg2+; Fluorescent; UV-Visible; Sensor
ABSTRACT
A colorimetric and fluorometric dual-model probe for mercury (II) ion was developed employing fluorescein hydrazide (FH) in ethanol-HEPES solution (1:1, v/v, pH 8.0). The probe exhibited high selectivity and sensitivity for Hg2+ detection using UV/Vis and fluorescence spectroscopy. Addition of Hg2+ caused a visual color change from colorless to coloured and a fluorescence change from colorless to bright green. Other metal ions did not interfere with the detection of Hg2+.
1. Introduction
The development of selective and sensitive imaging tools capable of monitoring heavyand transition-metal ions has attracted considerable attention because of the wide use of these metal ions and their subsequent impact on the environment and nature [1-3]. Mercury pollution specifically is a topic of recent concern [4-6] because mercury contamination is widespread and originates from a variety of natural and anthropogenic sources including oceanic and volcanic emission [7,8], gold mining [9], solid waste incineration, and the combustion of fossil fuels [10]. Once introduced into the marine environment, bacteria convert inorganic mercury into methylmercury, which enters the food chain and accumulates in higher organisms, especially in large edible fish [11]. Mercury can accumulate in the human body and may cause wide variety of diseases even in a low concentration, such as prenatal brain damage, kidney failure [12], serious cognitive and motion disorders, and Minamata disease [13].
Many types of mercury sensors have been developed based on small fluorescent organic molecules [14-20], proteins [21], oligonucleotides [22,23], genetically engineered cells [24], conjugated polymers [25], foldamers [26], membranes [27], electrodes [28], and nanomaterials [29-32]. Recently, considerable efforts have been made to develop a colorimetric or fluorescent molecular probe for mercury ions [33-37]. Many of these systems are based on well established and unique molecular frameworks, such as crown ethers [38,39], calix[4] arenes [40], cyclams [41], squaraines [42], 8-hydroxyquinolines [43] 1,4-disubstituted azines [44], thioureas [45], 1,3-dithiole- 2-thione [46]. Before fluorescein hydrazide or other fluorescein derivations were used as sensors for Cu2+ [39,40]. However, in current work, we employed fluorescein derivation (Figure 1) to design and construct colorimetric and fluorometric dual-channel assay to specifically detect Hg2+ the presence of a wide range of other cations and anions in ethanol-HEPES (1:1, v/v, pH 8.0) solution. It is noted that FH was used as Cu2+ sensor by Chen et al. in pH 7.2 Tris buffer before, which there are many differences from those in the manuscript: a) sensor conditions are different; b) the sensor targets are different; c) UVVisible spectra are different; d) the system color changes are different; e) fluorescence properties and intensity are different. These studies have demonstrated for the first time a controllable and multifunctional chemosensor in different buffer conditions. Thus, the results are significant and interesting as a new generation of chemosensors produced.
2. Experimental
2.1. Reagents and Chemicals
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was purchased from Sigma-Aldrich. FH was synthesized using a modification of a literature method. HEPES solutions were adjusted to pH 8.0 by adding
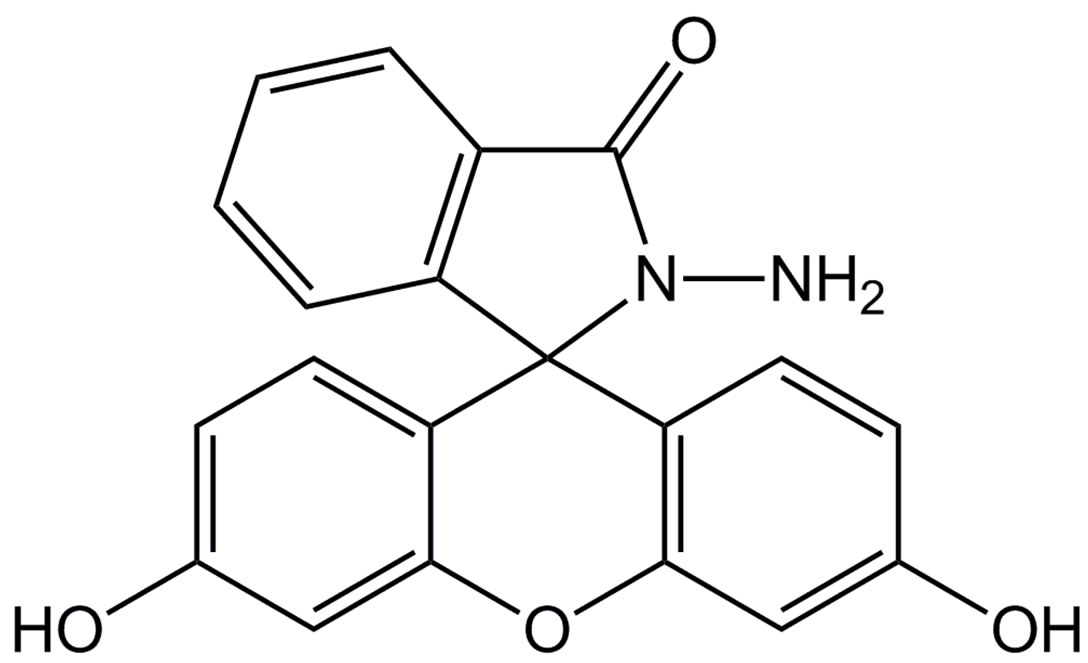
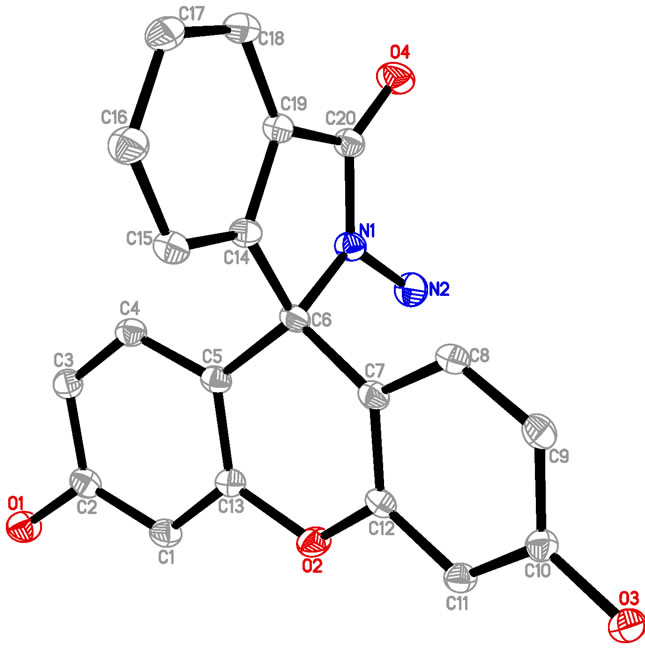
Figure 1. The structures of FH.
NaOH (0.1 M) to aqueous HEPES (10 mM). Cationic salts were purchased from Shanghai Experiment Reagent Co., Ltd (Shanhai, China). All the common chemicals used were of analytical grade.
2.2. Apparatus
A Mettler Toledo pH meter (Mettler-Toledo International Inc, Switzerland) was used to determined pH. The UVVisible spectra were recorded on a Cary 50 Bio UVVisible spectrophotometer (Agilent, Santa Clara, CA). Fluorescence spectra were measured using a Cary Eclipse fluorescence spectrophotometer (Agilent, Santa Clara, CA). A PO-120 quartz cuvette (10 mm) was purchased from Huamei Experiment Instrrument Plants (Shanhai, China). 1H NMR and 13C NMR spectra were recorded on a Bruker DRX-300MHz NMR spectrometer (Billerica, MA). A light yellow single crystal of FH was mounted on a glass fiber for data collection. Cell constants and an orientation matrix for data collection were obtained by least-squares refinement of diffraction data from reflections with 2.04˚ - 27.4˚ for FH using a Bruker SMART APEX CCD automatic diffractometer. Data were collected at 173 K using Mo Kα radiation (λ = 0.710713 Å) and the ω-scan technique and corrected for Lorentz and polarization effect (SADABS) [50]. The structures were solved by direct methods (SHELX97) [51], and subsequent difference Fourier map and then refined on F2 using a full-matrix least-squares procedure and anisotropic displacement parameters.
