Surgical Science
Vol. 4 No. 6 (2013) , Article ID: 32284 , 6 pages DOI:10.4236/ss.2013.46059
Serum Intestinal Fatty Acid Binding Protein in Patients with Small Bowel Obstruction*
1Division of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences,Niigata, Japan
2DS Pharma Biomedical Co., Ltd., Osaka, Japan
Email: †kandat@tsrh.jp
Copyright © 2013 Kaoru Sakamoto et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 28, 2013; revised April 29, 2013; accepted May 7, 2013
Keywords: Biomarker; I-FABP; Small Bowel Obstruction; Strangulation
ABSTRACT
Purpose: The aims of this pilot study were to reveal the biological characteristics of serum I-FABP and explore its clinical utility as a biomarker in patients with small bowel obstruction (SBO). Methods: Serum I-FABP levels were measured in 37 consecutive patients with SBO between 2007 and 2008. Serum I-FABP levels were compared between ischemia (n = 10) and non-ischemia (n = 27) groups. Serum I-FABP levels were longitudinally analyzed in 21 patients who showed high (>2.0 ng/ml) serum I-FABP levels. The relationship between serum I-FABP level and length of damaged bowel was also analyzed. Results: Median serum I-FABP levels were 9.2 ng/ml in the ischemia group and 1.9 ng/ml in the non-ischemia group (p < 0.0001). The elevated I-FABP levels rapidly decreased after therapeutic intervention and normalized on the third day in all patients. Linear regression analysis revealed a positive correlation between I-FABP levels and lengths of surgically excised bowels (y = 2.527x − 7.660, r = 0.604, p = 0.0018). By setting the cutoff level at 7.2 ng/ml, the diagnostic ability of serum I-FABP was 70.0% in terms of sensitivity, 92.6% in terms of specificity, and 86.5% in terms of accuracy. Conclusion: Serum I-FABP sensitively reflects bowel damage in SBO patients and seems to be a potential biomarker for detecting small-bowel ischemia.
1. Introduction
Small bowel obstruction (SBO) is one of the most common diseases in abdominal surgery. SBO associated with ischemia, i.e., strangulated obstruction of the small bowel, is a life-threatening condition. A delay in diagnosis can lead to irreversible full-thickness bowel necrosis that requires massive resection of the small bowel and is associated with a high risk of death [1]. Timely diagnosis of strangulated SBO is essential to improve both prognosis and quality of life of SBO patients. Although advances in radiological imaging technology, including multidetector computed tomography (MDCT), have made it easier to diagnose strangulated SBO than before, the accurate diagnosis of strangulated SBO remains difficult even for diagnostic radiologists and experienced surgeons [2-4]. Furthermore, it is costly to conduct MDCT in all patients with SBO to determine that the obstructed bowel loops are affected by ischemia.
Organ-specific biomarkers are widely used for the diagnosis of various diseases by blood analysis (alanine aminotransferase for hepatic injury, creatine phosphokinase [CPK] for myocardial infarction, and amylase for pancreatitis), because they are non-invasive, affordable, and easily applicable. Bowel-specific biomarkers are needed to identify patients who are significantly at a high risk of strangulated SBO. However, no diagnostically satisfactory blood marker has been established yet in bowel diseases [5].
Intestinal fatty acid binding protein (I-FABP), a cytosolic protein with a molecular mass of approximately 15 kDa [6], distributes in the small bowel mucosa from the duodenum to the distal segment of the ileum [7,8]. The cytohistologic features of I-FABP, namely, a cytosolic protein with a low molecular mass and specific localization in the intestinal epithelium, make I-FABP a potentially beneficial blood marker for the diagnosis of small bowel disease.
We have hypothesized that I-FABP is a promising biomarker for small bowel ischemia and explored its diagnostic utility in the last twenty years. Rodent experimental models showed that serum I-FABP levels rapidly rose at the very early stages of small bowel ischemia [9]. Clinical studies of patient blood samples revealed that serum I-FABP levels were elevated in patients with occlusion of the superior mesenteric artery and strangulated SBO [10,11]. Very recently, we conducted a multicenter validation study to evaluate the clinical usefulness of serum I-FABP measurement and demonstrated that I-FABP is a useful biomarker for the diagnosis of small bowel disease as well as small bowel ischemia in patients presenting with acute abdomen [12].
