Advances in Aging Research
Vol.2 No.4(2013), Article ID:37726,7 pages DOI:10.4236/aar.2013.24019
Neuroprotective role of 17β estradiol with tachykinin neuropeptide NKB and Aβ (25 - 35) in aging female rat brain
![]()
1School of Life Sciences, Jawaharlal Nehru University, New Delhi, India; *Corresponding Author: scowsik@yahoo.com.com
2King George’s Medical University, Lucknow, India
Copyright © 2013 Rashmi Jha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 14 June 2013; revised 14 July 2013; accepted 21 July 2013
Keywords: Neurokinin B; Amyloid Beta (25 - 35); Estradiol; Neurodegenerative Diseases
ABSTRACT
The brain experiences structural, molecular and functional alterations during aging. In aging brain tissue, the oxidative stress increases due to decreased activity of antioxidant enzymes and increased oxidative stress leading to neurodegeneration associated with excitotoxicity. In the present study, we observed the effect of tachykinin neuropeptide Neurokinin B (NKB) and amyloid beta fragment Aβ (25 - 35) on the activity of Acetylcholine esterase (AChE) and Lipid peroxidation (LPO) in brains of 17β estradiol (E2) treated aging female rat synaptosomes of different age groups. An in-vitro incubation of E2 treated brain synaptosomes with Aβ (25 - 35) showed toxic effects on all the parameters. The treatment of NKB and combined NKB and Aβ (25 - 35) increased the AChE enzyme activity and decreased the level of LPO in E2 treated aging rats. The treatment of NKB and combined NKB and Aβ (25 - 35) in a concentration dependent manner reversed the effects of aging and Aβ (25 - 35) on AChE and LPO. The present finding suggests that E2 along with NKB reverse aging and Aβ (25 - 35) induced toxicity as well as AChE and LPO levels. The results of the current study showed a possible beneficial role of NKB with E2 in the age related neurological diseases.
1. INTRODUCTION
Aging is a progressive and deleterious process occurring in cells and tissues. Oxidative stress plays a pivotal role in the age-associated cognitive decline and neuronal loss in neurodegenerative diseases like Alzheimer’s (AD) and Parkinson’s (PD) [1,2]. The damage caused by the oxidative stress is due to the generation of free radicals [3].
The ovarian steroid hormone 17β estradiol (E2) is an essential hormone which protects neurons against Aβ toxicity, oxidative stress and excitotoxicity [4-7]. Thus, there has been growing interest in the action and functions of E2, particularly on whether it is neuroprotective for age related diseases and neurodegenerative conditions such as stroke, AD and PD [8].
Mammalian tachykinins comprise a family of regulatory peptides including substance P (SP), neurokinin A (NKA) and neurokinin B (NKB) [9,10]. Tachykinins are widely distributed within the peripheral and central nervous system and produce a diverse range of biological effects [11-14]. These tachykinin neuropeptides have significant sequence similarity with Aβ (25 - 35) which forms neurotoxin amyloids found to be nontoxic in neuronal cells [15]. The tachykinins are known to reduce oxidative stress in the brain [16-18], to reverse the neurotoxic effects of Aβ in neurons and to play a role in neurodegenerative diseases [19,20].
Acetylcholine esterase (AChE) is an essential neurontransmitter enzyme affected during aging and neurological disorders. The activity of AChE decreases with age and as reflected in reduced acetylcholine content in the brain [21]. Loss of this enzyme activity is also a marker of functional decline in the neuronal population. AChE inhibitors are used clinically in neurodegenerative diseases such as Myasthenia Gravis and glaucoma, and the cholinergic deficient glaucoma associated with AD [22].
Lipid Peroxidation (LPO) is an autocatalytic process and is a common consequence of cell death. The age related increase in the production of peroxidase may be derived from the membrane damage by hydrogen peroxide, and it is one of the major outcomes of free radical-mediated injury to the tissues. This may cause peroxidative damage during inflammation, cancer, toxicity of xenobiotics and aging. Malondialdehyde (MDA) is one of the end products in the LPO process [23,24].
The neuroprotective role of NKB on toxicity induced by Aβ (25 - 35) in aging rat brain synaptosomes is significant as reported earlier by Baquer and colleagues [17,18]. Earlier studies showed that E2 treatment had beneficial effects on lipid and antioxidant enzymes in aging rat tissues [25]. In the present study, we examine the neuroprotective effect of NKB and with E2 against Aβ (25 - 35) neurotoxicity and on the activity of AChE and LPO in the brain of aging female rats.
