Surgical Science
Vol.04 No.03(2013), Article ID 28600 ,8 pages
doi:10.4236/ss.2013.43038
Therapeutic Process of Gynecological Pelvic Abscess— Retrospective Review of 20 Cases
Department of Obstetrics and Gynecology, School of Medicine, Shimane University, Izumo, Japan
Email: *oride@med.shimane-u.ac.jp
Received January 17, 2013; revised February 18, 2013; accepted February 27, 2013
Keywords: Pelvic Abscess; Tubo-Ovarian Abscess; Endometrioma
ABSTRACT
Background: Conservative therapies of pelvic abscess are not highly effective and surgical treatment is usually required. This study reviewed cases of pelvic abscess treated at our hospital over a 3-year period to evaluate treatment efficacy. The medical records of 20 patients diagnosed with pelvic abscess and admitted to our hospital for treatment between November 2006 and December 2009 were retrospectively examined. Results: Mean age of the patients was 50 ± 16.6 years. Pelvic abscess occurred spontaneously in 13 patients and secondary to surgical manipulation in 7 patients. In the 13 patients with spontaneous abscess, 7 had undergone pelvic surgery and 2 had undergone insertion of an intrauterine contraceptive device. Concomitant endometriosis was present in 5 of the 13 (38.5%) patients. A positive bacterial culture from the abscess was obtained in 16 of 19 (84.2%) patients tested. Causative bacteria included 4 aerobic bacterial species detected in 7 patients and 11 anaerobic bacterial species detected in 10 patients. Although multiple antibiotics were administered in all cases, 19 of the 20 (95%) patients eventually required surgical intervention, which included total hysterectomy plus adnexectomy, drainage under laparotomy or drainage alone. Anaerobic bacteria were frequently detected as the causative bacteria. Conclusion: As treatment with antibiotics alone was ineffective in almost all cases, surgical treatment was required. Drainage might be the first-choice treatment for pelvic abscess to avoid invasive surgery.
1. Introduction
Pelvic inflammatory disease (PID) is caused by infection ascending from the vagina to the reproductive organs. It may be a progression of endometritis, adnexitis or pelvic peritonitis. In addition, it may occur as a complication of pelvic surgery. In PID, the spread and aggravation of inflammation can result in a pelvic abscess, such as a tuboovarian abscess (TOA). Pelvic abscess occurs as a result of the progression of pelvic infection or endomyometritis or as a complication of pelvic surgery. The clinical symptoms of pelvic abscess include fever and lower abdominal pain, and blood test findings of an increased white blood cell count (WBC) and increased C-reactive protein (CRP) level. It can be diagnosed with reasonable accuracy by a gynecological examination and imaging such as ultrasonography and magnetic resonance imaging.
With regard to treatment, conservative therapies, such as antibiotics, are not highly effective and surgical treatment is usually required. In our department, we have typically performed laparotomy such as adnexectomy and/ or total hysterectomy for the treatment of pelvic abscesses. In this study, we reviewed patient background, treatment and clinical outcome of the cases of pelvic abscess we have experienced thus far, with the aim of evaluating the efficacy of our current treatment approach and determining any improvements that can be made.
2. Patients and Methods
Twenty patients diagnosed with pelvic abscess who underwent medical treatment between November 2006 and December 2009 at the Department of Obstetrics and Gynecology, Shimane University Hospital were the subjects of this study. We retrospectively examined the medical records concerning patient background, laboratory data before and after medical care, therapeutic method and clinical outcome. This study was conducted in accordance with the Declaration of Helsinki.
3. Results
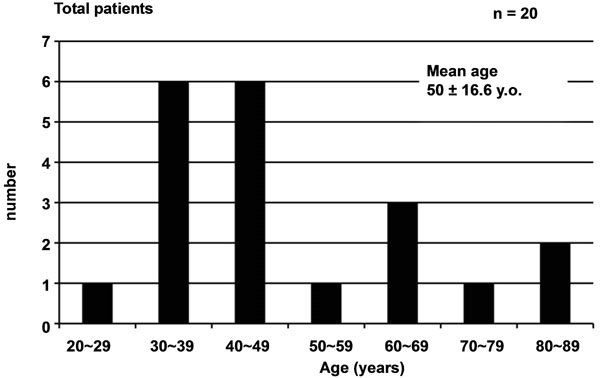
Among the 20 patients, pelvic abscess occurred spontaneously in 13 and secondary to surgical manipulation in 7. Mean age of the patients was 50 ± 16.6 years, with those in their 30s and 40s the most common. Mean age of the 13 patients with spontaneous pelvic abscess was 48.3 ± 15.9 years, with those in their 30s and 40s the most common, while that of the 7 patients with pelvic abscess secondary to surgical manipulation was 53.1 ± 18.7 years (Figure 1).