Evidence of the utility of I-FABP measurement for the diagnosis of small bowel ischemia was provided by researchers in the Unites States and Europe [13-16]. Today, I-FABP is considered one of the most promising blood markers for small bowel ischemia [5]. However, few studies have addressed the diagnostic utility of serum I-FABP in SBO patients [11,14]. Moreover, it remains unknown when serum I-FABP level reaches a peak after bowel injury, how long the high I-FABP level is maintained, and whether the level is related to the mass of insulted bowel or not. The lack of such knowledge that forms the basis of laboratory tests precludes appropriate interpretation of clinical data and therefore, the clinical application of serum I-FABP measurement is delayed in the management of SBO patients.
To address these clinical issues, we conducted a pilot study of serum I-FABP in SBO patients. In the present study, serum I-FABP levels were measured in SBO patients to explore the utility and limitations for the diagnosis of strangulated SBO. We also analyzed the relationship between I-FABP level and length of damaged bowel.
2. Patients and Methods
2.1. Study Design
This study was a prospective, single center, pilot study. Patients with SBO were enrolled between April 2007 and October 2008 at the Department of Surgery, Niigata University Hospital. For the diagnosis of SBO, both clinical symptoms and radiologic findings were needed, i.e., dilated bowel loops with multiple fluid levels that were shown by abdominal X-ray and/or computed tomography (CT). The attending physician was tasked to decide both treatment modality and surgical indication. Serum I-FABP levels of all patients were analyzed in a blinded manner at a single laboratory (DS Pharma Biomedical). The results of I-FABP measurement were not reported to the attending physician and therefore had no effect on the treatment decision-making in any patient.
This study was approved by the institutional review board of Niigata University Graduate School of Medical and Dental Sciences (No. 553) and written informed consent was obtained from all patients.
2.2. Patients
Thirty-seven patients were enrolled in the prospective study. There were 21 men and 16 women. Patient age at enrollment ranged from 22 to 93 years (median, 67 years). Seven patients underwent conservative treatment with only nihil per os (NPO) and/or a nasogastric tube (NGT), five underwent decompression using a long tube, and 24 underwent laparotomy. The remaining one patient received thrombolytic therapy for superior mesenteric artery occlusion. The clinical background of the 37 patients enrolled in this study is shown in Table 1.
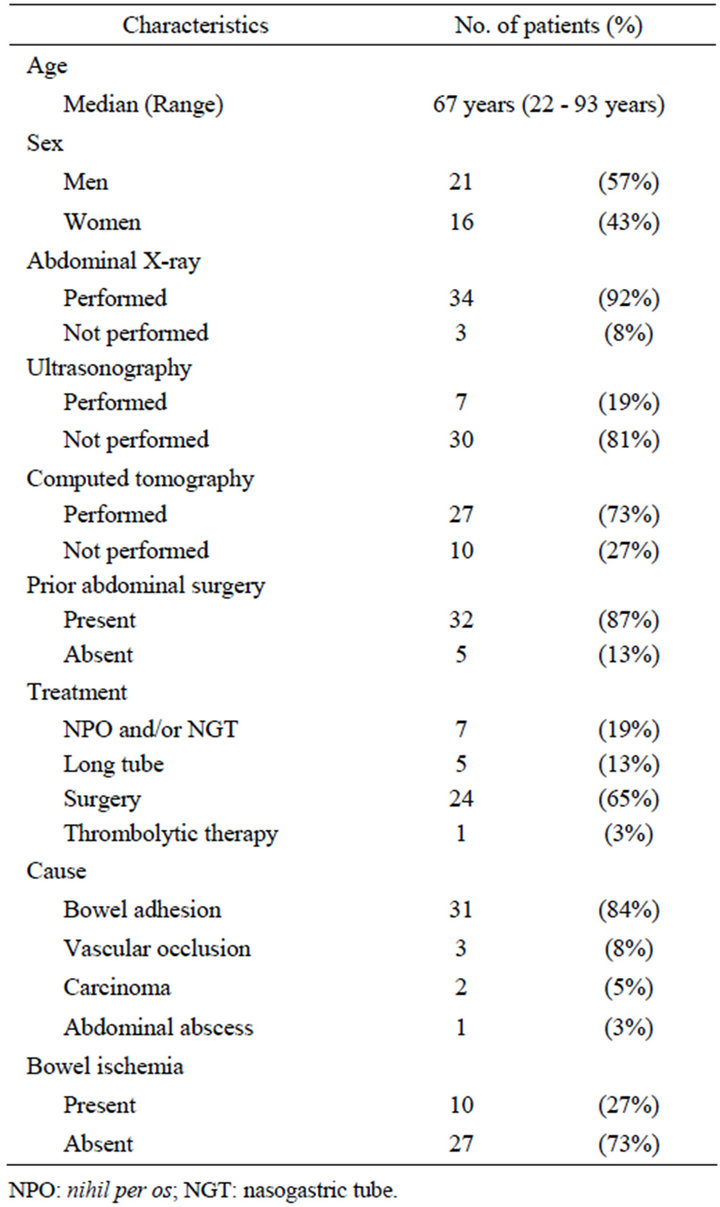
Table 1. Clinical background of 37 patients with small-bowel obstruction.