2.3. Preparation of FH
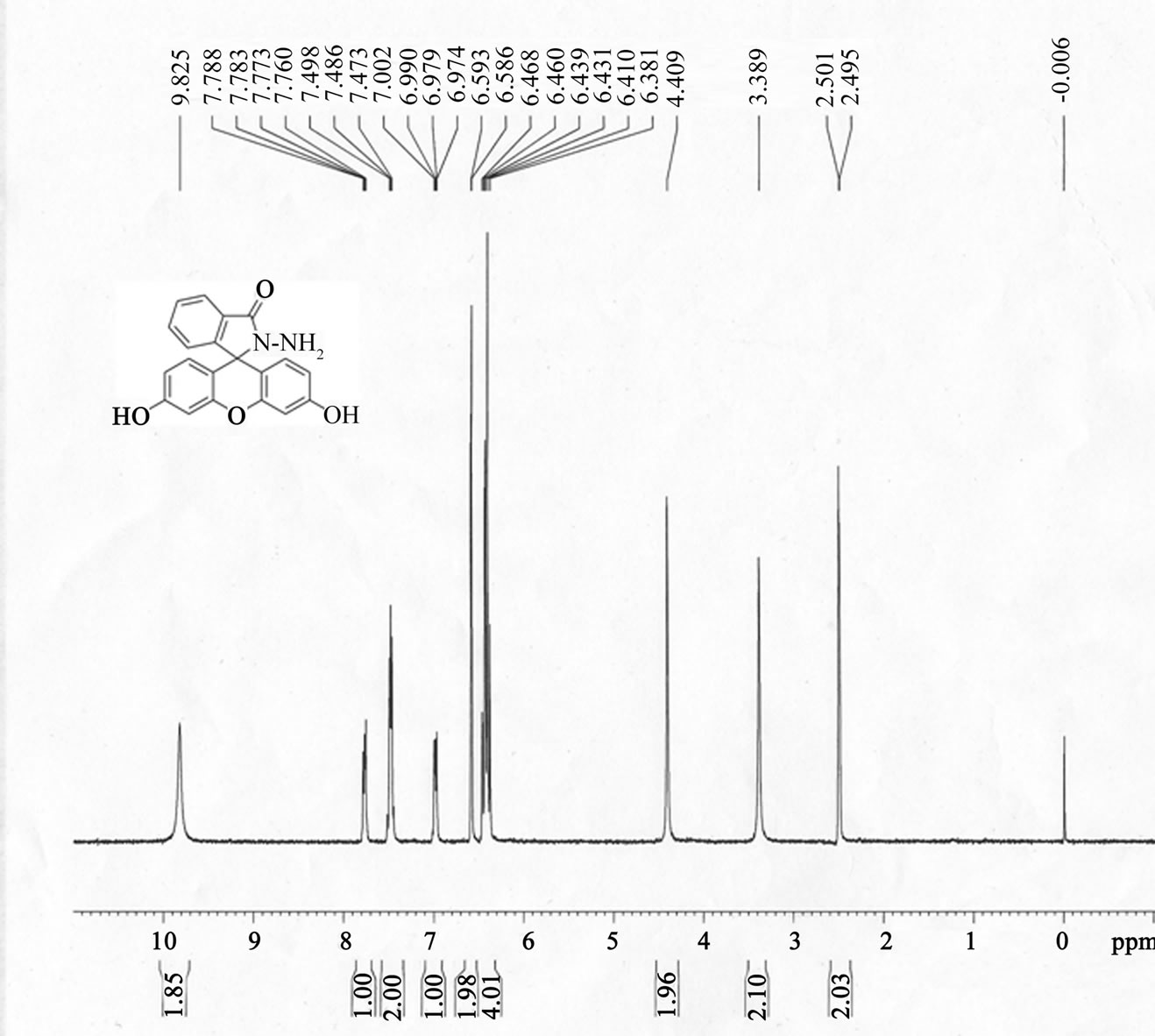
FH was prepared in high yield by reacting fluorescein with hydrazine hydrate in methanol (Scheme 1) according to the literature [52,53]. An excessive hydrazine hydrate (85%, 1.2 mL) was added to a 0.35 g of fluorescein dissolved in 20 ml of ethanol, and the reaction solution was refluxed in oil bath for 8 h. A brown oily product resulted from evacuating ethanol under reduced pressure. The solid product was precipitated by adding water and recrystallized from ethanol/water mixture, producing the fluorescein hydrazide (FH) as a yellow powder with 72% yield (0.25 g). The H2O/ethanol solution was allowed to evaporate slowly at room temperature for several days and yellow crystals suitable for X-ray crystallography were formed. 1H NMR ,(DMSO-d6): δ (ppm) 9.80 (s, 2H), 7.76 (m, 1H), 7.48 (m, 2H), 6.99 (m, 1H), 6.58 (s, 2H), 6.43 (d, 2H), 6.38 (d, 2H), 4.37 (s, 2H); 13C NMR (75 MHz, CDCl3): δ 24.25, 33.00, 113.31, 117.96, 121.58, 121.88, 123.80, 138.69, 156.24, 196.37 (Figure S1(a)); ESI-MS m/z 347.2 [FH+H]+ (calcd. 347.l) (Figure S1(b)); Elemental analysis (calcd.%) for C20H14N2O4: C, 69.36; N, 8.09; H, 4.07: Found: C, 69.30; N, 8.11; H, 4.01. Crystal data for C20H16N2O5 (Figure S1(c)): crystal size: 0.22 × 0.2 × 0.1, triclinic, space group P-1 (No. 2). a = 7.5959(15) Å, b = 10.690(2) Å, c = 11.028(2) Å, α = 104.34(3)˚, β = 109.09(3)˚, γ = 99.67(3)˚, V = 789.0(4) Å3, Z = 2, T = 173K, θmax = 25.0˚, 7521 reflections measured, 2762 unique (Rint = 0.0412). Final residual for 250 parameters and 2503 reflections with I > 2σ(I): R1 = 0.0622, wR2 = 0.1390 and GOF = 1.17.
2.4. General UV-Vis and Fluorescence Spectra Measurements
Since the chemosensor was not fully soluble in 100% aqueous media, ethanol was used as a solubilizing medium. FH stock solutions were prepared in ethanol. The UV-Vis and fluorescence spectra were obtained in mixed ethanol /HEPES aqueous buffer (1:1, v/v, 10 mM, pH 8.0) solution. Aqueous metal ion solutions were also prepared. Fluorescence measurements were carried out with a slit width of 10 nm.
2.5. Preparation of FH
The UV-Vis spectrum was characterized by a main band centred at 641 nm. The low detection threshold for Hg2+ was in the order of 10−6 - 10−5 M and at this level the colour change was very obvious. The fluorescence emission was measured for each sample by exciting at 450 nm and spectra and measuring from 475 - 700 nm. The sensitivity range for Hg2+ was 10−7 - 10−6 M.
3. Results and Discussion
3.1. UV-Vis Spectra
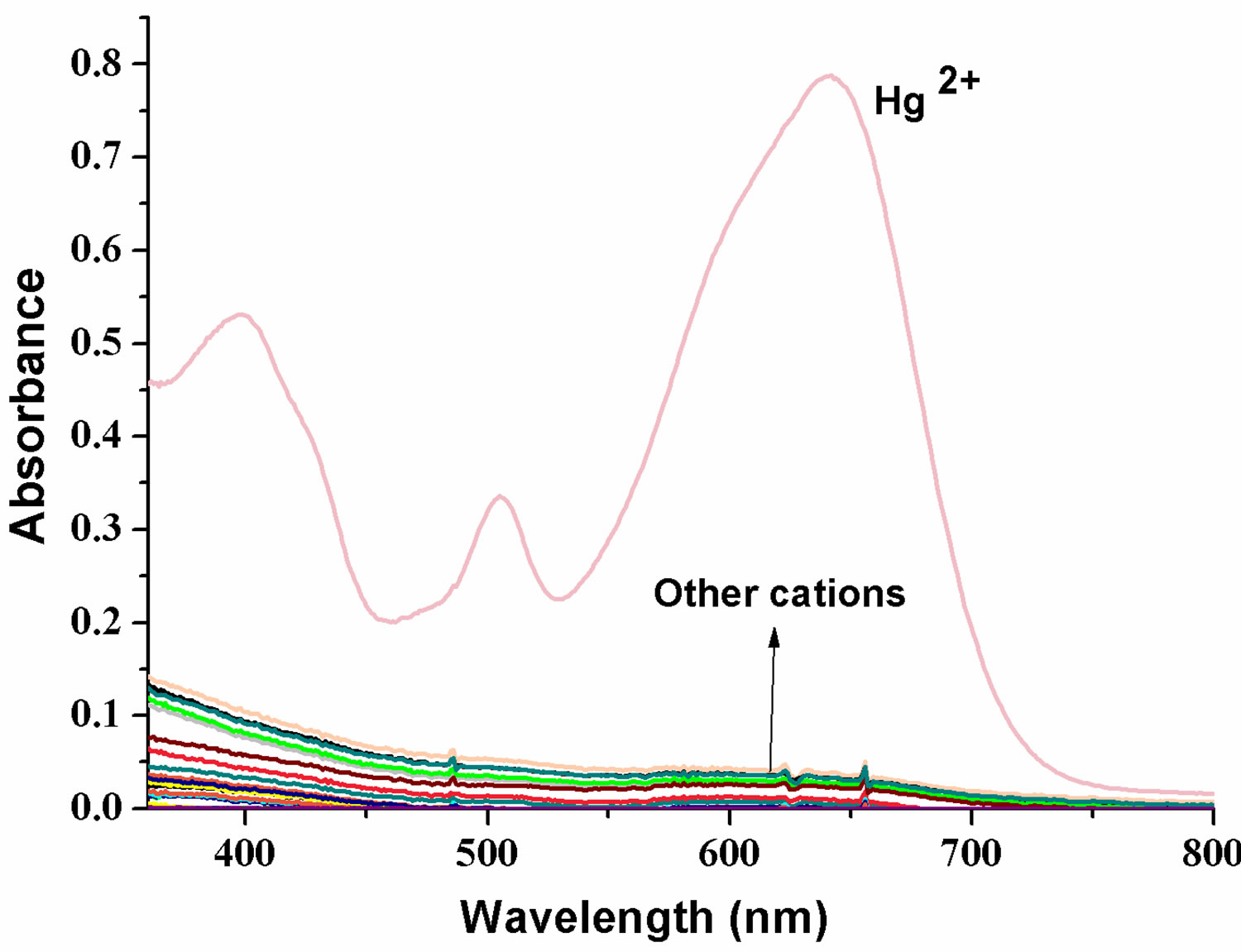
The complexation ability of FH with Hg2+ ion was investigated by UV-Vis absorption techniques. FH does not absorb in the range of 400 - 800 nm in a mixed solution of ethanol/HEPES (v/v = 1:1). Figure 2 shows the spectral changes of FH in ethanol/HEPES (v/v = 1:1) upon addition of various competitive metal ions, such as Hg2+,
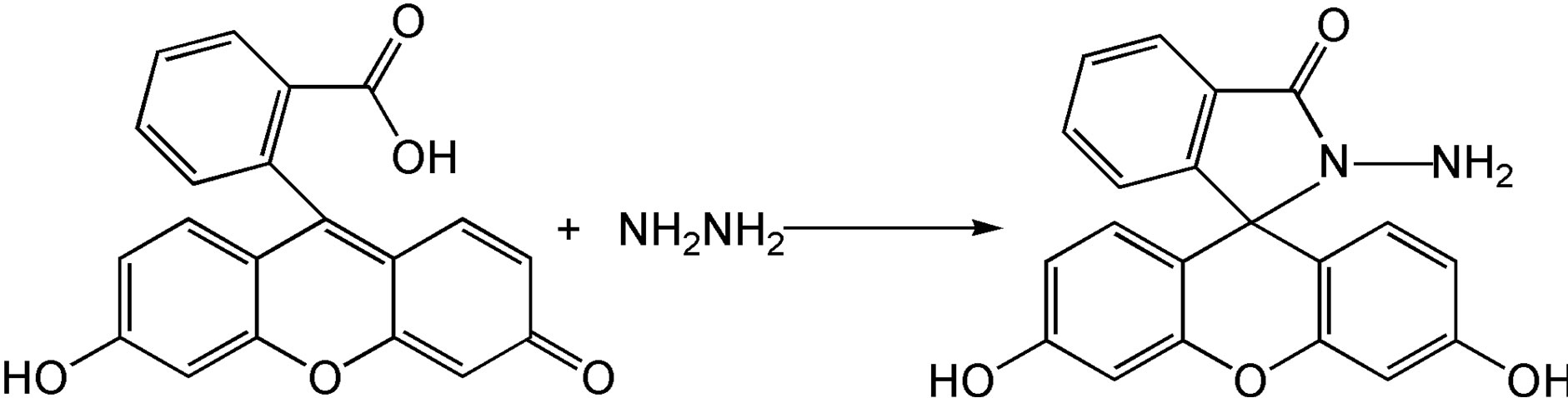
Scheme 1.Synthesis of probe.
