Surgical Science
Vol.5 No.5(2014), Article ID:46130,10 pages
DOI:10.4236/ss.2014.55037
Totally Endoscopic (Thoracoscopic and Laparoscopic) Radical Esophagectomy with Gastric Tube Reconstruction through a Small Neck Incision: An Early Experience with Thirty Egyptian Patients
Adel Denewer*, Adel Fathi, Ahmed Setit, Mohamed Hegazy, Ashraf Khater, Osama Hussein, Sameh Roshdy, Fayez Shahatto, Fathy Denewer
Department of Surgical Oncology, Oncology Center (OCMU), Faculty of Medicine, Mansoura University, Mansoura, Egypt
Email: *adeldenewer@mans.edu.eg
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 11 March 2014; revised 10 April 2014; accepted 17 April 2014
ABSTRACT
Background: Minimally invasive esophagectomy nowadays is replacing the classic open technique. Additional studies are needed to confirm its safety and efficacy. Methods: thirty patients with esophageal carcinoma were enrolled in this study. Patients were evaluated preoperatively and they underwent thoracoscopic and laparoscopic procedures for assessment of resectability. Resectablepatients underwent radical esophagectomy with gastric tube reconstruction through a four-cm neck incision. Results: 17 patients were operable and 13 patients were inoperable. The mean operative time for the whole procedure was 5.97 ± 1.66 hours. The mean blood loss was 250 ± 138.07 cc. The mean overall hospital stay was 17.47 ± 5.49 daysdays. Common postoperative complications included pneumonia (13.3%) pleural effusion (6.7%), cervical anastomotic leakage (10%), and wound infection (13.3%). One patient died in the early postoperative period. Conclusions: we conclude that totallyendoscopic (thoracoscopic and laparoscopic) esophagectomy is feasible and relatively safe technique. Beside its efficacy as an assessment tool, total esophagectomy and lymphadenectomy could be performed in the same time.
Keywords:Thoracoscopic, Laparoscopic, Radical Esophagectomy, Gastric Reconstruction, Esophagus Cancer

1. Background
Esophageal cancer is the 8th most common cancer and the 4th most common gastrointestinal malignancy. It is 6th leading cause of cancer deaths worldwide [1] . The incidence of the esophageal cancer has been increased over the past 3 decades [2] . In the United States and the western world, this profound increase has been due to the increase in the incidence of adenocarcinoma of the esophagus. This major epidemiologic shift is thought to be related to gastroesophageal reflux disease, obesity, and Barrett’s esophagus, the dominant risk factors for esophageal adenocarcinoma [2] [3] . The highest incidence rates are found in Asia and Sub-Saharan Africa and the lowest rates are found in Europe and North America. Generally, rates are much higher in men than in women [1] .
Radical surgery currently offers the most realistic chance of cure from cancer of the esophagus when spread beyond the most superficial epithelial layers but not extending beyond the locoregional lymph nodes [4] . Although the efficacy of esophagectomy with extended lymphadenectomy remains to be proven by a prospective randomized study, some reports have shown that better survival could be obtained after extended rather than conventional lymphadenectomy [5] .
The use of thoracoscopy and/or laparoscopy for esophageal resection was introduced in 1992 by Cushieri et al., aiming to further reduce pulmonary morbidity while potentially improving the oncological quality of the resection by enhancing visual control during the mediastinal dissection [6] .
Over the last two decades, minimally invasive approaches have been described in several studies as a procedure for the treatment of both benign and malignant diseases that seems equivalent to the standard open esophagectomy [7] [8] . However, it is still considered one of the most complex surgical procedures, and many questions still remain unanswered regarding the oncologic results [9] .
As the data about endoscopic esophagectomy are lacking among Egyptian in cancer patients, the aim of the current study is to assess the feasibility and oncological efficacy of thoracoscopically assisted esophagectomy in the management of esophageal cancer patients in a specialized oncology Center in North of Egypt.
2. Methods
2.1. Setting
This study was conducted at the Surgical Oncology Unit, Oncology Centre, Mansoura University (OCMU) during the period between August 2010 and October 2013.
2.2. Population
Thirty patients with esophageal carcinoma were enrolled in this study. All patients were investigated preoperatively by clinical history and physical examination; Computed Tomography (CT) of the chest, abdomen and pelvis (Brilliance CT 64 slice Philips Netherlands 2005); barium swallow; endoscopy with biopsy and pulmonary function tests. Patients were excluded from this study when they have 1) locally advanced (invading the trachea or the great vessels) or metastatic disease; 2) poor performance Status (3 & 4 according to WHO Criteria); or 3) a previous pulmonary or pleural surgery.