2.3. Blood Sampling and I-FABP Measurement
Blood (approximately 3 ml) was sampled from each patient at the time of diagnosis of SBO, and sampling was repeated in the morning every day until oral intake was started. In addition, samples were also collected 8 hours after interventional treatments, including long-tube decompression and surgery. Serum was immediately separated from each blood sample and stored frozen at −20˚C until measurement of I-FABP. Serum I-FABP levels were analyzed using the human I-FABP-specific enzyme-linked immunosorbent assay (ELISA) that we developed. This assay allowed quantification of serum I-FABP in the range of 0.1 to 50 ng/ml. Samples with a value of 50 ng/ml or more were diluted appropriately to determine the I-FABP level. In our previous study using this ELISA, the serum I-FABP level of 61 healthy subjects was 1.1 ± 0.9 ng/ml. Thus, 2.0 ng/ml was set as the upper limit of the normal range in this assay for serum I-FABP. The features of the ELISA used in this study are described in detail elsewhere [17].
2.4. Evaluation of Bowel Ischemia
The diagnosis of small bowel ischemia was made based on operative findings. We defined ischemia by the gross disturbance of blood flow in the bowel, regardless of extent and grade. All patients who responded to medical treatment without undergoing surgery were considered to be free of ischemia. One patient who underwent thrombolytic therapy with transarterial administration of urokinase was classified in the non-ischemic group, because arteriography showed that the involved small bowel was well visualized by perfusion through the collaterals.
2.5. Correlation between Serum I-FABP Level and Bowel Resection Length
To reveal whether serum I-FABP level is influenced by volume of damaged enterocytes, we analyzed the correlation by linear regression analysis using Pearson’s test. Because it was impossible to evaluate precisely damaged enterocyte volume in the clinical setting, we substituted the length of surgically excised small bowel for the damaged enterocyte volume.
2.6. Statistical Analysis
Serum I-FABP levels are expressed as median and range. The Mann-Whitney U test was used for intergroup comparisons of quantitative data and p < 0.05 was considered to be statistically significant.
A receiver operating characteristic (ROC) curve was depicted using JMP software version 6.0.0 (SAS Institute, Cary, NC), varying the potential cutoff levels of serum I-FABP concentration, and the Youden indices [sensitiveity-(1-specificity)] were sequentially calculated to determine the cutoff level of I-FABP. The concentration at which the Youden index showed a maximum value was selected as the cutoff level for the diagnosis of strangulated SBO.