 (a)
(a)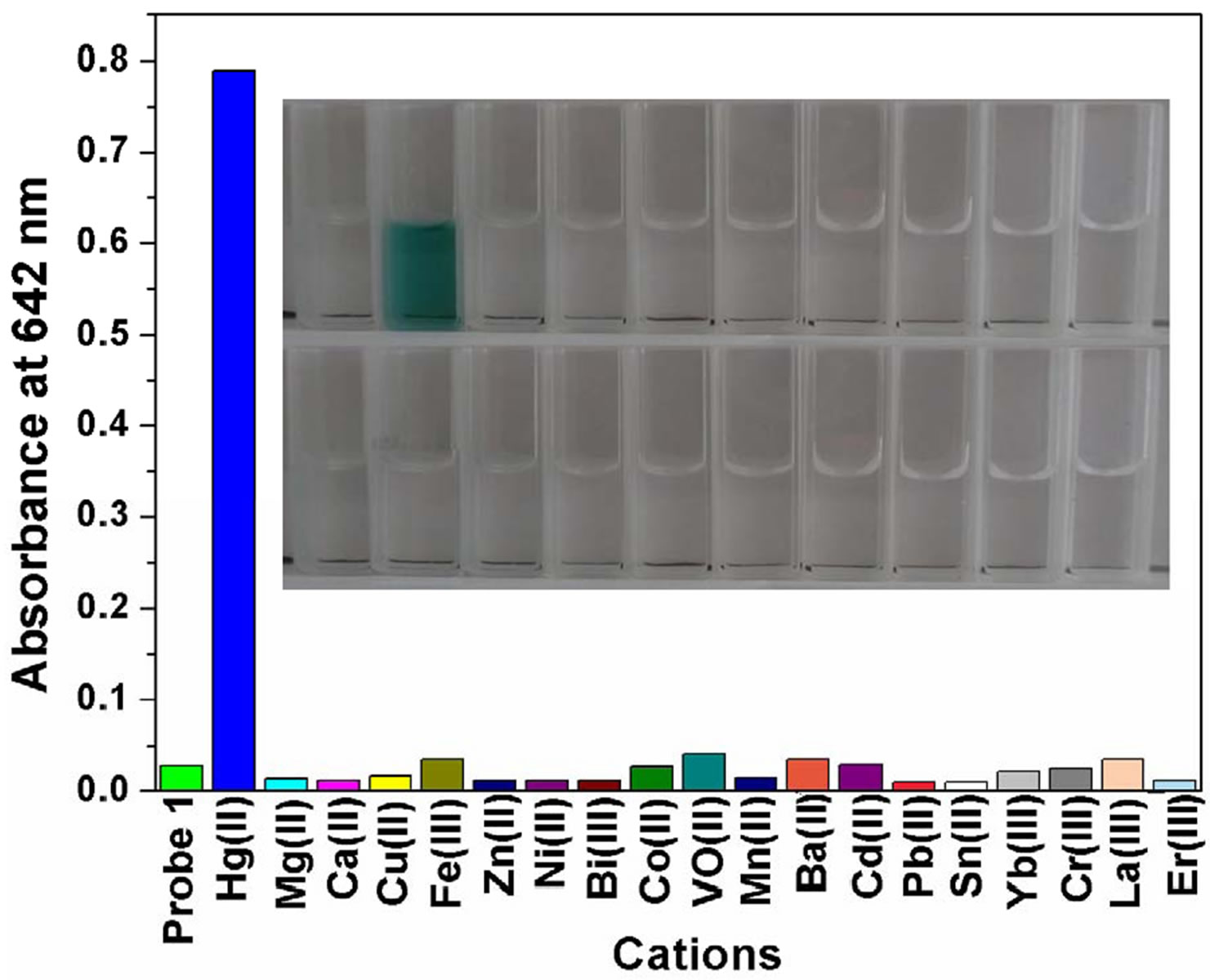 (b)
(b)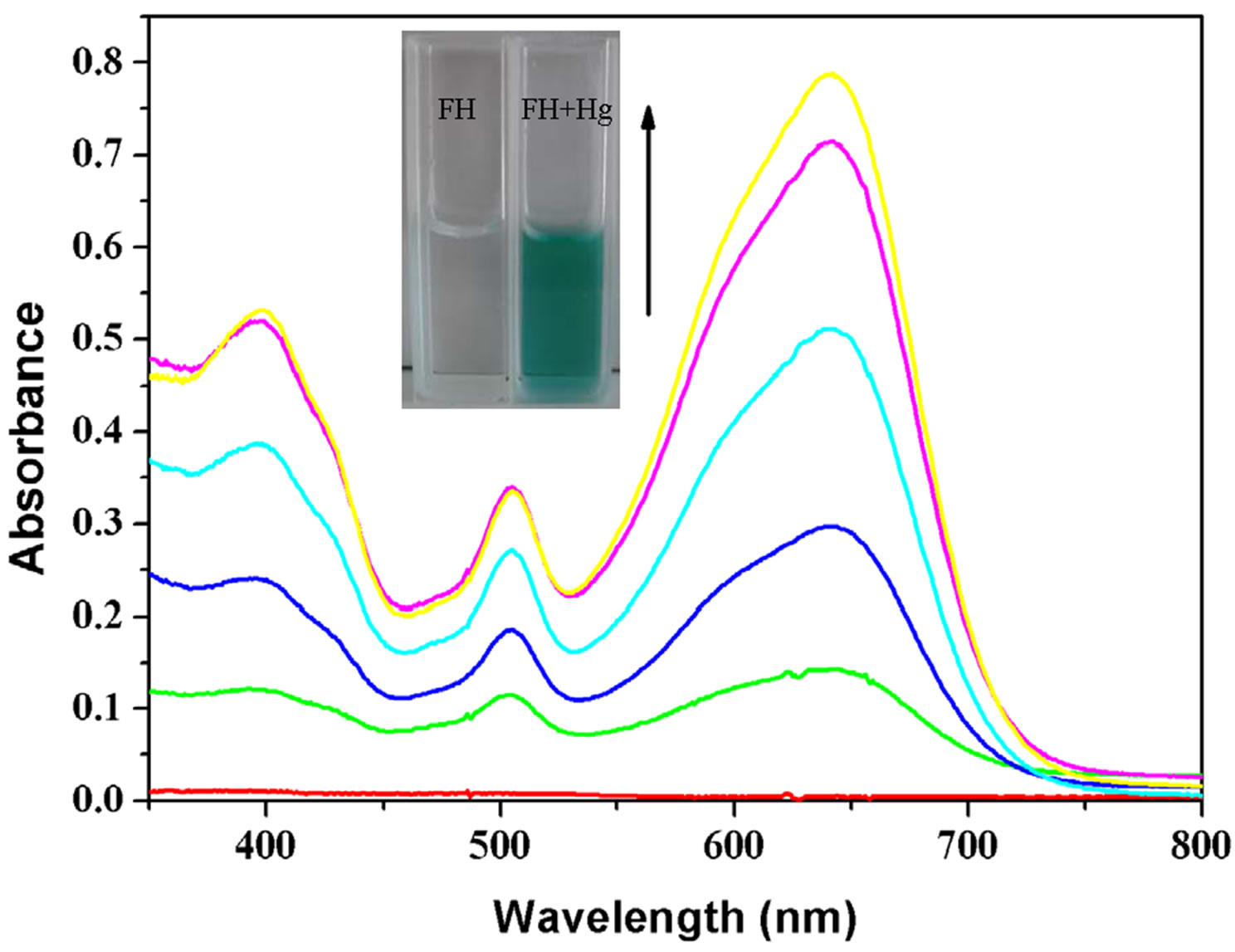 (c)
(c)
Figure 2. (a) UV-Visible spectra of FH (30 μM) in the presence of various metal ions in ethanol/HEPES solution (1:1, v/v, pH 8.0); (b) Optical density of the probe FH (30 μM) at 642 nm in the presence of various metal ions (250 μM) including: Hg2+, Mg2+, Ca2+, Cu2+, Fe3+, Zn2+, Ni2+, Bi3+, Co2+, VO2+, Mn2+, Ba2+, Cd2+, Pb2+, Sn2+, Yb3+, Cr3+, La3+, Er3+ etc. Inset: a color change photograph for Hg2+ and other cations. From left to right, then from top to bottom: FH (30 μM), and FH with Hg2+, Mg2+, Ca2+, Cu2+, Fe3+, Zn2+, Ni2+, Bi3+, Co2+, VO2+, Mn2+, Ba2+, Cd2+, Pb2+, Sn2+, Yb3+, Cr3+, La3+, Er3+ etc.; (c) Absorption spectral changes of FH (30 μM) in ethanol/HEPES buffer solution (1:1, v/v, 10 mM, pH 8.0) upon addition of Hg2+; Hg2+ added gradually with [Hg2+] = 0, 6, 12, 18, 24, 30 μM; each spectrum is recorded 3 h after Hg2+ addition. Inset: visual color changes of FH (30 μM) upon addition of Hg2+ in ethanol/HEPES buffer solution (1:1, v/v, 10 mM, pH 8.0).
Mg2+, Ca2+, Cu2+, Fe3+, Zn2+, Ni2+, Bi3+, Co2+, VO2+, Mn2+, Ba2+, Cd2+, Pb2+, Sn2+, Yb3+, Cr3+, La3+, Er3+, etc. From UV/Vis spectra (Figure 2(a)), we can clearly observe a new absorption band centered at 397, 504 and 641 nm for FH (30 µM) in the presence of 1 equiv of Hg2+. In contrast, other ions lead to almost no spectral changes (Figures 2(a) and (b)). In our present experiments, HgCl2 as a Hg2+ source was gradually added to the ethanol /HEPES (v/v = 1:1) FH solution. Notably, the new band at 397, 504 and 641 nm appeared and concomitantly grew with increasing Hg2+ concentration (Figure 2(c)).