2.3. Patient Preparation
All patients were admitted three days prior to surgery. Pulmonary exercises in the form of spirometry, steam inhalation, and nebulization were performed. Central venous accesses were inserted. Patients were hydrated and received total parenteral nutrition (TPN) three days prior to the surgery to improve the general condition.
2.4. Surgical Procedures
The choice of laparoscopic approach (abdominal or thoracic) was according to prior CT localization of the esophageal mass. Accordingly, thoracoscopy was the initial step for radiological evidence of thoracic esophageal tumors. After assessment, operable patients underwent thoracoscopic mobilization of the esophagus and nodal dissection and laparoscopic gastric mobilization and gastric tube creation.
All procedures were done with epidural and general anesthesia with single lung ventilation which was achieved by using a left-sided double lumen endotracheal tube. The double lumen tube was replaced by the regular endotracheal tube once the thoracic part of dissection was over, and the patient was placed in supine position.
After intubation, the patient was placed in the prone position over a sand bag under the chest and pelvis leaving the abdomen free to allow respiratory movements with the right arm raised cranially to expose the right axillary fossa.
2.4.1. The Thoracoscopic Part
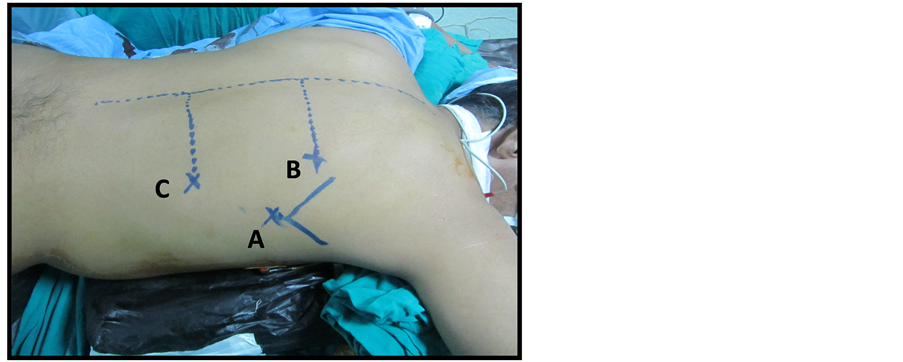
Three ports were used: two 10 mm and one 5 mm. Thirty degree camera was used throughout the whole procedure. The ports, positions were as follows; 10 mm port at the seventh intercostal space (ICS) below the inferior angle of the scapula for camera 1); 5 mm port at fifth ICS 7 cm right lateral to the spinous process for right working hand 2); 10 mm port at ninth ICS 7 cm right lateral to the spinous process for left working hand 3) (Figure 1(a)).
CO2 pneumothorax was created by closed Veress technique in the seventh ICS. This site was used later as a camera port, maintaining insufflations pressure around 6 to 8 mmHg. After entering pleural space, adhesiolysis was performed, by the ACE harmonic shear.
The first step was to assess resectability (by exclusion of lung or pleural metastases, invasion of vital structures or presence of fixed nodal disease).
The inferior pulmonary ligament was divided to allow proper mobilization of the esophagus. The visceral pleura below the azygos vein that covers the esophagus was grasped and divided longitudinally with electrocautery hook dissector starting posteriorly at the posterior edge of the esophagus, anterior to the azygos vein, to the anterior edge of the esophagus and from the costo-phrenic angle inferiorly to the arch of the azygos vein superiorly. This part of the pleura over the esophagus was excised en bloc with the tumor.
By retracting the esophagus ventrally, dissection continued separating the esophagus from the chest wall and descending thoracic aorta. In this step, the thoracic duct was visualized as a white glistening structure over the descending aorta and was protected. The vagal trunks were identified and divided. The left pleura was seen as a shining membrane and it was prone to injury in that situation. The infra-azygos dissection achieved a complete removal of the paraesophageal, subcarinal, hilar, and hiatal nodes. The esophagus was also separated all around from the pericardium, left pleura, arch of the aorta, and the descending aorta, and the azygos vein. The azygos vein was freed completely and was compressed by a non-toothed grasper for obliteration of the blood stream then it was sealed by a 10 mm Ligasure device (atlas) through a 10mm port (Figure 1(b)). Then, dissection was commenced above the level of the azygos with separation of the esophagus from the membranous portion of the trachea. By the end of this part of the operation, the entire esophagus from the thoracic inlet to the esophageal hiatus was mobilized (Figure 1(c)). Finally, insertion of one intercostal chest tube at the base of the lung through the 10mm port was done. Then the patient was turned and positioned for the next steps.