3. Results
3.1. Serum I-FABP Levels of SBO Patients with Bowel Ischemia
Based on surgical findings, we divided the 37 enrolled SBO patients into ischemia (n = 10) and non-ischemia (n = 27) groups and compared the serum I-FABP levels of the two groups using the data at the time of diagnosis of SBO (Figure 1).
The median serum I-FABP level was 9.2 ng/ml (range 3.3 - 871.1 ng/ml) in the ischemia group and 1.9 ng/ml (range 0.1 - 9.2 ng/ml) in the non-ischemia group. The serum I-FABP level of the ischemia group was significantly higher than that of the non-ischemia group (p < 0.0001).
3.2. Temporal Changes of Serum I-FABP Levels
Serum I-FABP levels were monitored in 36 patients with SBO, excluding one patient who postoperatively underwent hemodialysis. Of the 36 patients, 21 showed high serum I-FABP levels (>2.0ng/ml) at pretreatment.
The elevated serum I-FABP levels rapidly decreased within 8 hours after the treatment. Only two patients showed an increase in serum I-FABP level at 8 hours despite intervention: from 3.3 ng/ml to 3.8 ng/ml in one
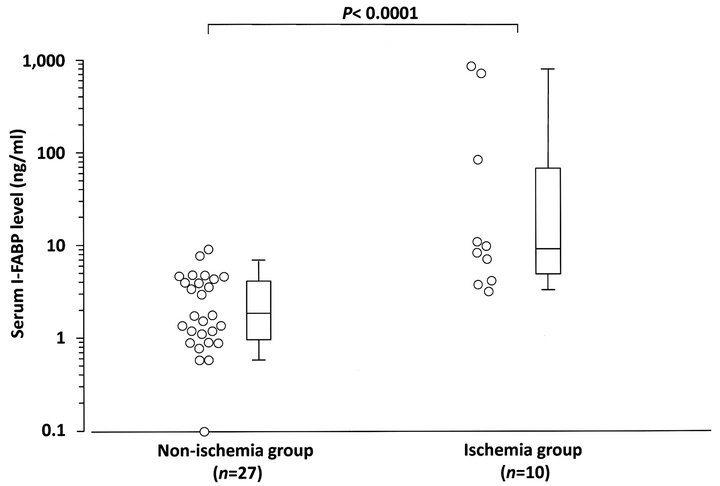
Figure 1. Distributions of serum I-FABP levels at the time of diagnosis of small bowel obstruction (SBO) in bowel ischemia and non-ischemia groups. Serum I-FABP levels of the ischemia group were significantly higher than those of the non-ischemia group (p < 0.0001). Open circles indicate serum I-FABP levels of patients at the time of diagnosis of SBO. Boxes and whiskers represent interquartile ranges (IQRs) and standard deviations of the serum I-FABP levels of each group, respectively. Horizontal lines within the boxes represent median values of serum I-FABP.
patient and from 3.5 ng/ml to 3.8 ng/ml in another one. The temporal changes of the 21 patients are shown in Figure 2. Serum I-FABP levels were normalized on the third day in all patients.
3.3. Correlation between Serum I-FABP Level and Bowel Resection Length
In this series, 24 patients underwent laparotomy for treatment of SBO. Of the 24 patients, 10 underwent surgical resection of damaged bowel loop owing to closed loop obstruction; two, for transient and reversible ischemia; three, for mucosal necrosis; and five, for full-thickness necrosis. In this series, no patient with simple mechanical obstruction underwent bowel resection. Linear regression analysis revealed a significant positive correlation between serum I-FABP levels and lengths of surgically excised bowel (y = 2.527x − 7.660, r = 0.604, p = 0.0018; Figure 3).