3.2. Fluorescence Spectra
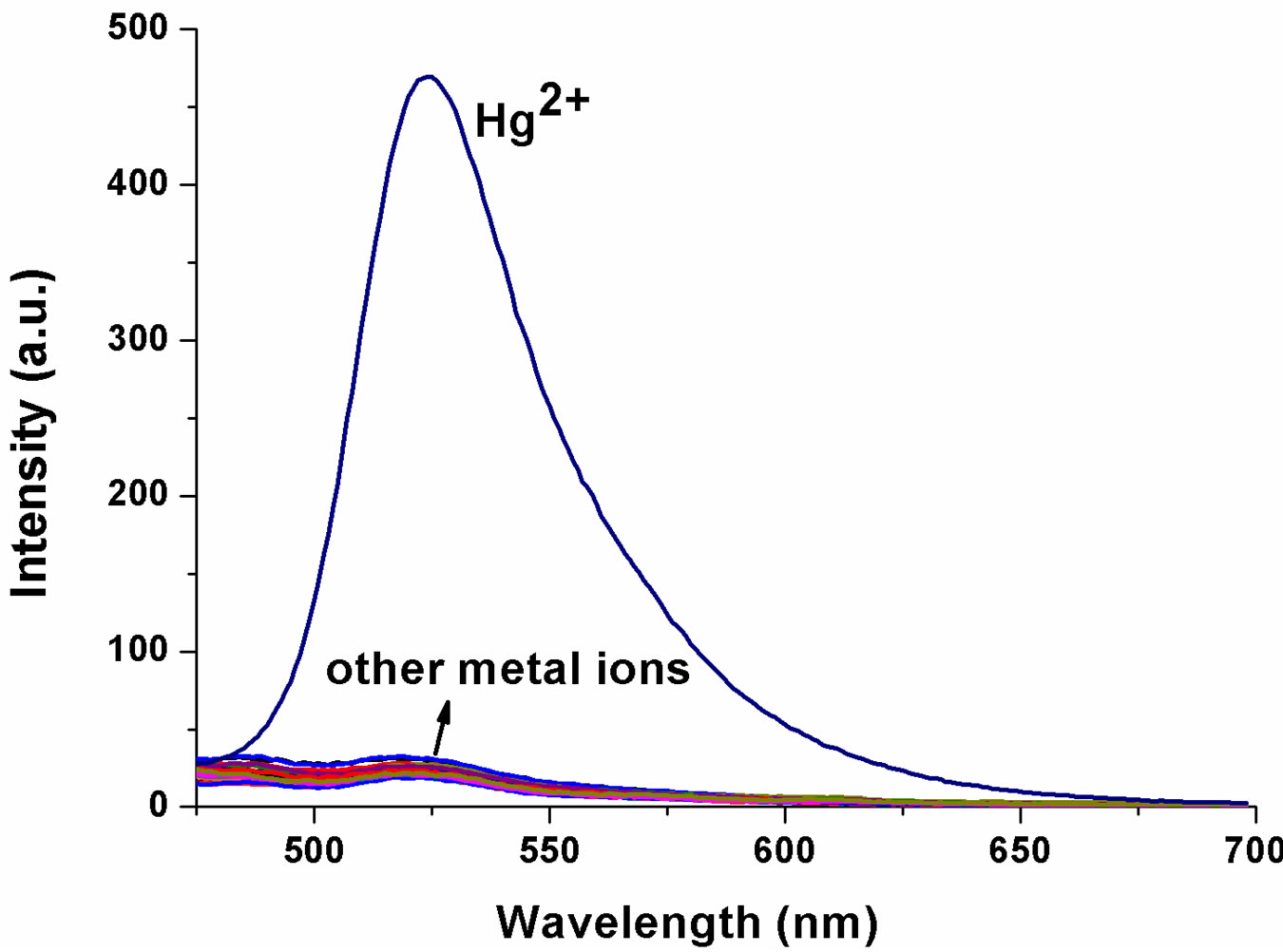
The ability of FH to selectively sense Hg2+ was determined analysis of the fluorescence spectra obtained with 1 μM of FH in ethanol/HEPES (v/v = 1:1) in the presence of a number of cations including Mg2+, Ca2+, Cu2+, Fe3+, Zn2+, Ni2+, Bi3+, Co2+, VO2+, Mn2+, Ba2+, Cd2+, Pb2+, Sn2+, Yb3+, Cr3+, La3+ and Er3+ etc. (100 equiv to Hg2+, respectively). The fluorescence spectra (Figure 3) show a similar result, which is consistent with that of UVVisible spectra. Addition of 3 equiv of Hg2+ ion results in an obviously enhanced fluorescence at 522 nm (OFFON), with an excitation at 460 nm while other ions induce no increase in fluorescence (Figure 3(a)). More interestingly, Hg2+-induced fluorescence-on change for the FH is visual with a solution color change from colorless to green under illumination with a 365 nm UV lamp (Figure 3(b)). As shown in Figure 3(c), a new emission band peak appears with the fluorescence intensity increasing with increase in Hg2+ concentration. Both UVVis and fluorescence results indicate that FH shows a good selectivity and sensitivity toward Hg2+ over other competitive cations.
Furthermore, a plot of fluorescence intensity when FH is titrated with 3 µM of Hg2+ shows good linearity (correlation coefficient of R = 0.9985) for a Hg2+ concentration range of 0.25 - 3 µM (Figure 4).
3.3. pH Dependent
The above-mentioned UV-Visible light absorption occurred at a pH of 8.0, which is close to physiological conditions. At a pH of 9.0, it seems that Hg2+ detection is possible, and that the absorbance was affected by solution alkalinity. At all other pH conditions, no notable change in either color or UV-Visible spectrum was noted (Figure 5).
3.4. Proposed Mechanism
To provide reasonable envidence of FH sensing of Hg2+ ion, electrospray ionization mass spectrometry (ESI-MS) analysis was conducted (Figure S2). Mass peaks at m/z 531.2 corresponding to [Fluorescein + Hg]+ are clearly
 (a)