2.4.2. The Laparoscopic Abdominal Part
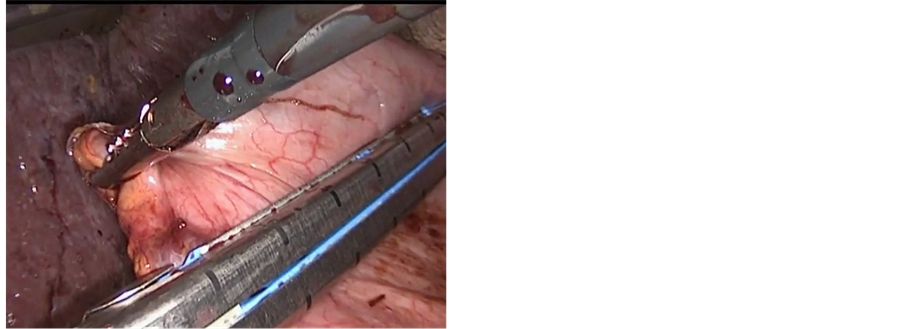
In the case with the lower third esophageal carcinoma, laparoscopy was the initial step of the procedure. The stomach was mobilized completely with preservation of the gastro-epiploic vessels and lymphadenectomy of the celiac trunk was performed. The left gastric artery was sealed a 10 mm Ligasure device (atlas). After complete mobilization of the stomach and the lower esophagus up to the crus of the diaphragm, the stomach was divided by an endo GIA stapler (Ethicon®) in order to create a gastric tube (5 to 7 cm width) along the greater curvature (Figure 2(a)). The gastric tube was left attached to the gastric fundus (Figure 2(b)).
2.4.3. The Cervical Part
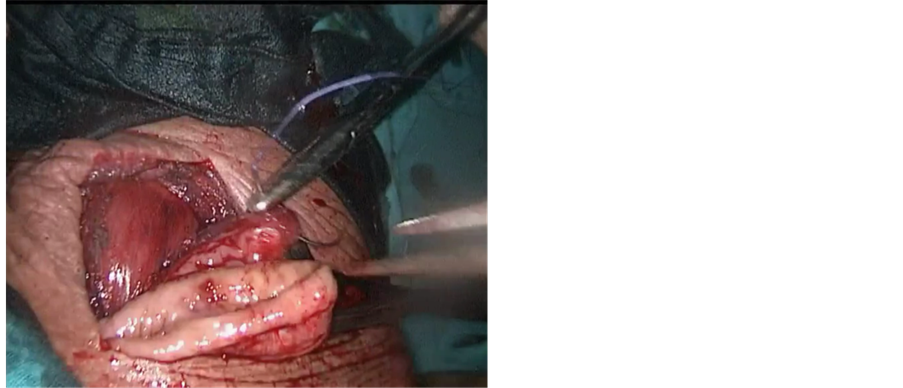
The neck was approached via a 4 cm incision at the anterior border of left sternocleidomastoid muscle. The esophagus was identified and dissection was continued posteriorly up to the prevertebral fascia. The left recurrent laryngeal nerve was identified and spared. A tape was passed around the esophagus to maintain traction (Figure 2(c)). Traction over the distal end of the esophagus was done to retrieve the specimen with the gastric tube attached to the specimen (Figure 2(d)). The nasogastric tube was removed and the esophagus was transected in the neck. Esophago-gastric tube anastomosis was performed using a3/0 Vicryl suture (Figure 3(a)).