3.4. Diagnosis of Small Bowel Ischemia
ROC analysis showed that the tentative serum I-FABP cutoff level for the diagnosis of small bowel ischemia was 7.2 ng/ml (Figure 4). By setting the cutoff level at 7.2 ng/ml, the diagnostic ability of serum I-FABP was found to be as follows: sensitivity, 70.0% (95% confidence
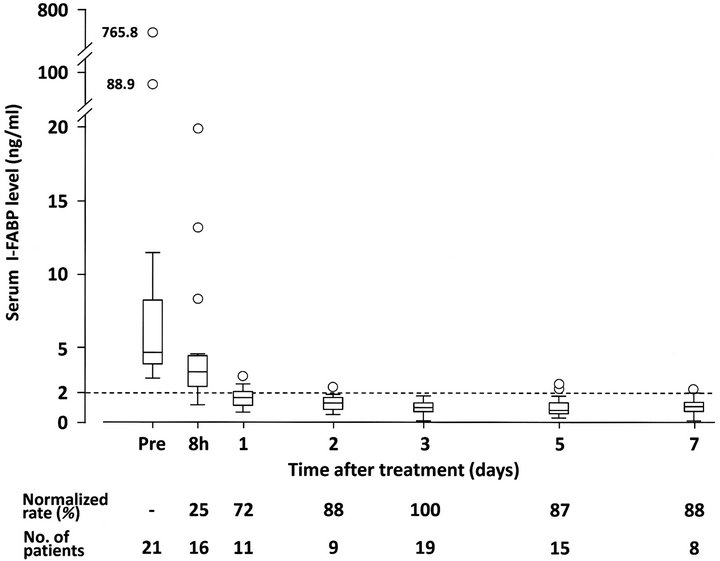
Figure 2. Temporal changes of serum I-FABP levels in 21 patients who showed high serum I-FABP levels (>2.0 ng/ml) at the time of diagnosis of small bowel obstruction (SBO). The elevated serum I-FABP levels rapidly decreased after the treatment and were normalized on the third day in all patients. Normalized rates were calculated as percentages of patients in whom serum I-FABP levels were normalized until that time. Boxes and whiskers represent interquartile ranges (IQRs) and 1.5 x IQR of the serum I-FABP levels at each time point, respectively. Horizontal lines within the boxes represent median values of serum I-FABP. Open circles indicate patients who showed high serum I-FABP levels outside 1.5 x IQR. Dotted line indicates the upper limit of normal serum I-FABP level (2.0 ng/ml).
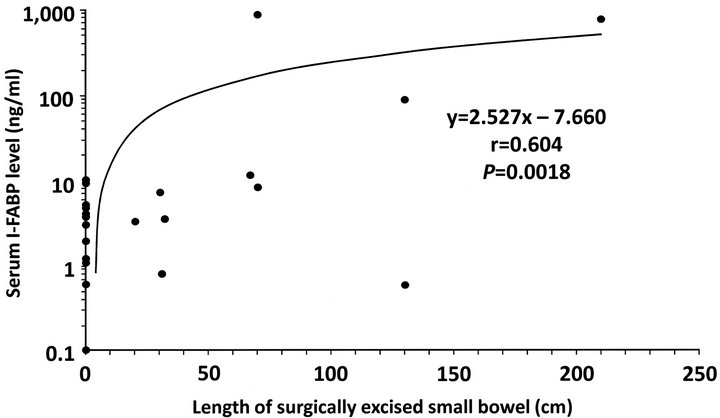
Figure 3. Correlation between serum I-FABP level and bowel resection length. Linear regression analysis revealed a significant positive correlation between serum I-FABP levels and lengths of surgically excised bowel (y = 2.527x − 7.660, r = 0.604, p = 0.0018).

Figure 4. Receiver operating characteristic (ROC) curve for serum I-FABP. ROC analysis of 37 patients with small bowel obstruction (SBO) showed that 7.2 ng/ml was the tentative serum I-FABP cutoff level for the diagnosis of small bowel ischemia.
interval: 39.7% - 89.2%); specificity, 92.6% (76.6% - 97.9%); accuracy, 86.5% (66.6% - 95.6%); positive likelihood ratio, 9.45 (2.24 - 38.1); and negative likelihood ratio, 0.32 (0.13 - 0.84).