(a)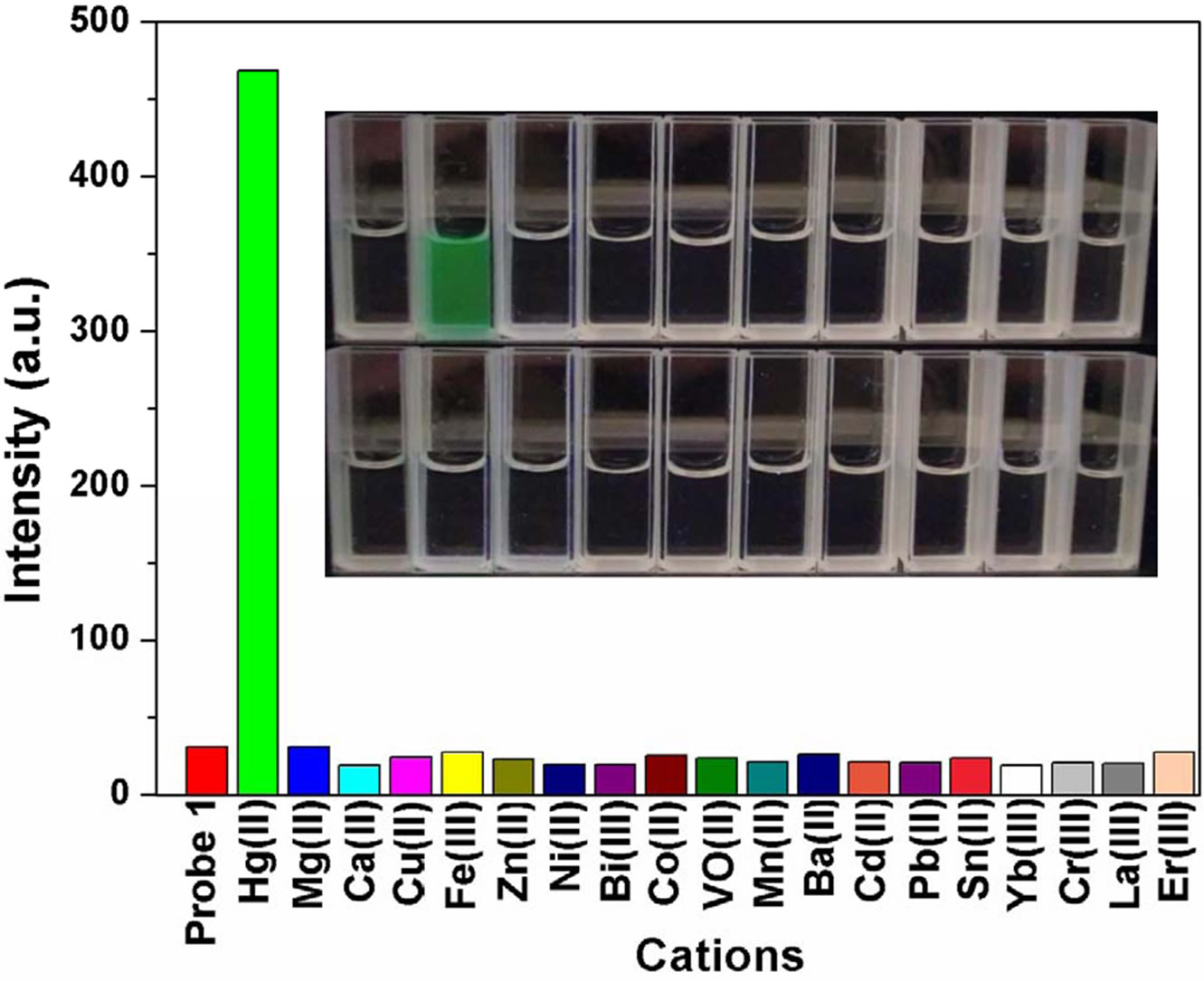 (b)
(b)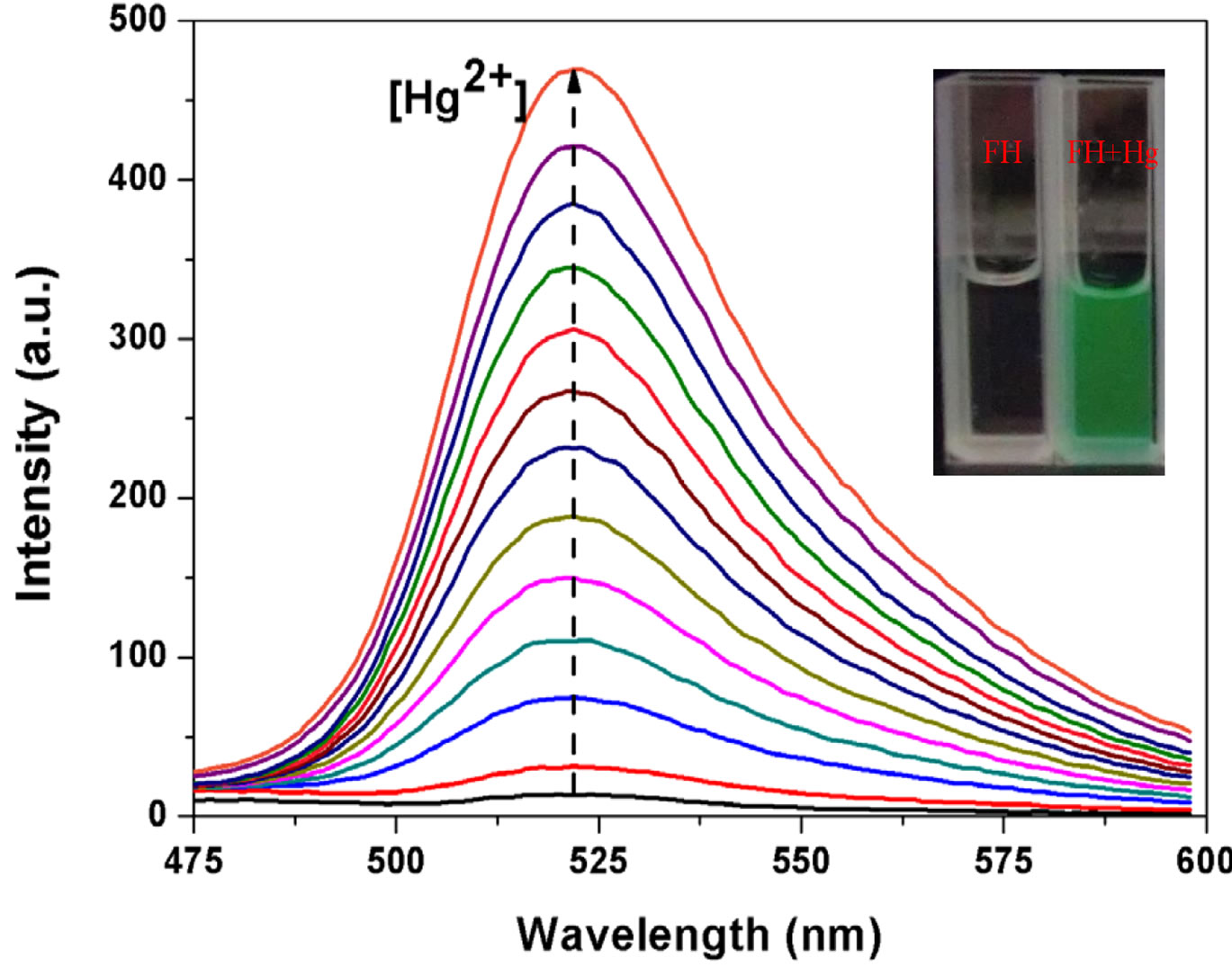 (c)
(c)
Figure 3. (a) Fluorescence spectra of FH (1 μM) in the presence of various metal ions in ethanol/HEPES solution (1:1, v/v, pH 8.0) (λex = 460 nm, λem = 522 nm, slit: 10 nm/10 nm) ; (b) Optical density of the probe FH (10 μM) at 522 nm in the presence of 300 μM various metal ions including: Hg2+, Mg2+, Ca2+, Cu2+, Fe3+, Zn2+, Ni2+, Bi3+, Co2+, VO2+, Mn2+, Ba2+, Cd2+, Pb2+, Sn2+, Yb3+, Cr3+, La3+, Er3+ etc. Inset: a visual fluorescence change photograph for Hg2+ and other cations under illumination with a 365 nm UV lamp. From left to right, then from top to bottom: FH (30 μM), and FH wit Hg2+, Mg2+, Ca2+, Cu2+, Fe3+, Zn2+, Ni2+, Bi3+, Co2+, VO2+, Mn2+, Ba2+, Cd2+, Pb2+, Sn2+, Yb3+, Cr3+, La3+, Er3+ etc.; (c) Fluorescence spectral changes of FH (1 μM) in ethanol/ HEPES buffer solution (1:1, v/v, 10 mM, pH 8.0) (λex = 460 nm, λem = 522 nm, slit: 10 nm/10 nm) upon addition of Hg2+; Hg2+ was added gradually with [Hg2+] = 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0 μM; Each spectrum is recorded 6 h after Hg2+ addition. Inset: visual fluorescence changes of FH upon addition of Hg2+ in ethanol/HEPES buffer solution (1:1, v/v, 10 mM, pH 8.0) under illumination with a 365 nm UV lamp.
observed, This provides direct evidence for the proposed response mechanism (Scheme 2). The hydrolysis complex with fluorescein anion is responsible for the above dual color and fluorescence changes.
4. Conclusion
In summary, we have demonstrated a simple Hg2+-selective chromogenic and fluorogenic chemodo-simeter system using a fluorescein hydrazide (FH) molecule in semi-aqueous solution. The sensor mechanism was proposed to be mercury-promoted hydrolysis procedure of

Figure 4. Plot of fluorescence intensity change of FH (1 μM) at 522 nm against Hg2+ concentration varied from 0.25 to 3 μM at λex/em = 460 - 522 nm.
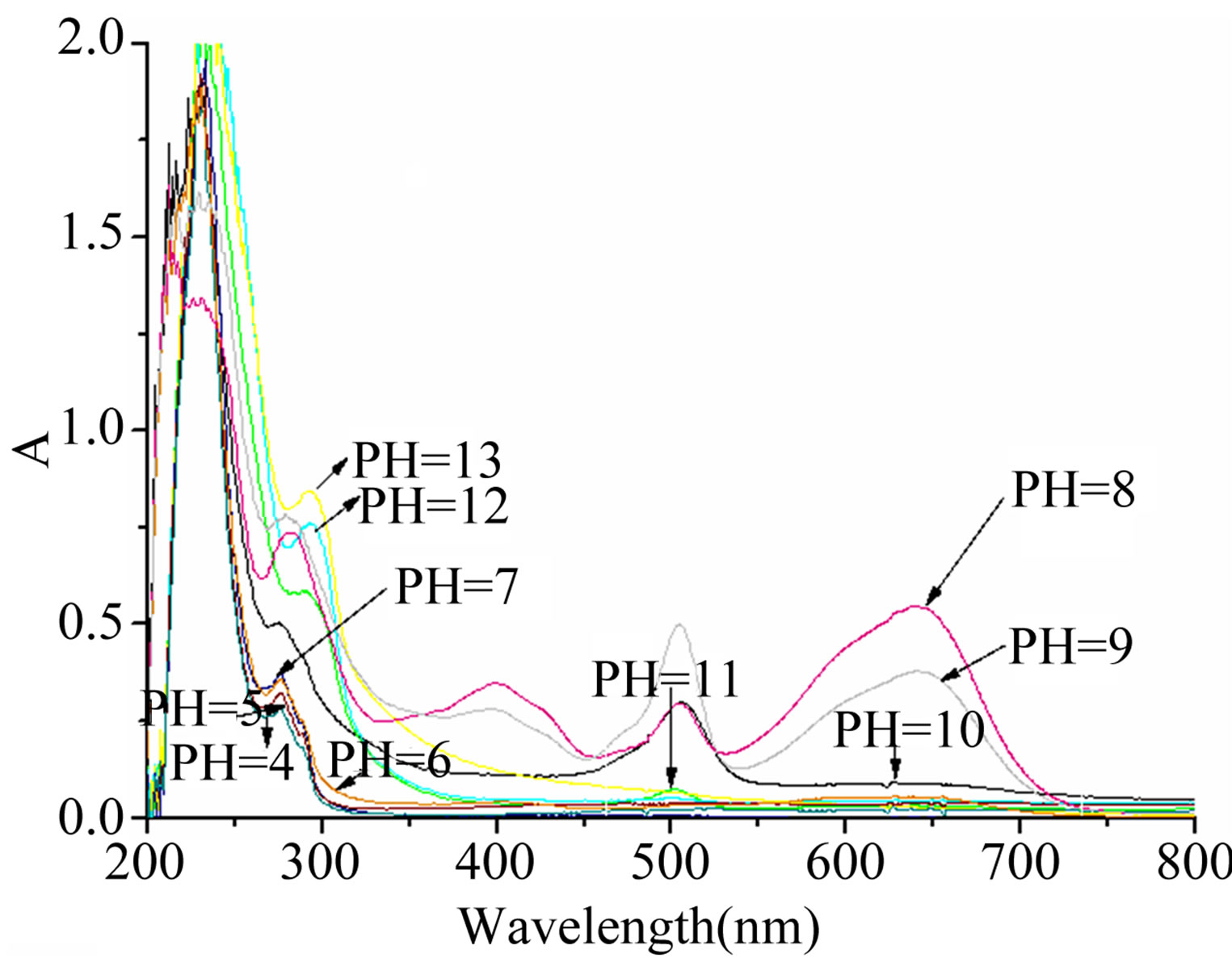
Figure 5. pH ranges for the measurement.