2. MATERIALS AND METHODS
2.1. Animals
The present study was conducted on female albino rats of the Wistar strain in different age groups (3, 12 and 24 months). Animals were maintained in the animal house facility of Jawaharlal Nehru University (JNU), New Delhi, India at a constant temperature of 25˚C, humidity 55% and 12h dark and light cycle. The animals were fed standard chow rat feed (Hindustan Leaver Ltd., India) and given tap water until the time of sacrifice. The JNUInstitutional Animal Ethics Committee (IAEC) of Jawaharlal Nehru University approved all the animal experiments; all institutional guidelines for care of animals were followed.
2.2. Hormone Administration
Subcutaneous injections of 17-β-estradiol (E2) (0.1 μg/g body weight) were given daily for one month to the aged rats (12 and 24 months old; n = 8 for each group). E2 was dissolved in propylene glycol in appropriate concentrations [26]. Three month old rats were used as a young control since earlier data from our laboratory showed reduced levels of E2 at 12 and 24 months as compared to the 3-month cyclic rats [27]. Control animals received an equal volume of vehicle. There was no treatment on the day of the sacrifice. Experimental animals of all the groups were sacrificed and brains were isolated for further study.
2.3. Preparation of Synaptosomes
The animals from control and E2 treated groups were sacrificed by cervical dislocation. The whole brain was excised and washed in ice-cold saline (0.9% NaCl). Tissue homogenates were prepared as described by Mayanil et al. 1982 [28]. Tissues were soaked, dried on blotting paper and weighed, minced and homogenized in nine volumes of homogenizing buffer containing 0.25 M sucrose, 0.02 M triethanolamine (pH 7.4) and 0.12 mM dithiothreitol. The pellet obtained after centrifugation at 12,000 (rpm) containing synaptosomes and mitochondria was taken for the present study. The whole procedure was carried out at 4˚C.
2.4. Treatment of Synaptosomes with NKB and Aβ (25 - 35)
Each sample containing ~100 µg protein of isolated rat brain synaptosomes was incubated with NKB, Aβ (25 - 35) and NKB+ Aβ (25 - 35) in microfuge tubes at 37˚C for 60 min in a shaking water bath with 0.1, 1 and 5 µM concentration of each of the peptides. All incubations performed in four combinations: Control (without any peptide), Aβ (25 - 35), NKB and NKB + Aβ (25 - 35) in three age groups of control and E2 treated 12 month and 24 month rats at three peptide concentrations
2.5. Measurement of Acetylcholine Esterase (AChE) Activity
The AChE activity was measured in synaptosomes according to the method described by Ellman et al. 1961 [29] with minor modifications such as measuring of the rate of production of thiocholine as the acetyl thiocholine was hydrolyzed. The activity was measured in the synaptosomes, isolated from 3, 12 and 24-month-old rat controls (without E2 treatment) and E2 treated rat brain in the presence and absence of NKB and Aβ (25 - 35) and combined NKB and Aβ (25 - 35). The change in optical density (OD) was recorded at 412 nm and the enzyme activity was calculated as micromoles of thiocholine formed per minute/mg protein (µM/min/mg protein) at room temperature.
2.6. Measurement of Lipid Peroxidation (LPO)
The degradation products of peroxidised lipids from MDA were measured in the homogenates of the whole brain synaptosomes. The amount of MDA was measured spectrophotometrically at 532 nm by using a thiobarbituric acid reactive substance (TBARS) essentially by the method of Genet et al. 2002 [30]. Results were expressed as nmole of MDA per mg of protein.
2.7. Statistical Analysis
Data have been presented as mean ± standard error of mean (SEM). The data were analyzed using one way ANOVA to test for differences between different treatments at different age groups. Differences between the means of the individual groups were assessed by Dunnett’s multiple comparisons test. A value of p < 0.05 was considered to be statistically significant.
2.8. Chemicals
All substrates, standards, NKB and Aβ (25 - 35) peptide fragment were purchased from Sigma Chemicals Company, USA. All other chemicals were of analytical grade and purchased from SRL and Qualigens, India.