2.5. Patients Follow up
All patients were referred to postoperative adjuvant chemotherapy and radiotherapy as indicated and they were
 (a)
(a)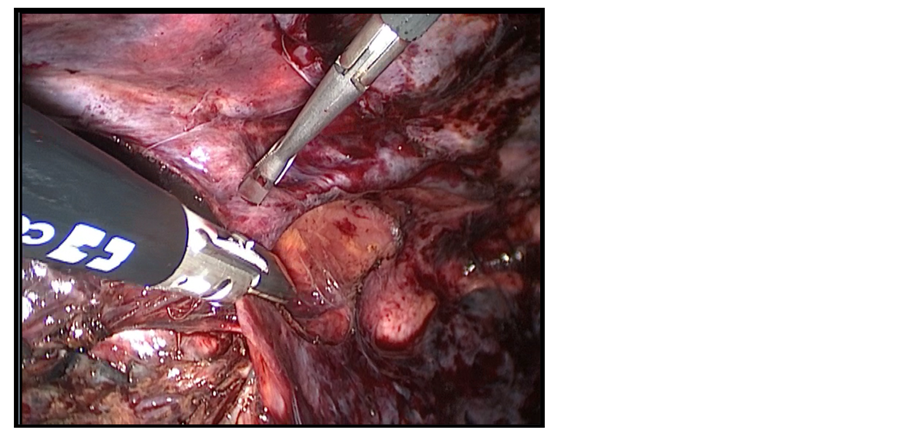 (b)
(b)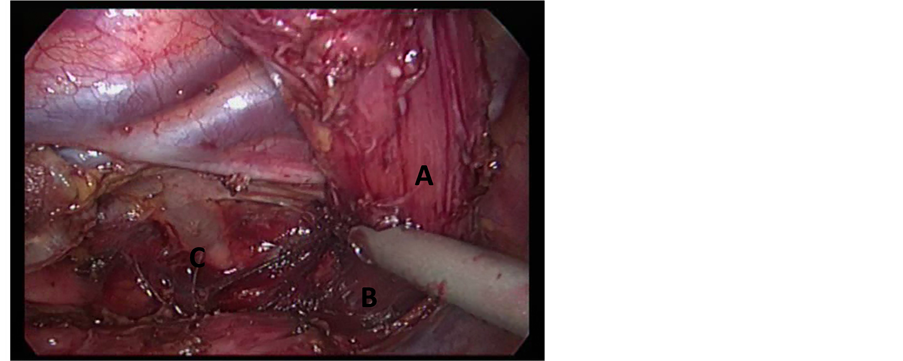 (c)
(c)
Figure 1. (a) Patient and Port position; (b) Division of the azygos vein by Ligasure device 10 mm; (c) Mobilization of the esophagus (A) to the thoracic inlet (B), thoracic duct is visualized (C).
 (a)
(a)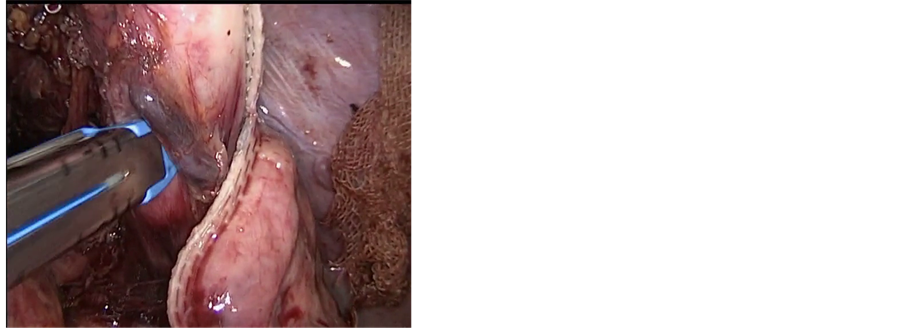 (b)
(b)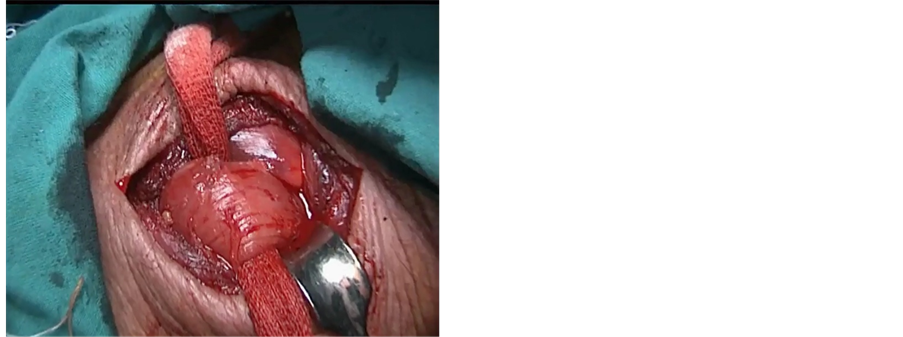 (c)
(c)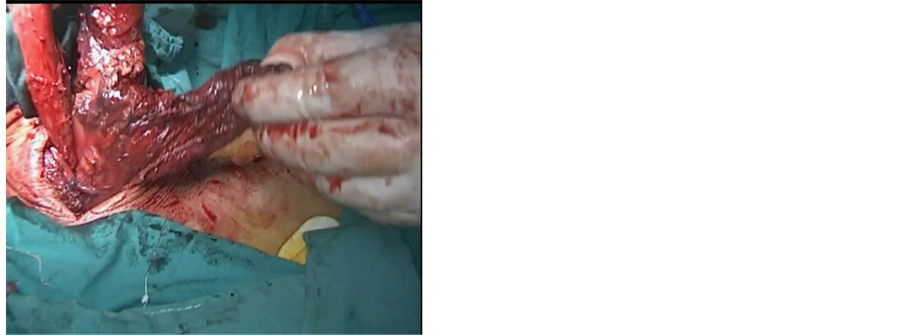 (d)
(d)
Figure 2. (a) Creation of gastric tube with endo GIA stapler; (b) Gastric tube still attached to the gastric fundus; (c) Mobilization of the esophagus through small neck incision; (d) Extraction of the esophagus from the neck incision which is still attached to the gastric tube.
followed up closely for detection of recurrence or metastasis. All patients were followed up regularly; biweekly in the first month, bimonthly in the following three months, monthly for six months, and every six months thereafter. Patients were censored if they developed the outcome (death, recurrence or metastasis) or at the study closure (October 2013). Follow up was carried out thought a full history, laboratory investigation and radiologic investigation including CT scanning of the chest and barium swallow (Figure 3(b)).
2.6. Statistical Analyses
Data were presented as mean and standard deviation (SD) for continuous data and proportion for categorical data. All statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA).
3. Results