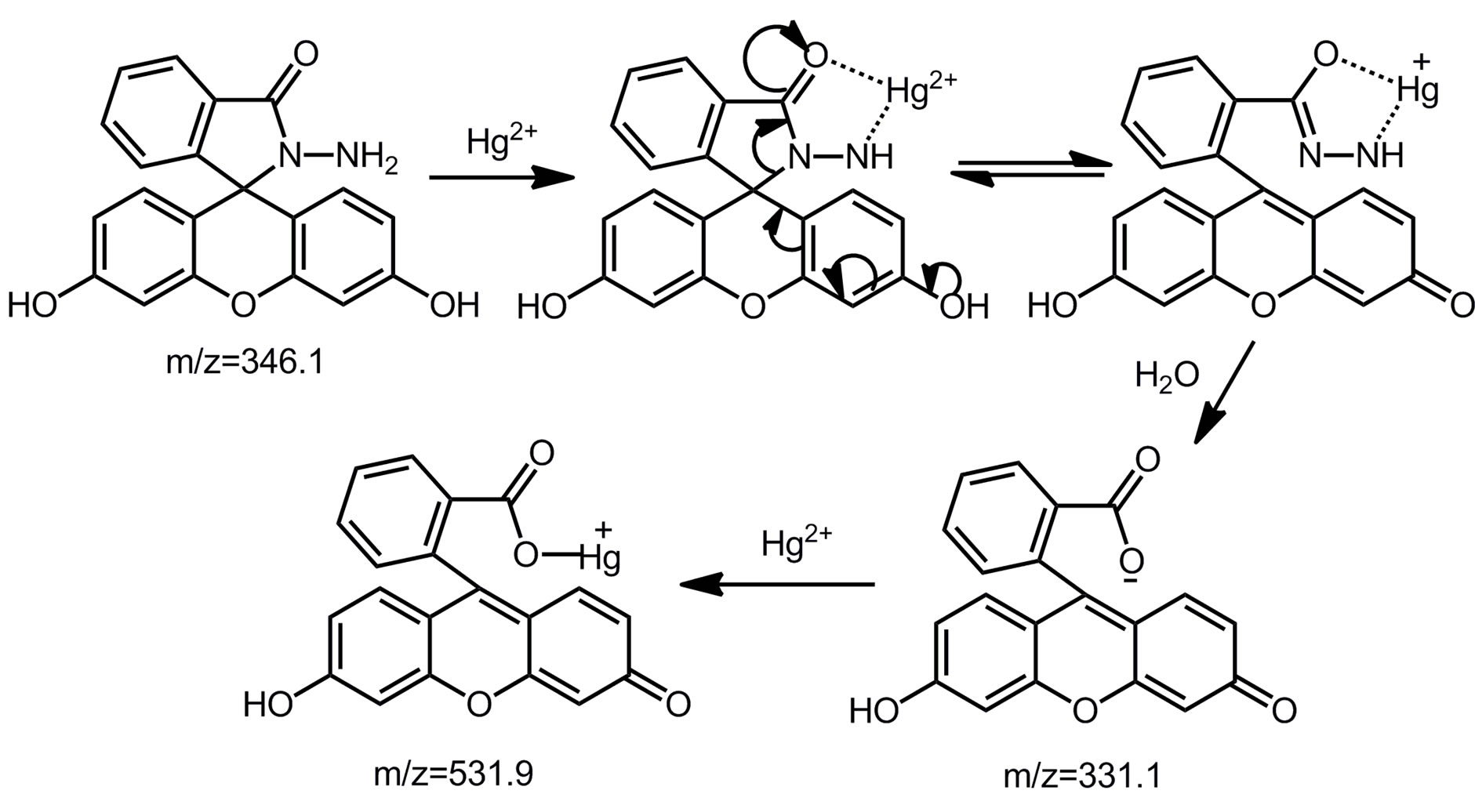
Scheme 2.Proposed detection mechanism of FH for Hg2+.
FH. This is another case of chemodosimeters as Rhodamine B hydrazide sensor for Cu (II) [44]. Visual color and fluorescence response suggests the probe’s practicability for further environmental and biological mecury ions detection.
5. Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 21072119, 21102086), the Shanxi Province Science Foundation for Youths (No. 2012021009-4), the Shanxi Province Foundation for Returnee (No. 2012-007), the Taiyuan Technology star special (No. 12024703).
REFERENCES
- A. P. De Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, “ChemInform Abstract: Signaling Recognition Events with Fluorescent Sensors and Switches,” Chemical Reviews, Vol. 97, No. 5, 1997, pp. 1515-1566. doi:10.1002/chin.199744337
- X. Peng, J. Du, J. Fan, J. Wang, Y. Wu, J. Zhao, S. Sun and T. Xu, “A Selective Fluorescent Sensor for Imaging Cd2+ in Living Cells,” Journal of the American Chemical Society, Vol. 129, No. 6, 2007, pp. 1500-1501. doi:10.1021/ja0643319
- M. Royzen, Z. Dai and J. W. Cannry, “Ratiometric Displacement Approach to Cu (II) Sensing by Fluorescence,” Journal of the American Chemical Society, Vol. 127, No. 6, 2005, pp. 1612-1613. doi:10.1021/ja0431051
- P. M. Bolger and B. A. Schwetz, “Mercury and Health,” The New England Journal of Medicine, Vol. 347, No. 22, 2002, pp. 1735-1736. doi:10.1056/NEJMp020139
- H. H. Harris, I. J. Pickering and G. N. George, “The Chemical Form of Mercury in Fish,” Science, Vol. 301, No. 5637, 2003, p. 1203. doi:10.1126/science.1085941
- T. W. Clarkson, L. Magos and G. J. Myers, “The Toxicology of Mercury-Current Exposures and Clinical Manifestations,” The New England Journal of Medicine, Vol. 349, No. 18, 2003, pp. 1731-1737. doi:10.1056/NEJMra022471
- A. Renzoni, F. Zino and E. Franchi, “Mercury Levels along the Food Chain and Risk for Exposed Populations, Environmental Research,” Vol. 77, No. ER983832, 1998, pp. 68-72. doi:10.1006/enrs.1998.3832
- D. B. Gil, M. I. Rodriguez-Cáceres, M. del C. HurtadoSánchez and A. M. de la Pena, “Fluorescent Determination of Hg2+ in Water and Fish Samples Using a Chemodosimeter Based in a Rhodamine 6G Derivative and a Portable Fiber-Optic Spectrofluorimeter,” Applied Spectroscopy, Vol. 64, No. 5, 2010, pp. 520-527. doi:10.1366/000370210791211600
- O. Malm, “Gold Mining as a Source of Mercury Exposure in the Brazilian Amazon,” Environmental Research, Vol. 77, 1998, pp. 73-78, Article ID: 983828. doi:10.1006/enrs.1998.3828
- E. M. Nolan and S. J. Lippard, “Turn-On and Ratiometric Mercury Sensing in Water with a Red-Emitting Probe,” Journal of the American Chemical Society, Vol. 129, No. 18, 2007, pp. 5910-5918. doi:10.1021/ja068879r
- S. Hardy and P. Jones, “Capillary Electrophoresis Determination of Methylmercury in Fish and Crab Meat after Extraction as the Dithizone Sulphonate Complex,” Journal of Chromatography A, Vol. 791, No. 1-2, 1997, pp. 333-338. doi:10.1016/S0021-9673(97)00829-7
- Z. D. Wang, J. H. Lee and Y. Lu, “Highly Sensitive ‘TurnOn’ Fluorescent Sensor for Hg2+ in Aqueous Solution Based on Structure-Switching DNA,” Chemical Communication, No. 45, 2008, pp. 6005-6007. doi:10.1039/b812755g
- M. Harada, “Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution,” Critical Reviews in Toxicology, Vol. 25, No. 1, 1995, pp. 1-24. doi:10.3109/10408449509089885
- L. Prodi, C. Bargossi, M. Montalti, N. Zaccheroni, N. Su, J. S. Bradshaw, R. M. Izatt and P. B. Savage, “An Effective Fluorescent Chemosensor for Mercury Ions,” Journal of the American Chemical Society, Vol. 122, No. 28, 2000, pp. 6769-6770. doi:10.1021/ja0006292
- X. Guo, X. Qian and L. Jia, “A Highly Selective and Sensitive Fluorescent Chemosensor for Hg2+ in Neutral Buffer Aqueous Solution,” Journal of the American Chemical Society, Vol. 126, No. 8, 2004, pp. 2272-2278. doi:10.1021/ja037604y
- Y. K. Yang, K. J. Yook and J. Tae, “A Rhodamine-Based Fluorescent and Colorimetric Chemodosimeter for the Rapid Detection of Hg2+ Ions in Aqueous Media,” Journal of the American Chemical Society, Vol. 127, No. 48, 2005, pp. 16760-16761. doi:10.1021/ja054855t
- M. H. Ha-Thi, M. Penhoat, V. Michelet and I. Leray, “Highly Selective and Sensitive Phosphane Sulfide Derivative for the Detection of Hg2+ in an Organoaqueous Medium,” Organic Letters, Vol. 9, No. 6, 2007, pp. 1133- 1136. doi:10.1021/ol070118l
- E. M. Nolan and S. J. Lippard, “Turn-On and Ratiometric Mercury Sensing in Water with a Red-Emitting Probe,” Journal of the American Chemical Society, Vol. 129, No. 18, 2007, pp. 5910-5918. doi:10.1021/ja068879r
- Z. X. Han, H. Y. Luo, X. B. Zhang, R. M. Kong, G. L. Shen and R. Q. Yua, “A Ratiometric Chemosensor for Fluorescent Determination of Hg(2+) Based on a New Porphyrin-Quinoline Dyad,” Spectrochimica Acta Part A, Vol. 72, No. 5, 2009, pp. 1084-1088. doi:10.1016/j.saa.2009.01.003
- Y. M. Li, X. L. Zhang, B. C. Zhu, J. Xue and L. L. Yan, “A Disulfide-Linked Naphthalimide Dimer for Hg(II) Detection in Aqueous Solution,” The Journal of Fluorescence, Vol. 21, No. 4, 2011, pp. 1343-1348. doi:10.1007/s10895-010-0820-0
- S. V. Wegner, A. Okesli, P. Chen and C. He, “Design of an Emission Ratiometric Biosensor from MerR Family Proteins: A Sensitive and Selective Sensor for Hg2+,” Journal of the American Chemical Society, Vol. 129, No. 12, 2007, pp. 3474-3475. doi:10.1021/ja068342d
- K. C. Chiang, C. Huang, C. W. Liu and H. T. Chang, “Oligonucleotide-Based Fluorescence Probe for Sensitive and Selective Detection of Mercury(II) in Aqueous Solution,” Analytical Chemistry, Vol. 80, No. 10, 2008, pp. 3716-3721. doi:10.1021/ac800142k
- C. X. Tang, Y. Zhao, X. W. He and X. B. Yin, “A ‘TurnOn’ Electrochemiluminescent Biosensor for Detecting Hg2+ at Femtomole Level Based on the Intercalation of Ru(phen)32+ into ds-DNA,” Chemical Communications, Vol. 46, No. 47, 2010, pp. 9022-9024. doi:10.1039/c0cc03495a
- M. Virta, J. Lampinen and M. Karp, “A LuminescenceBased Mercury Biosensor,” Analytical Chemistry, Vol. 67, No. 3, 1995, pp. 667-669. doi:10.1021/ac00099a027
- X. Liu, Y. Tang, L. Wang, J. Zhang, S. Song, C. Fan and S. Wang, “Optical Detection of Mercury (II) in Aqueous Solutions Using Conjugated Polymers and Label-Free Oligonucleotides,” Advanced Materials, Vol. 19, No. 11, 2007, pp. 1471-1474. doi:10.1002/adma.200602578