3. RESULTS
3.1. Acetylcholine Esterase (AChE)
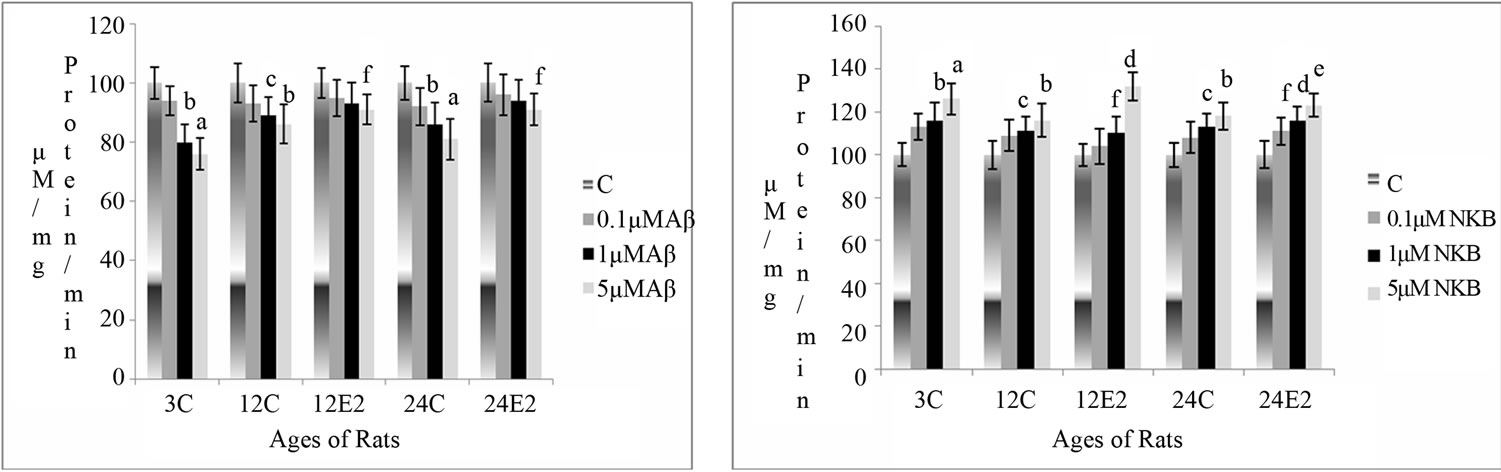
Changes in AChE activity were measured in rat brain synaptosomes, in different age groups with and without E2 treatment, at different concentrations of Aβ (25 - 35), NKB and NKB + Aβ (25 - 35). The results are shown in Figures 1(a)-(c).
3.1.1. Effect E2 and Varying Concentrations of Aβ (25 - 35) on AChE Activity
The AChE activity was found to be reduced in the synaptosomes of control (without E2) rat brain incubated with different concentrations of Aβ (25 - 35). However, there was less marked decrease in the AChE activity in the synaptosomes of E2 treated rats. In the synaptosomes of 12 and 24-month control rats (without E2), a decrease in activity was observed. Synaptosomes of 12 and 24- month rats with E2 treated showed less decrease (p < 0.05 and p < 0.05) with incubation of 5 µM of Aβ (25 - 35) in as compared to age matched control. The results are shown in Figure 1(a).
3.1.2. Effect of E2 and Varying Concentrations of NKB on AChE Activity
The activity of AChE was found to increase in all the age groups with incubation of different concentrations of NKB. This increase in enzyme activity was more significant in E2 treated rats with increasing peptide concentrations as compared with that of age matched control. The enzyme activity increased (p < 0.01) with incubation of 5 μM of peptide in synaptosomes isolated from brains
 (a)
(a)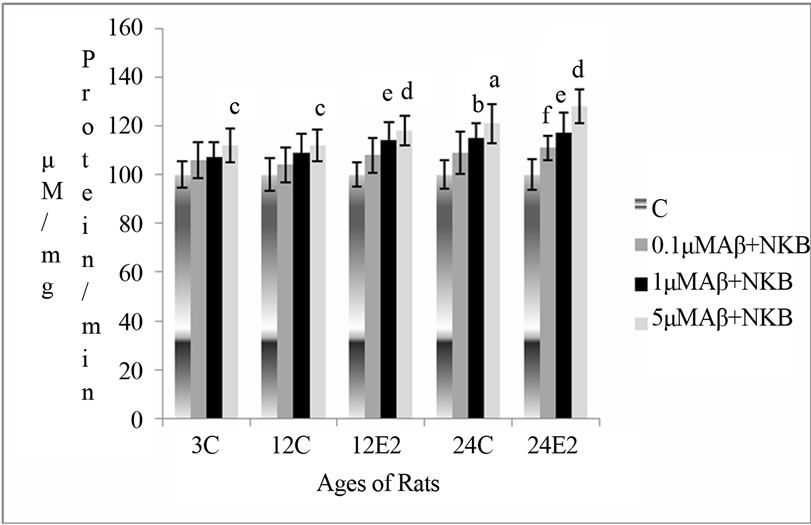 (b)
(b)
Figure 1. Percentage changes in the AChE activity in synaptosomes of 3, 12 and 24 months control (C) and estradiol (E2) treated aging female rats in presence of (a) Aβ (25 - 35) (b) NKB and (c) NKB + Aβ (25 - 35). Peptide concentrations are 0.1, 1.0 and 5.0 µM. Statistical significance: ap < 0.001, bp < 0.01, cp < 0.05 comparing age matched control (untreated) versus peptide treated; dp < 0.001, ep < 0.01, fp < 0.05 comparing E2 treated versus peptide treated.
of 12-month-old control rats (without E2). The activity increased significantly with addition or incubation of 1 and 5 μM of NKB (p < 0.05 and p < 0.001) in the synaptosomes of E2 treated 12-month-old rats. Increase in AChE activity was highly significant in E2 treated group of 24-month-old rat using a dose of 1 and 5 µM of NKB (p < 0.001 and p < 0.01). The results are shown in Figure 1(b).