4. Discussion
Clinical evidence of the utility of serum I-FABP measurement for the diagnosis of small bowel ischemia has been accumulating at a rapid pace [9-16]. We have recently shown the clinical utility of serum I-FABP as a blood marker for the diagnosis of small-bowel ischemia in a large-scale validation study [12]. That study also uncovered several issues that need to be resolved prior to the clinical application of this small bowel biomarker, including temporal changes after bowel injury, the relationship between serum I-FABP level and extent of insulted bowel, and the ability to discriminate strangulated obstruction from simple obstruction in SBO patients. The present study was conducted to acquire supplemental evidence for the resolution of those clinical issues.
We have previously shown that bowel ischemia rapidly caused the release of I-FABP from damaged enterocytes in rodent experimental models. Serum I-FABP level rose to 300 ng/ml 1 hour after the transient 30-minute occlusion of the superior mesenteric artery and returned to normal within 4 hours [9]. In human, however, little data are available on the biodynamics of serum I-FABP release by damaged enterocytes. Derikx et al. [18], utilizing 5-cm-long jejunal segments harvested by pancreaticoduodenectomy, measured arteriovenous differences in I-FABP concentrations. In their study, 30- minute ischemia followed by reperfusion caused rapid elevation of I-FABP concentrations in venous blood from the ischemic jejunum, reaching 4 ng/ml, and then the abnormally elevated I-FABP concentrations returned to normal as swiftly as 60 min after the reperfusion. In the present study, the elevated I-FABP levels rapidly decreased within 8 hours after appropriate treatment and completely normalized within 3 days. These findings clinically underpinned the rapid and real-time responding feature of I-FABP, which was previously shown in experimental models, extending the possibility that serum I-FABP measurement may be used as a blood biomarker to monitor whether enterocyte damage is continuing or diminished.
To use I-FABP as a bowel biomarker, it is necessary to clarify the relationship between serum I-FABP level and the extent and/or grade of small bowel injury. Theoretically, serum I-FABP increase is greater as the extent of small bowel injury is increased. However, no study has experimentally demonstrated that relationship. The length of bowel affected by ischemia is largely changeable by bowel dilation in SBO. Ischemia grade widely ranges from reversible ischemia to transmural necrosis and the ischemia grade is variable in a single strangulated loop. It is practically impossible to evaluate precisely the extent and grade of bowel ischemia during SBO surgery. Thus, we alternatively used the length of bowel surgically excised in this study, as we considered it a more objective parameter. The present study has shown that serum I-FABP level is related to the extent of insulted bowel, although the excised bowel length is used as surrogate. Studies using animal experimental models will be necessary to clarify this pathophysiological issue of I-FABP.
Our validation study of the diagnosis of small bowel ischemia, the subjects of which were patients with acute abdomen, showed that serum I-FABP had high diagnostic utility comprehensively, but was inferior to CPK and lactate dehydrogenase in terms of specificity: 73.8% vs. 82.8% and 77.7%, respectively [12]. In that study, a fraction of patients with simple SBO also yielded high serum I-FABP levels, probably because the biomarker was so sensitive that the microcirculation in the expanded bowel wall was disturbed by the increased luminal pressure. Thus, it remains unclear whether or not the serum I-FABP assay can efficiently differentiate patients with bowel strangulation in the SBO patient population.
ROC analysis in the present study revealed that the cutoff for I-FABP should be set at a higher level (7.2 ng/ml) than that determined based on the data of patients with acute abdomen (3.1 ng/ml). Through this, the diagnostic ability of I-FABP was satisfactorily improved: sensitivity, 70.0%; specificity, 92.6%; accuracy, 86.5%; positive likelihood ratio, 9.45; and negative likelihood ratio, 0.32. In a series of studies that evaluated the diagnostic ability of MDCT for strangulated SBO [2-4], sensitivity was 85% - 89%, specificity was 67% - 96%, and accuracy was 79%. Those studies not only revealed the high performance of MDCT for the diagnosis of strangulated SBO, but also pointed out the existence of significant interobserver variability, which was dependent on the expertise of observers. In this study, serum I-FABP measurement showed promising diagnostic ability for strangulated SBO, which was compatible with that of MDCT. The measurement of this blood marker may complement the drawback of MDCT by providing quantitative information.