The mean age of the patients was 58.97 ± 12.02 years (30 - 80 years). They were 16 males (53.3%) and 14 females (46.7%). After pulmonary function tests, 14 patients (46.7%) were normal and 16 patients (53.3%) showed either obstructive, restrictive pulmonary disorders or both. Those patients received chest measures before the operation (Table 1).
3.1. Histopathological Examination
Reports confirmed SCC pathology in 16 patients (53.3%) and adenocarcinoma in 14 patients (46.7%). Fourteen patients (46.7%) were grade II and 16 patients (53.3%) were grade III. The most common site was lower third, being affected in 18 patients (60%). The mean tumor size was 5.65 ± 2.29 cm (range 2 - 12 cm). The most common clinical stage was stage II in 17patients (56.7%). Only 5patients (16.7%) received neoadjuvant chemoradiotherapy (Table 2).
3.2. Thoracoscopic and Laparoscopic Assessment
17 patients (56.7%) were operable and 13 (43.3%) patients were found to be inoperable due to either small pleural nodules (n = 2), peritoneal nodules (n = 2), encasement of thoracic aorta (n = 1), encasement of celiac trunk (n = 1), presence of fixed lymph nodes (n = 6) or infiltration to the trachea (n = 1). The mean time of the thoracoscopic part was 3.38 h ± 0.88 h (range 2 h - 5 h) while in the abdominal part it was 2.59 h ± 0.78 h (range 1.5 h - 4 h). The mean operative blood loss was 250 cc ± 138.07 cc (range100 cc - 500 cc) and there were no patients who received intraoperative blood transfusion. In onepatient (3.3%), left pleural injury occurred due to sever adhesions between the tumor and pleura. This injury did not affect the patient general condition intra operatively (Table 3).
 (a)
(a)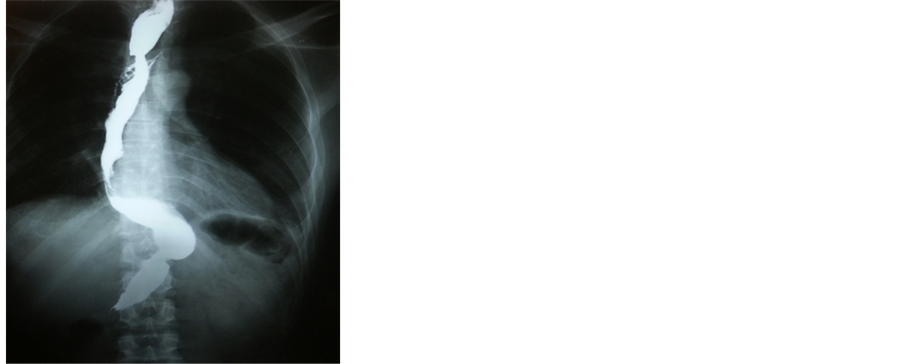 (b)
(b)
Figure 3. (a) Starting anastomosis between gastric tube and upper esophagus. Starting anastomosis between gastric tube and upper esophagus; (b) One year postoperative barium swallow showing the gastric tube.