3.1.3. Effect of E2 and Varying Concentrations of Combination of NKB and Aβ (25 - 35) on AChE Activity
The AChE activity was found to increase with a combination of Aβ (25 - 35) and NKB in synaptosomes of control (without E2 treatment) rat brain, but this increase was more significant in synaptosomes of E2 treated 12 and 24-month aging rats. The combined dose of NKB and Aβ (25 - 35) at 1 and 5 µM concentration in 12- month E2 treated rats significantly raised AChE activity as compared to the control of matching age (p < 0.01 and p < 0.001). The combined dose of NKB and Aβ (25 - 35) at 0.1, 1 and 5 µM concentration in synaptosomes of 24 month E2 treated rats showed significantly raised activity as compared to the controls of matching age (p < 0.05, p < 0.01 and p < 0.001). The results are shown in Figure 1(c).
3.2. Lipid Peroxidation (LPO)
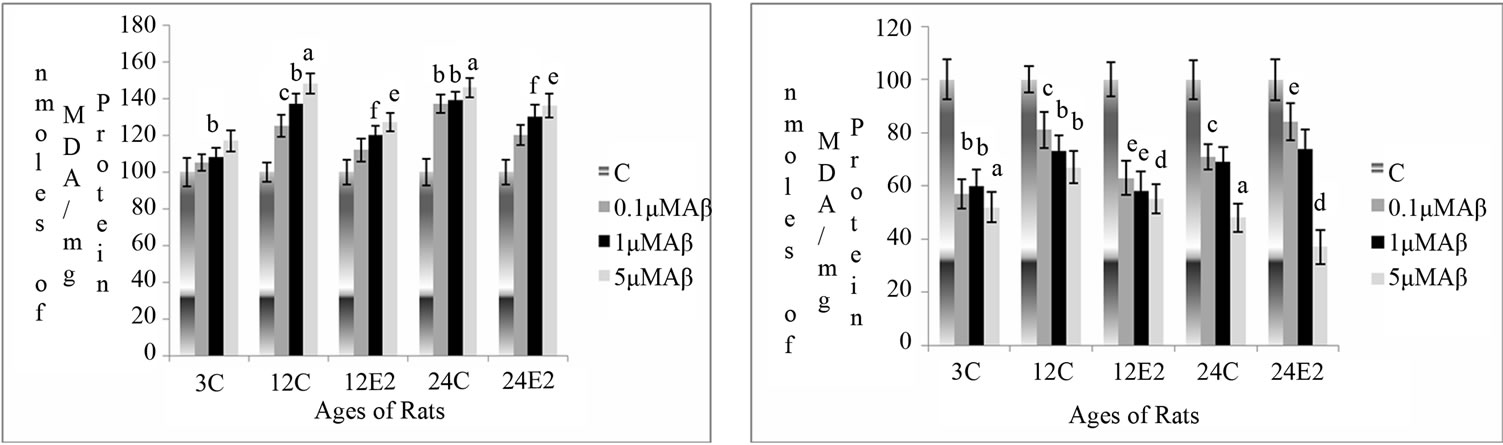
Changes in LPO activity were measured as a function of the formation of MDA in rat brain synaptosomes, in different age groups with and without E2 treatment, at different concentrations of Aβ (25 - 35), NKB and NKB + Aβ (25 - 35). The results are shown in Figures 1(a)- (c).
3.2.1. Effect of E2 and Varying Concentrations of Aβ (25 - 35) on MDA Level
The MDA level increased in brain synaptosomes of control (without E2 treatment) rats with different concentration of Aβ (25 - 35) incubations, but there was only a slight increase in the MDA levels in the synaptosomes of E2 treated rats. However, there was less increase (p < 0.01) with the incubations of 5 μM Aβ (25 - 35) in synaptosomes isolated from 12-month E2 treated rats as compared to age matched controls (p < 0.01). The synaptosomes of 24-month-old E2 treated rats, incubated with 1 and 5 µM of Aβ showed less marked increase in the activity by as compared to the age matched control values (p < 0.05 and p < 0.01). The results are shown in Figure 2(a).