In summary, we revealed the nature of serum I-FABP in a pilot study with SBO patients as subjects. I-FABP was swiftly released from the injured small bowel in strangulated SBO and serum I-FABP level was related to the length of the small bowel segment that was necessary for excision. This bowel-specific biomarker showed promising results in terms of discriminating patients with strangulated obstruction from SBO patients. We are planning a multicenter study to demonstrate the diagnostic utility of serum I-FABP measurement for bowel strangulation in SBO patients.
5. Conclusion
Serum I-FABP sensitively reflects bowel damage in SBO patients and seems to be a potential biomarker for detecting small-bowel ischemia.
REFERENCES
- D. Hashimoto, M. Hirota, T. Matsukawa, Y. Yagi and Baba H, “Clinical Features of Strangulated Small Bowel Obstruction,” Surgery Today, Vol. 42, No. 11, 2012, pp. 1061-1065. doi:10.1007/s00595-012-0207-8
- M. Assenza, G. Ricci, V. Macciucca-Mde, E. Polettini, E. Casciani, M. L. De Cicco, et al., “Comparison among Preoperative Single-slice CT and Multi-Slice CT in Simple, Closed Loop and Strangulating Bowel Obstruction,” Hepatogastroenterology, Vol. 54, No. 79, 2007, pp. 2017-2023.
- K. Kato, K. Mizunuma, M. Sugiyama, S. Sugawara, T. Suzuki, M. Tomabechi, et al., “Interobserver Agreement on the Diagnosis of Bowel Ischemia: Assessment Using Dynamic Computed Tomography of Small Bowel Obstruction,” Japanese Journal of Radiology, Vol. 28, No. 10, 2010, pp. 727-732. doi:10.1007/s11604-010-0500-7
- A. Blachar, S. Barnes, S. Z. Adam, G. Levy, I. Weinstein, R. Precel, et al., “Radiologists’ Performance in the Diagnosis of Acute Intestinal Ischemia, Using MDCT and Specific CT Findings, Using a Variety of CT Protocols,” Emergency Radiology, Vol. 18, No. 5, 2011, pp. 385-394. doi:10.1007/s10140-011-0965-4
- N. J. Evennett, M. S. Petrov, A. Mittal and J. A. Windsor, “Systematic Review and Pooled Estimates for the Diagnostic Accuracy of Serological Markers for Intestinal Ischemia,” World Journal of Surgery, Vol. 33, No. 7, 2009, pp. 1374-1383. doi:10.1007/s00268-009-0074-7
- D. H. Alpers, A. W. Strauss, R. K. Ockner, N. M. Bass and J. I. Gordon, “Cloning of a cDNA Encoding Rat Intestinal Fatty Acid-Binding Protein,” Proceedings of the National Academy of Sciences of the United of America, Vol. 81, No. 2, 1984, pp. 313-317. doi:10.1073/pnas.81.2.313
- L. B. Agellon, M. J. Toth and A. B. Thomson, “Intracellular Lipid Binding Proteins of the Small Intestine,” Molecular and Cellular Biochemistry, Vol. 239, No. 1-2, 2002, pp. 79-82. doi:10.1023/A:1020520521025
- M. N. Pelsers, Z. Namiot, W. Kisielewski, A. Namiot, M. Januszkiewicz, W. T. Hermens, et al., “Intestinal-Type and Liver-Type Fatty Acid-binding Protein in the Intestine. Tissue Distribution and Clinical Utility,” Clinical Biochemistry, Vol. 36, No. 7, 2003, pp. 529-535. doi:10.1016/S0009-9120(03)00096-1