Table 1. Demographic data of thirty cases of esophageal carcinoma.
Table 2. Tumor characteristics.
3.3. Postoperative Stay and Complications
Overall hospital stay was 17.47 ± 5.49 days (range 10 - 30 days). Four patients developed pneumonia (13.3%) that was treated by antibiotics, good oxygen ventilation and nebulizer (Table 4). Two patients developed pleural effusion (6.7%); one of them was at the right side and was developed after removal of the chest tube. The effusion was then aspirated. The other one developed left pleural effusion in the same patient who had intraoperative
Table 3. Intraoperative details.
Table 4. Overall hospital stay and postoperative complications.
pleural injury. They were also managed conservatively by aspiration. Three patients (10%) developed cervical anastomotic leakage that was managed by conservative measures (TPN, liberal drainage, and proper antibiotic). The management was enough in one patient (in whom the fistula sealed spontaneously). On the other hand, one patientrequired surgical intervention and secondary repair with feeding jejunostomy and the third patient died after 18 days of operation due to sever mediastinitis. One patient (3.3%) developed deep venous thrombosis (DVT) postoperatively and he was treated by the standard regimen of anticoagulants. Four patients (13.3%) developed wound infection that was managed by antibiotics according the results of culture and sensitivity.
Postoperative pathological examination revealed only two patients (6.7%) who had infiltrated lower safety margin while the majority of patients (93.3%) had negative safety margin. Lymph nodes were pathologically positive in 10 out of the 17 operable cases (58.8%) and were negative in the remaining 7 cases (41.2%). The mean total number of L.N. harvest (thoracoscopic and abdominal part) was 17.12 ± 2.32 and the mean number of the mediastinal L.N. which was resected by thoracoscopy was 8.29 + 2.02. Overall survival (OS) and disease free survival were assessed in the remaining 16 cases. The median overall survival was 15 ± 5.81 month (range 6 - 24 month). Overall survival was significantly affected by the clinical stage (P = 0.016). The median disease free survival was 13.88 + 5.88 month (6 - 24). Disease free survival was significantly affected by the tumor size (P = 0.040), neoadjuvant therapy (P = 0.029), lymph node status (P = 0.020) and the clinical staging of cases (P = 0.007).
4. Discussion
We are reporting a feasible and a relatively safe thoracoscopic and laparoscopic esophagectomy among a series of Egyptian patients with esophageal carcinoma. Comparing our population to previous studies showed a relatively lower age. The mean age in the current study was around 59 years while in most series the mean age was ranging from 60 - 67 years [8] . The finding may indicate a younger age of detection in our population compared with western populations which could be attributed to higher level of pollution in Nile Delta region in Egypt [10] .
In the current study, the mean operative time of the total procedure was around 360 minutes, which is comparable to the time reported by several investigators. For example, Gao et al. conducted minimally invasive esophagectomy (MIE) in 96 patients and the mean operative time was 330 minutes [11] . Close enough, Kinjo et al. conducted hybrid MIE in 34 patients with an operative time of 264 minutes [12] and Schoppmann et al. conducted MIE in 31 patients with a mean operative time of 411 minutes [13] . The mean blood loss in this study was 250 ± 138.07 cc. This was relatively lower than reported by Kinjo et al. who reported a mean blood loss of 536 cc [12] and Gao et al. who reported an average amount of 346 cc [11] . On the other hand, the current blood loss was slightly higher than that of Berger et al. who reported an average amount of 182 cc [14] .
In our study, we used the ligasure only for cutting of the azygos vein. But in other studies, either clipping or double ligation with sutures was used as reported by Palanivelu et al. [15] or by using endo GIA stapler as reported by Berger et al. [14] . We found this new modification is less time consuming and less costly with no risk of bleeding found intraoperative or postoperative which may need further evaluation.