3.2.2. Effect of E2 and Varying Concentrations of NKB on MDA Level
The MDA levels decreased in synaptosomes isolated from different age groups of control rat brain with increasing concentration of NKB. However, there was a significant decrease in MDA levels in the synaptosomes of E2 treated group rats incubated with different concentration of NKB. The synaptosomes isolated from 12- month E2 treated rats showed decreased in MDA levels when incubated with 1 and 5 µM concentrations of NKB as compared to without any peptide incubation in 12- month E2 treated rats (p < 0.01 and p < 0.001). In the synaptosomes of 24-month E2 treated rats, the level of MDA decreased when incubated with 5 µM concentration of NKB as compared with as matched control (p < 0.001). The results are shown in Figure 2(b).
3.2.3. Effect of E2 and Varying Concentrations of Combined NKB and Amyloid (25 - 35) on MDA Level
The MDA level decreased with incubation of combined doses of Aβ (25 - 35) and NKB in the synaptosomes isolated from rats without E2 treatment, whereas this decrease was significantly greater in synaptosomes isolated from of E2 treated 12- and 24-month aging rats. In 12-month-old E2 treated rats the MDA level significantly decreased when synaptosomes were incubated with 5 μM of the combined dose of NKB and Aβ (25 - 35) as compared to the control of matching age group (p < 0.001). Synaptosomes isolated from 24-month-old E2 treated rats showed decrease in the MDA level with incubation of 5 μM concentration of the combined dose of NKB and Aβ (25 - 35) as compared to without E2 treated 24-month rats control (p < 0.001). The results are shown in Figure 2(c).
4. DISCUSSION
In the present study, AChE activity was assessed in the presence of different concentrations of Aβ (25 - 35), NKB and combined treatment of NKB and Aβ (25 - 35) in synaptosomes from different age groups of untreated (without E2) and E2 treated rats. The AChE activity was found to be inhibited in all the age groups as a function of different concentration of Aβ (25 - 35) incubation when compared with age matched controls. The percentage decrease with the Aβ (25 - 35) peptide was lower in the E2 treated rats as compared to control (without E2) 12- and 24-month-old rats. These results show that E2 shows significant response and neuroprotective role against oxidative stress produced by Aβ (25 - 35). The activity of AChE increased in all the age groups with an increase in the NKB peptide concentration, as compared with that of age matched control synaptosomes which supported the results of Mantha et al. 2006 [18]. However incubation of synaptosomes of E2 treated rats with various concentrations of NKB increased the activity of
 (a)
(a)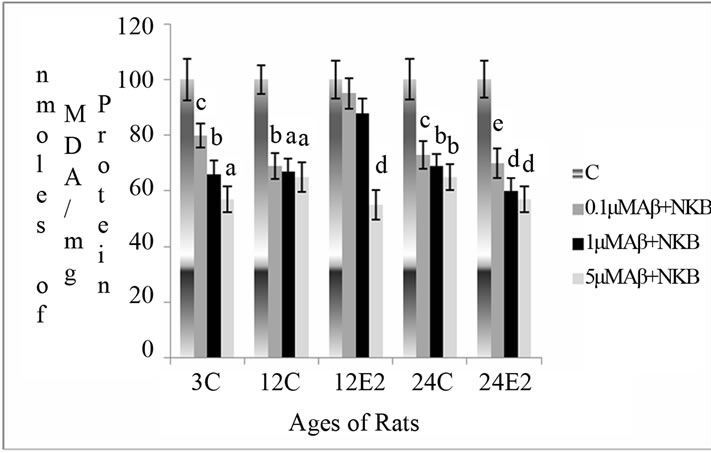 (b)
(b)
Figure 2. Percentage changes in lipid peroxidation (MDA level) in synaptosomes of 3, 12 and 24 months control (C) and estradiol (E2) treated aging female rats in presence of (a) Aβ (25 - 35) (b) NKB and (c) NKB + Aβ (25 - 35). Peptide concentrations are 0.1, 1.0 and 5.0 µM. Statistical significance: ap < 0.001, bp < 0.01, cp < 0.05 comparing age matched control (untreated) versus peptide treated; dp < 0.001, ep < 0.01, fp < 0.05 comparing E2 treated versus peptide treated.