- Y. Zhao and Z. Zhong, “Tuning the Sensitivity of a Foldamer-Based Mercury Sensor by Its Folding Energy,” Journal of the American Chemical Society, Vol. 128, No. 31, 2006, pp. 9988-9989. doi:10.1021/ja062001i
- W. H. Chan, R. H. Yang and K. M. Wang, “Development of a Mercury Ion-Selective Optical Sensor Based on Fluorescence Quenching of 5,10,15,20-Tetraphenylporphyrin,” Analytica Chimica Acta, Vol. 444, No. 2, 2011, pp. 261-269. doi:10.1016/S0003-2670(01)01106-0
- V. Ostatna and E. Palecek, “Self-Assembled Monolayers of Thiol-End-Labeled DNA at Mercury Electrodes,” Langmuir, Vol. 22, No. 15, 2006, pp. 6481-6484. doi:10.1021/la061424v
- G. K. Darbha, A. Ray and P. C. Ray, “Gold NanoparticleBased Miniaturized Nanomaterial Surface Energy Transfer Probe for Rapid and Ultrasensitive Detection of Mercury in Soil, Water, and Fish,” ACS Nano, Vol. 1, No. 3, 2007, pp. 208-214. doi:10.1021/nn7001954
- D. Li, A. Wieckowska and I. Willner, “Optical Analysis of Hg2+ Ions by Oligonucleotide-Au Nanoparticles Hybrids and DNA-Based Machines,” Angewandte Chemie International Edition, Vol. 47, No. 21, 2008, pp. 3927- 3931. doi:10.1002/ange.200705991
- X. Xue, F. Wang and X. Liu, “One-Step, Room Temperature, Colorimetric Detection of Mercury (Hg2+) Using DNA/Nanoparticle Conjugates,” Journal of the American Chemical Society, Vol. 130, No. 11, 2008, pp. 3244-3245. doi:10.1021/ja076716c
- L. Wang, J. Zhang, X. Wang, Q. Huang, D. Pan, S. Song and C. Fan, “Gold Nanoparticle-Based Optical Probes for Target-Responsive DNA Structures,” Gold Bull, Vol. 41, No. 1, 2008, pp. 37-41. doi:10.1007/BF03215621
- X. J. Zhu, S. T. Fu, W. K. Wong, J. P. Guo and W. Y. Wong, “A Near-Infrared Fluorescent Chemodosimeter for Mercuric Ion Based on an Expanded Porphyrin,” Angewandte Chemie International Edition, Vol. 118, No. 19, 2006, pp. 3222-3226. doi:10.1002/ange.200600248
- J. Wang and X. Qian, “A Series of Polyamide Receptor Based PET Fluorescent Sensor Molecules: Positively Cooperative Hg2+ Ion Binding with High Sensitivity,” Organic Letters, Vol. 8, No. 17, 2006, pp. 3721-3724. doi:10.1021/ol061297u
- L. Praveen, V. B. Ganga, R. hirumalai, T. T. Sreeja, M. L. P. Reddy and R. L. Varma, “A New Hg2+-Selective Fluorescent Sensor Based on a 1,3-Alternate Thiacalixarene Anchored with Four 8-Quinolinoloxy Groups,” Inorganic Chemistry, Vol. 46, No. 16, 2007, pp. 6277-6282. doi:10.1021/ic070259j
- E. M. Nolan and S. J. Lippard, “Tools and Tactics for the Optical Detection of Mercuric Ion,” Chemical Reviews, Vol. 108, No. 50, pp. 3443-3480. doi:10.1002/chin.200850280
- M. H. Lee, S. Lee, S. H. Kim, C. Kang and J. S. Kim, “Nanomolar Hg(II) Detection Using Nile Blue Chemodosimeter in Biological Media,” Organic Letters, Vol. 11, No. 10, 2009, pp. 2101-2104, doi:10.1021/ol900542y
- S. Yoon, E. W. Miller, Q. He, P. H. Do and C. J. Chang, “A Bright and Specific Fluorescent Sensor for Mercury in Water, Cells, and Tissue,” Angewandte Chemie International Edition, Vol. 46, No. 35, 2007, pp. 6658-6661. doi:10.1002/anie.200701785
- M. Zhu, M. Yuan, X. Liu, J. Xu, J. Lv, C. Huang, H. Liu, Y. Li, S. Wang and D. Zhu, “Visible Near-Infrared Chemosensor for Mercury Ion,” Organic Letters, Vol. 10, No. 7, 2008, pp. 1481-1484. doi:10.1021/ol800197t
- A. B. Othman, J. W. Lee, J. S. Wu, J. S. Kim, R. Abidi, P. Thuery, J. M. Strub, A. V. Dorsselaer and J. Vicens, “Calixarene-based, Hg2+-Induced Intramolecular Fluorescence Resonance Energy Transfer Chemosensor,” Journal of Organic Chemistry, Vol. 72, No. 20, 2007, pp. 7634-7640. doi:10.1021/jo071226o
- S. H. Kim, J. S. Kim, S. M. Park and S. K. Chang, “Hg2+- Selective OFF-ON and Cu2+-Selective ON-OFF Type Fluoroionophore Based upon Cyclam,” Organic Letters, Vol. 8, No. 3, 2006, pp. 371-374. doi:10.1021/ol052282j
- J. V. Ros-Lis, R. Martinez-Manez, K. Rurack, F. Sancenon, J. Soto and M. Spieles, “Highly Selective Chromogenic Signaling of Hg2+ in Aqueous Media at Nanomolar Levels Employing a Squaraine-Based Reporter,” Inorganic Chemistry, Vol. 43, No. 17, 2004, pp. 5183- 5185. doi:10.1021/ic049422q
- Y. Cheng, M. Zhang, H. Yang, F. Li, T. Yi, C. Huang, “Azo Dyes Based on 8-Hydroxyquinoline Benzoates: Synthesis and Application as Colorimetric Hg2+-Selective Chemosensors,” Dyes and Pigments, Vol. 76, No. 3, 2008, pp. 775-783. doi:10.1016/j.dyepig.2007.01.022
- A. Caballero, R. Martinez, V. Lloveras, I. Ratera, J. Vidal-Gancedo, K. Wurst, A. Tárraga, P. Molina and J. Veciana, “Highly Selective Chromogenic and Redox or Fluorescent Sensors of Hg2+ in Aqueous Environment Based on 1,4-Disubstituted Azines,” Journal of the American Chemical Society, Vol. 127, No. 45, 2005, pp. 15666- 15667. doi:10.1021/ja0545766
- G. Hennrich, W. Walther, U. Resch-Genger and H. Sonnenschein, “Cu(II)- and Hg(II)-Induced Modulation of the Fluorescence Behavior of a Redox-Active Sensor Molecule,” Inorganic Chemistry, Vol. 40, No. 4, 2001, pp. 641-644. doi:10.1021/ic000827u
- G. Zhang, D. Zhang, S. Yin, X. Yang, Z. Shuaia and D. Zhu, “1,3-Dithiole-2-thione Derivatives Featuring an Anthracene Unit: New Selective Chemodosimeters for Hg(II) ion,” Chemical Communication, Vol. 16, 2005, pp. 2161- 2163. doi:10.1039/b417952h
- E. M. Nolan, M. E. Racine and S. J. Lippard, “Selective Hg(II) Detection in Aqueous Solution with Thiol Derivatized Fluoresceins,” Inorganic Chemistry, Vol. 45, No. 6, 2006, pp. 2742-2749. doi:10.1021/ic052083w
- X. Q. Chen and H. M. Ma, “A Selective FluorescenceOn Reaction of Spiro form Fluorescein Hydrazide with Cu(II),” Analytica Chimica Acta, Vol. 575, No. 2, 2006, pp. 217-222. doi:10.1016/j.aca.2006.05.097
- T. Li, Z. Yang, Y. Li, Z. Liu, G. Qi and B. Wang, “A Novel Fluorescein Derivative as a Colorimetric Chemosensor for Detecting Copper(II) Ion,” Dyes and Pigments, Vol. 88, No. 1, 2011, pp. 103-108. doi:10.1016/j.dyepig.2010.05.008
- G. M. Sheldrick, “SADABS,” University of Gottingen, Gottingen, 1997.
- G. M. Sheldrick, “Program for the Refinement of Crystal Structure,” University of Goettingen, Gottingen, 1997
- X. F. Yang, D. B. Wu and H. Li, “Sensitive Determination of Cobalt(II) Using a Spiro Fluorescein Hydrazide as a Chemiluminogenic Reagent,” Microchimica Acta, Vol. 149, No. 1-2, 2005, pp. 123-129. doi:10.1007/s00604-004-0285-4
- V. Dujols, F. Ford and A. W. Czarnik, “A LongWavelength Fluorescent Chemodosimeter Selective for Cu(II) Ion in Water,” Journal of the American Chemical Society, Vol. 119, No. 31, 1997, pp. 7386-7387.