The mean overall hospital stay in the current study was approximately 17.5 days. This was comparable to that observed by Mamidanna et al. who reported a hospital stay of 15 days [16] and Gao et al. reported a hospital stay of 12.6 days [11] . However, it was relatively shorter than reported by Kinjo et al. who reported a mean overall hospital stay of 23 days [12] . In our study, the postoperative hospital stay was longer in the patients that developed postoperative pneumonia rather than the other patients and it was also longer in patients of cervical anastomotic leakage.
Postoperative pneumonia was reported as the most frequent morbidity after esophagectomy [7] . Similarly, the incidence of postoperative pneumonia in our study was the most frequent complication at 13.3%. The percentage was comparable to that observed by Gao et al. who reported 13.5% [11] . On the other hand, it was lower than the rates reported by Mamidanna et al. and Kinjo et al. who reported approximately 30% [12] [16] . The higher rate of pneumonia emphasized our efforts to request smoking cessation six weeks before the surgical resection, good preoperative chest measures, and using of intraoperative positive pressure once the thoracic esophagus has been completely mobilized.
The early postoperative mortality in the current study was low at 3.3%. This was comparable to what was observed in previous studies. For example, early postoperative mortality was 2.1% in Gao et al. study [11] , 4% in Mamidanna et al. study [16] , and 7.7% in By Berger et al. study [14] . The median OS in the current study was 15 months (6 - 24). This was considerably lower than seen in some reports. Smithers et al. reported a median OS of 25 months in total MIE and 31 months in hybrid MIE [17] . Additionally, the median OS after MIE was estimated at 35 months by by Zingg et al. [18] and 51 months by Sundaram et al. [19] [20] . The average number of lymph nodes harvest in our study was 17. It ranged between 14 and 24 in different studies [12] [14] [20] . In a systematic review of 17 studies in 2012, the number of lymph nodes resected via MIE was 16 (5.7 - 33.9) [8] . In our study, 93.3% of patients had a negative safety margin. This was similar to that reported by previous studies which ranged between 91% and 97% [12] [14] .
In conclusion, totally endoscopic (thoracoscopic and laparoscopic) oesophagectomy is feasible and safe. Beside its efficacy as an assessment tool, total esophagectomy and lymphadenectomy could be performed at the same time.
Acknowledgements
We thank Dr. Aiman El-Saed; professor of community medicine, Mansoura University, for his support throughout this study.
References
- Ferlay, J., Shin, H.R., Bray, F., Forman, D., Mathers, C. and Parkin, D.M. (2010) Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127, 2893-2917. http://dx.doi.org/10.1002/ijc.25516
- Holmes, R.S. and Vaughan, T.L. (2007) Epidemiology and Pathogenesis of Esophageal Cancer. Seminars in Radiation Oncology, 17, 2-9. http://dx.doi.org/10.1016/j.semradonc.2006.09.003
- Wei, J.T. and Shaheen, N. (2003) The Changing Epidemiology of Esophageal Adenocarcinoma. Seminars in Gastrointestinal Disease, 14, 112-27.
- Lerut, T., Coosemans, W., De Leyn, P., Van Raemdonck, D., Nafteux, P. and Moons, J. (2001) Optimizing Treatment of Carcinoma of the Esophagus and Gastroesophageal Junction. Surgical Oncology Clinics of North America, 10, 863- 884.
- Akiyama, H., Tsurumaru, M., Udagawa, H. and Kajiyama, Y. (1994) Radical Lymph Node Dissection for Cancer of the Thoracic Esophagus. Annals of Surgery, 220, 364-372; Discussion 372-363.
- Cuschieri, A., Shimi, S. and Banting, S. (1992) Endoscopic Oesophagectomy through a Right Thoracoscopic Approach. Journal of the Royal College of Surgeons of Edinburgh, 37, 7-11.
- Ben-David, K., Sarosi, G.A., Cendan, J.C., Howard, D., Rossidis, G. and Hochwald, S.N. (2012) Decreasing Morbidity and Mortality in 100 Consecutive Minimally Invasive Esophagectomies. Surgical Endoscopy, 26, 162-167. http://dx.doi.org/10.1007/s00464-011-1846-3
- Dantoc, M.M., Cox, M.R. and Eslick, G.D. (2012) Does Minimally Invasive Esophagectomy (Mie) Provide for Comparable Oncologic Outcomes to Open Techniques? A Systematic Review. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract, 16, 486-494.