AChE significantly. There was higher increase in the activities of the enzyme in the synaptosomes of NKB incubated E2 treated young and old rats as compared to synaptosomes of rats without E2 treatment. These results indicate that NKB showed more significant results with E2 treated synaptosomes as compared to their individual application indicating that NKB is more effective and has stimulatory action in the presence of E2. The combined dose of NKB and Aβ (25 - 35) incubation was effective in increasing the activity of AChE in the synaptosomes of E2 treated aging rat brain. The results of the present study demonstrate that NKB along with E2 showed antiaging and protective potential against aging and Aβ (25 - 35) induced toxicity. NKB with E2 significantly attenuate a decrease in acetylcholinesterase activity in the aged rat brain. Almeida et al. 2004 [9] proposed that tachykinin peptides SP, NKA and NKB mediate noradrenergic and cholinergic excitatory neurotransmission. It has been reported that tachykinin peptides release AChE in a dose dependent manner [31] and that NKB was effective enough to release AChE in neuronal cells [32].
In the present study, the MDA level, which is an index of LPO, was found to increase with aging rat brain. This result supported some earlier studies which showed a significant increase in the levels of MDA in aged rats [33]. However, E2 treated groups showed a significant decrease in the level of MDA in aging rats. This finding supports the study of Kumar and coworkers [5], which also showed decreased MDA levels. There are additional reports [34] which support that E2 has a protective role in decreasing the lipid peroxidative mediated damage.
Here, we studied the MDA formation as a function of different concentration of Aβ (25 - 35), NKB and the combined dose of NKB and Aβ (25 - 35) in synaptosomes of various age groups of untreated and E2 treated rats. The MDA level increased with age as compared to young 3-month control rats in the brain synaptosomes. Present findings are in concurrence with the results of Rodriguez and Ruiz [35] who showed that peroxidative damage in plasma increased with the aging process in healthy human subjects. When we measured the MDA level in brain synaptosomes incubated with various concentration of Aβ (25 - 35), it resulted in an elevation of its level in a dose dependent manner. However, an insignificant increase in MDA level was observed in synaptosomes of E2 treated rats incubated with Aβ (25 - 35) as compared to that of without E2 rats. These observations indicate that Aβ (25 - 35) showed less toxicity in the presence of E2 as compared to individual application. Estrogens give antioxidant effect due to its ability to donate an H+ atom to a peroxyl radical, thereby inhibiting lipid peroxidation [36,37]. When we incubated the synaptosomes of E2 treated rats with various concentration of NKB incubations, there was a significant sharp decrease in the MDA formation as compared with that of the age matched untreated controls (without E2) indicating that E2 functions as radical scavengers in the presence of NKB and inhibits lipid peroxidation. In the incubation of combined dose of NKB and Aβ (25 - 35), the brain synaptosomes of E2 treated rats with an increase in peptide concentrations, showed a significant decrease in MDA levels, and it also reversed the amyloid induced increase in MDA formation. Thus it is suggested NKB is more effective with E2 and protects against Aβ (25 - 35) induced toxicity in aging rat brain synaptosomes.
In the present study, it is clearly shown that hormone treatment to aging animals reduces risk factors associated with aging by increasing the activity of AChE and decreasing the levels of MDA. Moreover, it is also quite apparent that E2 is more effective with NKB and may reduce the onset of age related neurological disorders. These results convincingly indicate that NKB treatment in synaptosomes of E2 treated rats significantly protect cells with Aβ-induced toxicity. These results demonstrate that NKB plays a protective role in AChE activity and decreases lipid peroxidative mediated damage. It further suggests that NKB may exert neuroprotective effect when combined with E2. In summary, the protective effects of E2 and NKB are related to elevation of AChE and inhibition of lipid peroxidation.
5. CONCLUSION
This study demonstrates the combination treatment of NKB and E2 have significantly positive effects as compared to their individual application. This suggests that interaction between peptides and estradiol may exist. It can be concluded that neuroprotection by estrogen with neurokinin B will be useful for pharmacological modification of the aging process, and for applying new strategies for control of age related neurodegenerative diseases.
6. ACKNOWLEDGEMENTS
Financial grant from University Grant Commission, New Delhi, India in the form of project is gratefully acknowledged.
REFERENCES
- Markesbery, W.R. (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biology & Medicine, 23, 134-147. http://dx.doi.org/10.1016/S0891-5849(96)00629-6
- Jenner, P. (1998) Oxidative mechanisms in nigral cell death in Parkinson’s disease. Movement Disorders, 13, 24-34.
- Stocker, R. and Frie, B. (1991) Endogenous antioxidant defense in human blood plasma. In: Sies, H., Ed., Oxidative Stress: Oxidants and Antioxidants, Academic Press, London, 213-243.