Supporting Information (SI)
 (a)
(a) 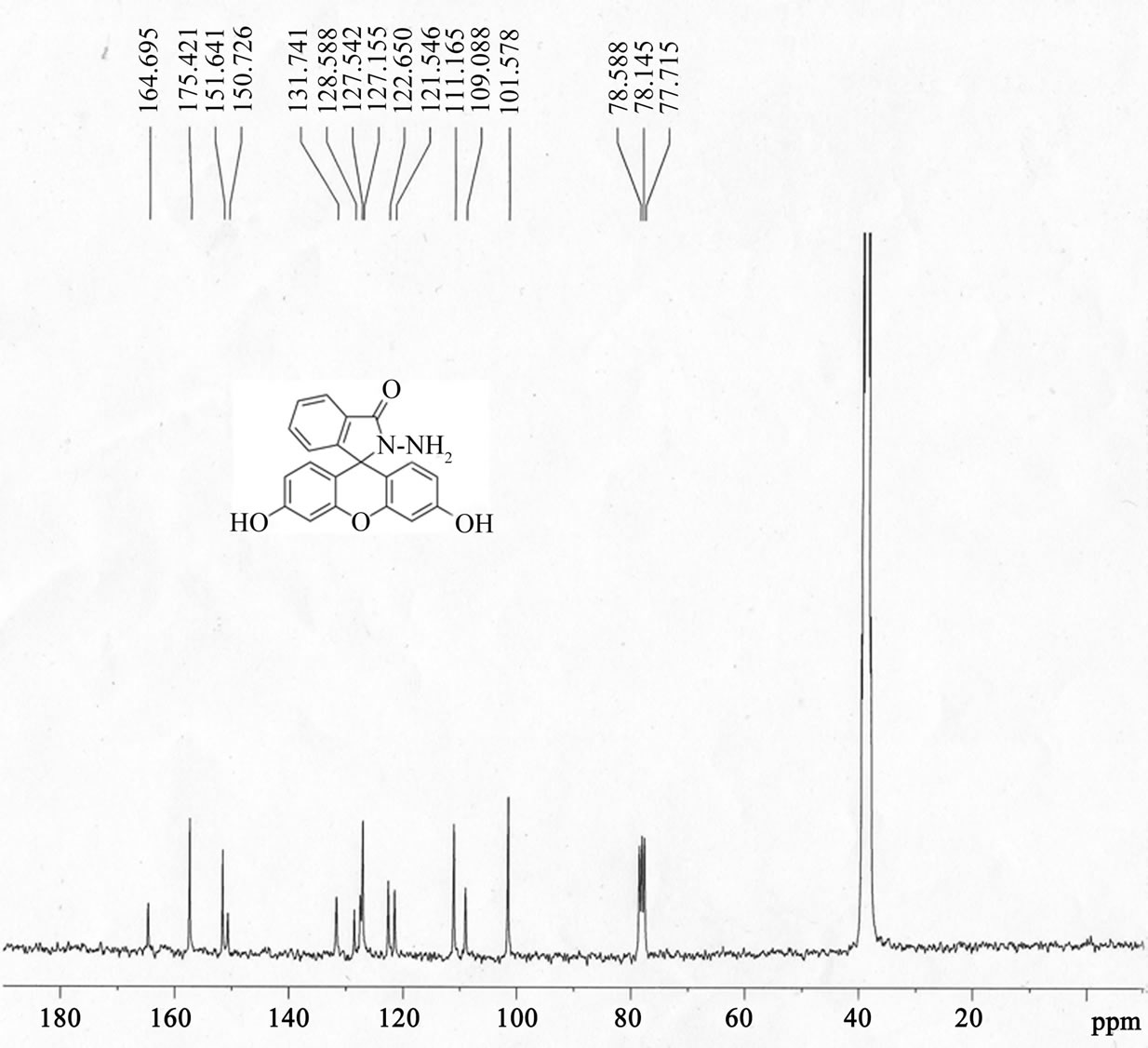
 (b)
(b) 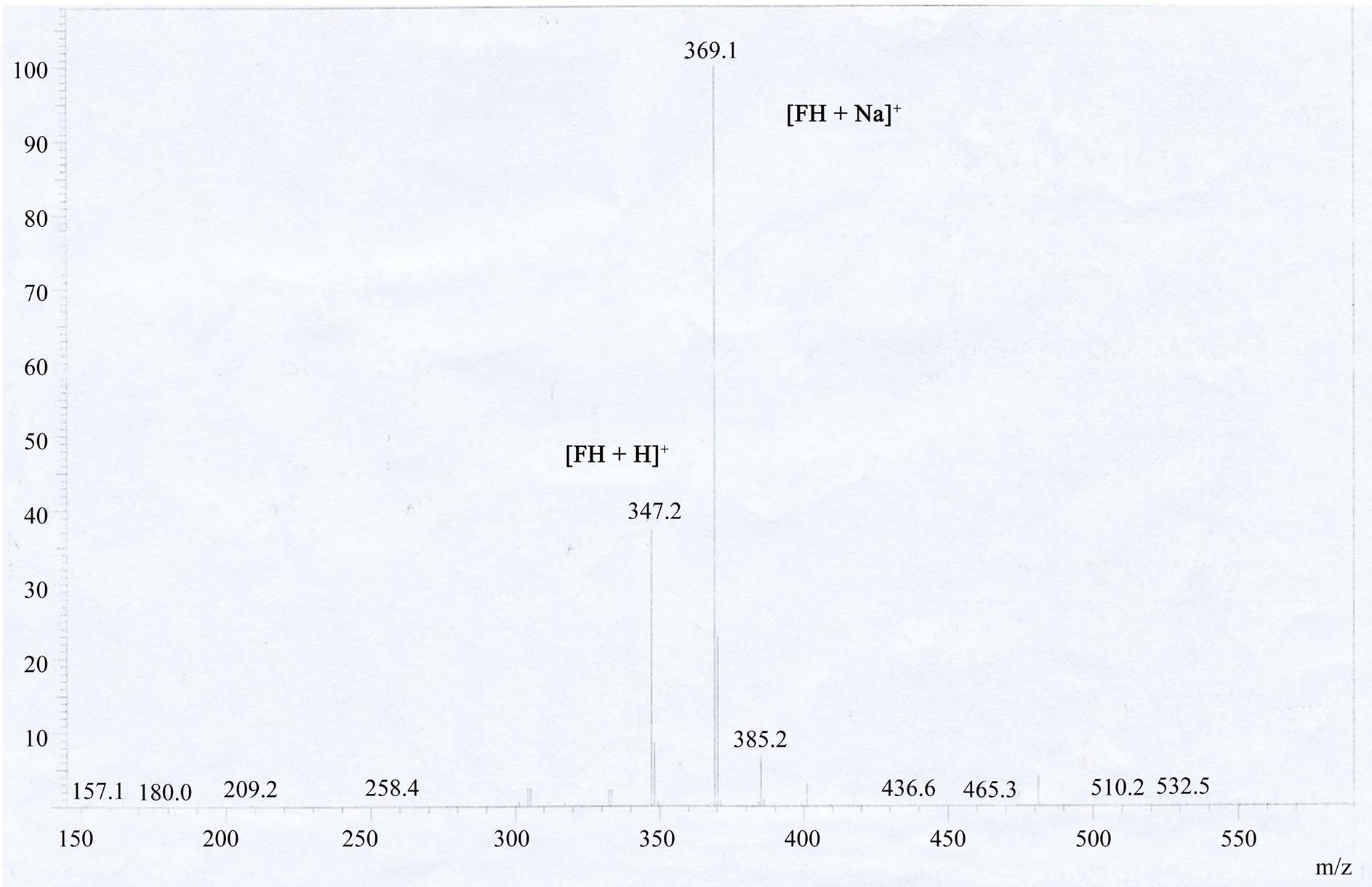 (c)
(c)
Figure S1H NMR , 13C NMR, ESI-MS of fluorescein hydrazide (FH). (a) 1H NMR (300 MHz, 25˚C, DMSO-d6, TMS as an interior criterion): δ 9.83 (s, 2H), 7.78 (m, 1H), 7.49 (m, 2H), 6.98 (m, 1H), 6.59 (s, 2H), 6.38-6.47 (m, 4H), 4.41 (s, 2H); (b) 13C NMR (75 MHz, DMSO-d6): δ 78.15 (Cspiro), 101.58, 109.09, 111. 17, 121.55, 122.65, 127.16, 127.54, 128.59, 131.74, 150.73, 151.64, 157.42, 164.70; The ESI-MS spectra of fluorescein hydrazide (FH).
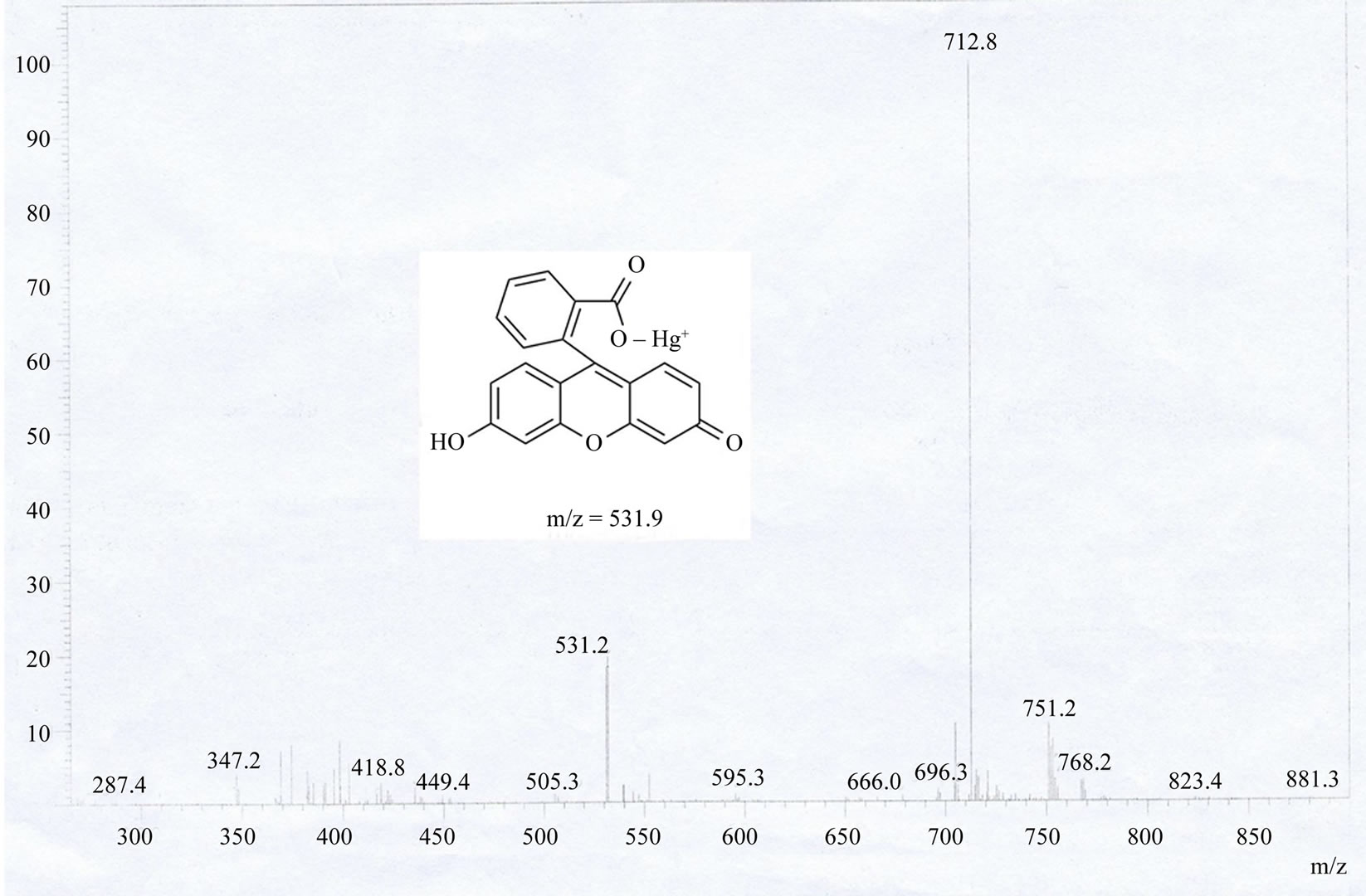
Figure S2.ESI-MS spectra analytic of fluorescein hydrazide (FH) sensing to Hg2+ ion (fluorescein-Hg complex).
NOTES
*Contributed equally to this work.
#Corresponding authors.