- T. Kanda, Y. Nakatomi, H. Ishikawa, M. Hitomi, Y. Matsubara, T. Ono, et al., “Intestinal Fatty Acid-Binding Protein as a Sensitive Marker of Intestinal Ischemia,” Digestive Diseases and Sciences, Vol. 37, No. 9, 1992, pp. 1362-1367. doi:10.1007/BF01296004
- T. Kanda, T. H. Fujii, M. Fujita, Y. Sakai, T. Ono and K. Hatakeyama, “Intestinal Fatty Acid-Binding Protein is Available for Diagnosis of Intestinal Ischaemia: Immunochemical Analysis of Two Patients with Ischaemic Intestinal Diseases,” Gut, Vol. 36, No. 5, 1995, pp. 788-791. doi:10.1136/gut.36.5.788
- T. Kanda, H. Fujii, T. Tani, H. Murakami, T. Suda, Y. Sakai, et al., “Intestinal Fatty Acid-binding Protein Is a Useful Diagnostic Marker for Mesenteric Infarction in Humans,” Gastroenterology, Vol. 110, No. 2, 1996, pp. 339-343. doi:10.1053/gast.1996.v110.pm8566578
- T. Kanda, A. Tsukahara, K. Ueki, Y. Sakai, T. Tani, A. Nishimura, et al., “Diagnosis of Ischemic Small Bowel Disease by Measurement of Serum Intestinal Fatty Acid-Binding Protein in Patients with Acute Abdomen: A Multicenter, Observer-Blinded Validation Study,” Journal of Gastroenterology, Vol. 46, No. 4, 2011, pp. 492- 500. doi:10.1007/s00535-011-0373-2
- G. Gollin, C. Marks and W. H. Marks, “Intestinal Fatty Acid Binding Protein in Serum and Urine Reflects Early Ischemic Injury to the Small Bowel,” Surgery, Vol. 113, No. 5, 1993, pp. 545-551.
- D. R. Cronk, T. P. Houseworth, D. G. Cuadrado, G. S. Herbert, P. M. McNutt and K. S. Azarow, “Intestinal Fatty Acid Binding Protein (I-FABP) for the Detection of Strangulated Mechanical Small Bowel Obstruction,” Current Surgery, Vol. 63, No. 5, 2006, pp. 322-325. doi:10.1016/j.cursur.2006.05.006
- D. van Noord, P. B. Mensink, R. J. de Knegt, M. Ouwendijk, J. Francke, A. J. van Vuuren, et al., “Serum Markers and Intestinal Mucosal Injury in Chronic Gastrointestinal Ischemia,” Digestive Diseases and Sciences, Vol. 56, No. 2, 2011, pp. 506-512. doi:10.1007/s10620-010-1303-5
- G. Thuijls, K. van Wijck, J. Grootjans, J. P. Derikx, A. A. van Bijnen, E. Heineman, et al., “Early Diagnosis of Intestinal Ischemia Using Urinary and Plasma Fatty Acid Binding Proteins,” Annals of Surgery, Vol. 253, No. 2, 2011, pp. 303-308. doi:10.1097/SLA.0b013e318207a767
- H. Funaoka, T. Kanda, S. Kajiura, Y. Ohkaru and H. Fujii, “Development of a High-specificity Sandwich ELISA System for the Quantification of Human Intestinal Fatty Acid-Binding Protein (I-FABP) Concentrations,” Immunological Investigations, Vol. 40, No. 3, 2011, pp. 223- 242. doi:10.3109/08820139.2010.534216
- J. P. Derikx, R. A. Matthijsen, A. P. de Bruïne, A. A. van Bijnen, E. Heineman, R. M. van Dam, et al., “Rapid Reversal of Human Intestinal Ischemia-Reperfusion Induced Damage by Shedding of Injured Enterocytes and Reepithelialisation,” Public Library of Science One, Vol. 3, No. 10, 2008, Article ID: e3428. doi:10.1371/journal.pone.0003428
NOTES
*Conflict of Interest Statement: The authors declare no conflict of interest. This study was partially supported by DS Pharma Biomedical Co., Ltd.
#KS and TK should be considered equal first authors.
†Corresponding author.