- Brann, D.W., Krishnan, D., Chandramohan, W., Virendra, B.M. and Mohammad, M.K. (2007) Neurotrophic neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids, 72, 381-405. http://dx.doi.org/10.1016/j.steroids.2007.02.003
- Kumar, P., Taha, A., Kale, R.K., Cowsik, S.M. and Baquer, N.Z. (2011) Physiological and biochemical effects of 17β estradiol in aging female rat brain. Experimental Gerontology, 46, 597-605. http://dx.doi.org/10.1016/j.exger.2011.02.008
- Kumar, P., Kale, R.K., McLean, P. and Baquer N.Z. (2011) Protective effects of 17β estradiol on altered age related neuronal parameters in female rat brain. Neuroscience Letters, 502, 56-60. http://dx.doi.org/10.1016/j.neulet.2011.07.024
- Meyers, B., Agostino, D.A., Walker, J. and Kritzer, M.F. (2010) Gonadectomy and hormone replacement exert regionand enzyme isoform-specific effects on monoamine oxidase and catechol-o-methyltransferase activity in prefrontal cortex and neostriatum of adult male rats. Neuroscience, 165, 850. http://dx.doi.org/10.1016/j.neuroscience.2009.11.013
- Henderson, V.W. (2010) Action of estrogens in the aging brain: Dementia and cognitive aging. Biochimica et Biophysica Acta, 1800, 1077-1083. http://dx.doi.org/10.1016/j.bbagen.2009.11.005
- Almeida, T.A., Rojo, J., Nieto, P.M., Pinto, F.M., Hernandez, M., Martin, J.D. and Candenas, M.L. (2004) Tachykinins and tachykinin receptors: Structure and activity relationships. Current Medicinal Chemistry, 11, 2045- 2081. http://dx.doi.org/10.2174/0929867043364748
- Patacchini, R., Lecci, A., Holzer, P. and Maggi, C.A. (2004) Newly discovered tachykinins raise new questions about their peripheral roles and the tachykinin nomenclature. Trends in Pharmacological Sciences, 25, 1-3. http://dx.doi.org/10.1016/j.tips.2003.11.005
- Maggi, C.A. and Meli. A. (1988) The sensory-efferent function of capsaicin-sensitive sensory neurons. General Pharmacology, 19, 1-43. http://dx.doi.org/10.1016/0306-3623(88)90002-X
- Regoli, D., Boudon, A. and Fauchere, J.L. (1994) Receptors and antagonists for substance P and related peptides. Pharmacological Reviews, 46, 551-599.
- Maggi, C.A. (1997) Tachykinins as peripheral modulators of primary afferent nerves and visceral sensitivity. Pharmacological Research, 36, 153-169. http://dx.doi.org/10.1006/phrs.1997.0219
- Patak, E.N., Pennefather, J.N. and Story, M.E. (2000) Effects of tachykinins on uterine smooth muscle. Clinical and Experimental Pharmacology and Physiology, 27, 922-927. http://dx.doi.org/10.1046/j.1440-1681.2000.03362.x
- Singh, K.P. and Maji, S.K. (2012) Amyloid-like fibril formation by tachykinin neuropeptides and its relevance to amyloid β-protein aggregation and toxicity. Cell Biochemistry and Biophysics, 64, 29-44. http://dx.doi.org/10.1007/s12013-012-9364-z
- Turska, E., Lachowicz, L. and Wasiak, T. (1985) Effect of analogues of substance P fragments on the MAO activity in rat brain. General Pharmacology, 16, 293-295. http://dx.doi.org/10.1016/0306-3623(85)90088-6
- Mantha, A.K., Moorthy, K., Cowsik, S.M. and Baquer, N.Z. (2006) Neuroprotective role of neurokinin B (NKB) on amyloid β (25 - 35) induced toxicity in aging rat brain synaptosomes: Involvement in oxidative stress and excitotoxicity. Biogerentology, 7, 1-17. http://dx.doi.org/10.1007/s10522-005-6043-0
- Mantha, A.K., Moorthy, K., Cowsik, S.M. and Baquer, N.Z. (2006) Membrane associated functions of neurokinin B (NKB) on Aβ (25 - 35) induced toxicity in aging rat brain synaptosomes. Biogerentology, 7, 19-33. http://dx.doi.org/10.1007/s10522-005-6044-z
- Kowall, N.W., Beal, M.F., Buscigliot, J., Duffyt, L.K. and Yankner, N.W. (1991) An in vivo model for the neurodegenerative effects of β amyloid and protection by substance P. Neurobiology, 88, 7247-7251.