- Benzoni, E., Terrosu, G., Bresadola, V., Uzzau, A., Intini, S., Noce, L., Cedolini, C., Bresadola, F. and De Anna, D. (2004) A Comparative Study of the Transhiatal Laparoscopic Approach versus Laparoscopic Gastric Mobilisation and Right Open Transthoracic Esophagectomy for Esophageal Cancer Management. Journal of Gastrointestinal and Liver Diseases, 16, 395-401.
- Elewa, H.H. (2010) Potentialities of Water Resources Pollution of the Nile River Delta, Egypt. The Open Hydrology Journal, 4, 1-13. http://dx.doi.org/10.2174/1874378101004010001
- Gao, Y., Wang, Y., Chen, L. and Zhao, Y. (2011) Comparison of Open Three-Field and Minimally-Invasive Esophagectomy for Esophageal Cancer. Interactive Cardiovascular and Thoracic Surgery, 12, 366-369. http://dx.doi.org/10.1510/icvts.2010.258632
- Kinjo, Y., Kurita, N., Nakamura, F., Okabe, H., Tanaka, E., Kataoka, Y., Itami, A., Sakai, Y. and Fukuhara, S. (2012) Effectiveness of Combined Thoracoscopic-Laparoscopic Esophagectomy: Comparison of Postoperative Complications and Midterm Oncological Outcomes in Patients with Esophageal Cancer. Surgical Endoscopy, 26, 381-390. http://dx.doi.org/10.1007/s00464-011-1883-y
- Schoppmann, S.F., Prager, G., Langer, F.B., Riegler, F.M., Kabon, B., Fleischmann, E. and Zacherl, J. (2010) Open versus Minimally Invasive Esophagectomy: A Single-Center Case Controlled Study. Surgical Endoscopy, 24, 3044- 3053. http://dx.doi.org/10.1007/s00464-010-1083-1
- Berger, A.C., Bloomenthal, A., Weksler, B., Evans, N., Chojnacki, K.A., Yeo, C.J. and Rosato, E.L. (2011) Oncologic Efficacy Is not Compromised, and May Be Improved with Minimally Invasive Esophagectomy. Journal of the American College of Surgeons, 212, 560-568. http://dx.doi.org/10.1016/j.jamcollsurg.2010.12.042
- Palanivelu, C., Prakash, A., Senthilkumar, R., Senthilnathan, P., Parthasarathi, R., Rajan, P.S. and Venkatachlam, S. (2006) Minimally Invasive Esophagectomy: Thoracoscopic Mobilization of the Esophagus and Mediastinal Lymphadenectomy in Prone Position—Experience of 130 Patients. Journal of the American College of Surgeons, 203, 7-16. http://dx.doi.org/10.1016/j.jamcollsurg.2006.03.016
- Mamidanna, R., Bottle, A., Aylin, P., Faiz, O. and Hanna, G.B. (2012) Short-Term Outcomes Following Open versus Minimally Invasive Esophagectomy for Cancer in England: A Population-Based National Study. Annals of Surgery, 255, 197-203. http://dx.doi.org/10.1097/SLA.0b013e31823e39fa
- Smithers, B.M., Gotley, D.C., Martin, I. and Thomas, J.M. (2007) Comparison of the Outcomes between Open and Minimally Invasive Esophagectomy. Annals of Surgery, 245, 232-240. http://dx.doi.org/10.1097/01.sla.0000225093.58071.c6
- Zingg, U., McQuinn, A., DiValentino, D., Esterman, A.J., Bessell, J.R., Thompson, S.K., Jamieson, G.G. and Watson, D.I. (2009) Minimally Invasive versus Open Esophagectomy for Patients with Esophageal Cancer. The Annals of Thoracic Surgery, 87, 911-919. http://dx.doi.org/10.1016/j.athoracsur.2008.11.060
- Sundaram, A., Geronimo, J.C., Willer, B.L., Hoshino, M., Torgersen, Z., Juhasz, A., Lee, T.H. and Mittal, S.K. (2012) Survival and Quality of Life after Minimally Invasive Esophagectomy: A Single-Surgeon Experience. Surgical Endoscopy, 26, 168-176. http://dx.doi.org/10.1007/s00464-011-1850-7
NOTES

*Corresponding author.