- Yankner, B.A., Duffy, L.K. and Kirschner, D.A. (1990) Neurotrophic and neurotoxic effects of amyloid protein: Reversal by tachykinin neuropeptides. Science, 250, 279- 282. http://dx.doi.org/10.1126/science.2218531
- Pradhan, S.N. (1980) Central neurotransmitters and aging. Life Sciences, 26, 1643-1656. http://dx.doi.org/10.1016/0024-3205(80)90172-1
- Tripathy, A. and Srivastava, U.C. (2008) Acetylcholinesterase: A versatile enzyme of nervous system. Annual Review of Neuroscience, 15, 106-111. http://dx.doi.org/10.5214/ans.0972.7531.2008.150403
- Hagihara, M., Nishigaki, I., Maseki, M. and Yagi, K. (1984) Age dependent changes in lipid peroxide levels in the lipoprotein fractions of human serum. The Journals of Gerontology, 39, 269-272. http://dx.doi.org/10.1093/geronj/39.3.269
- Kurata, M., Suzuki, M. and Agar, N.S. (1993) Antioxidant systems and erythrocyte life-span in mammals. Comparative Biochemistry and Physiology, 106, 477-487.
- Moorthy, K., Yadav, U.C.S., Siddiqui, M.R., Mantha, A.K., Basir, S.F., Sharma, D., Cowsik, S.M. and Baquer, N.Z. (2005) Effect of hormone replacement therapy innormalizing age related neuronal markers in different age groups of naturally menopausal rats. Biogerontology, 6, 345-356. http://dx.doi.org/10.1007/s10522-005-4810-6
- Moorthy, K., Yadav, U.C.S., Siddiqui, M.R., Basir, S.F., Sharma, D. and Baquer, N.Z. (2004) Effect of estradiol and progesterone treatment on carbohydrate metabolizing enzymes in tissues of aging female rats. Biogerontology, 5, 249-259. http://dx.doi.org/10.1023/B:BGEN.0000038026.89337.02
- Moorthy, K., Sharma, D., Basir, S.F. and Baquer, N.Z. (2005) Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Experimental Gerontology, 40, 295-302. http://dx.doi.org/10.1016/j.exger.2005.01.004
- Mayanil, C.S., Kazmi, S.M. and Baquer, N.Z. (1982) Na+, K+-ATPase and Mg2+-ATPase activities in different regions of rat brain during alloxan diabetes. Journal of Neurochemistry, 39, 903-908. http://dx.doi.org/10.1111/j.1471-4159.1982.tb11475.x
- Ellman, G.L., Courtney, K.D., Andres Jr., V. and Featherstone, R.M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88-95. http://dx.doi.org/10.1016/0006-2952(61)90145-9
- Genet, S., Kale, R.K. and Baquer, N.Z. (2002) Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (TSP foenum-graecum). Molecular and Cellular Biochemistry, 236, 7-12. http://dx.doi.org/10.1023/A:1016103131408
- Guzman, R.G., Kendrick, K.M. and Emson, P.C. (1993) Effect of substance P on acetylcholine and dopamine release in the rat striatum: A microdialysis study. Brain Research, 622, 147-154. http://dx.doi.org/10.1016/0006-8993(93)90813-3
- Yau, W.M., Mandel, K.G., Dorsett, J.A. and Youther, M.L. (1992) Neurokinin3 receptor regulation of acetylcholine release from myenteric plexus. American Journal of Physiology, 263, 659-664.
- Uysal, M., Seckin, S., Kocak-Toker, N. and Oz, H. (1989) Increased hepatic lipid peroxidation in aged mice. Mechanisms of Ageing and Development, 48, 85-89. http://dx.doi.org/10.1016/0047-6374(89)90028-6
- Martins, D.B., Mazzanti, C.M., Franca, R.T., Pagnoncelli, M., Costa, M.M., De Souza, E.M., Goncalves, J., Spanevello, R., Schmatz, R., Da Costa, P., Mazzanti, A., Beckmann, D.V., Cecimda, S., Schetinger, M.R. and Lopes, S.T. (2012) 17-β estradiol in the actylcholinesterase activity and lipid peroxidation in the brain and blood of ovariectomized adult and middle-aged rats. Life Sciences, 90, 351-359. http://dx.doi.org/10.1016/j.lfs.2011.12.006
- Rodriguez, M.M.A. and Ruiz, T. (1992) Homeostasis between lipid peroxidation and antioxidant enzymes activties in health human aging. Mechanisms of Ageing and Development, 66, 213-222. http://dx.doi.org/10.1016/0047-6374(92)90137-3
- Subbiah, M.T., Kessel, B., Agrawal, M., Rajan, R., Abplanalp, W. and Rymaszewski, Z. (1993) Antioxidant potential of specific estrogens on lipid peroxidation. The Journal of Clinical Endocrinology & Metabolism, 77, 1095-1097. http://dx.doi.org/10.1210/jc.77.4.1095
- Vina, J., Sastre, J., Pallardo, F. and Borras, C. (2003) Mitochondrial theory of aging: Importance to explain why females live longer than males. Antioxidants & Redox Signaling, 5, 549-556. http://dx.doi.org/10.1089/152308603770310194