Background data for the 13 patients with spontaneous abscess are summarized in Table 1. Two patients had a previous history of diabetes and 1 patient was receiving steroid treatment for autoimmune disease. Seven patients
 (a)
(a)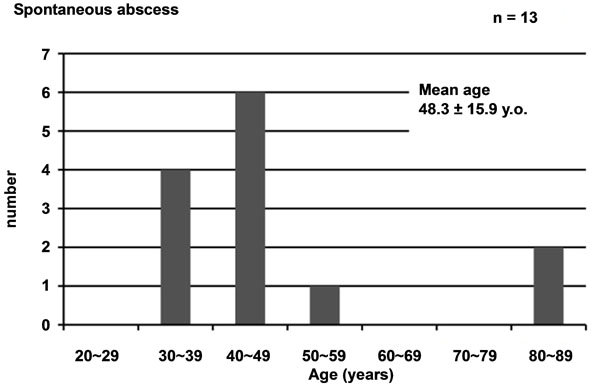 (b)
(b)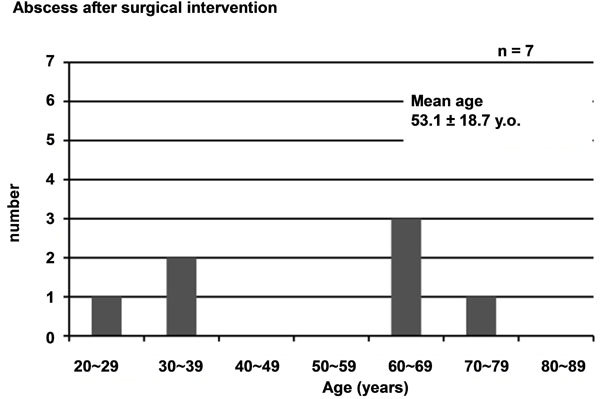 (c)
(c)
Figure 1. Age distribution of cases evaluated. (a) For all 20 cases of pelvic abscess; (b) For 13 cases of spontaneous abscess and (c) For 7 cases after surgical manipulation.
had undergone pelvic surgery and 2 had undergone insertion of an intrauterine contraceptive device (IUD). Concomitant endometriosis was present in 5 of the 13 (38.5%) patients. Table 2 summarizes the post-admission clinical course of the 13 patients. Many of the patients presented with chief complaints of fever and lower abdominal pain. Mean time from symptom onset to presentation was 11.2 ± 15.5 days. One patient presented 60 days after symptom onset. On admission, WBC and CRP values were generally high. All 13 patients were treated with antibiotics after admission, but WBC and CRP were improved only in 5 (45.5%) of 11 patients (excluding 2 who underwent immediate surgical treatment). Overall, 12 (92.3%) of the 13 patients with spontaneous pelvic abscess required surgical treatment, with a mean time from admission to surgery of 5.3 ± 5.9 days. One patient (case 9) who did not receive surgery achieved remission by antibiotic treatment alone. Table 3 summarizes the surgical treatment of these 13 patients with spontaneous pelvic abscess. Right adnexal abscess was most commonly observed, in 8 patients, on laparotomy. Left adnexal abscess was observed in 4 patients.
Douglas’ abscess was also found in 2 patients. Curative surgical procedures, such as adnexectomy and total hysterectomy, were performed in 11 of the 12 patients receiving surgical treatment. Adhesion was associated with increased operation time and bleeding volume, with a mean operation time of 221.5 ± 70.7 h and a mean bleeding volume of 832.3 ± 645.8 ml. In case 5, the patient had severe adhesion and experienced a large amount of intraoperative bleeding due to use of aspirin 100 mg/ day, and therefore only palliative drainage was performed. Recurrence was noted shortly after surgery. Aspirin was then discontinued and total hysterectomy and bilateral adnexectomy were performed. The time from surgery to hospital discharge ranged from 11 to 49 days, with a mean of 19.8 ± 10.9 days.
Among the 7 patients who developed pelvic abscess after receiving surgical manipulation, the procedures that led to abscess formation were surgery for gynecologic cancer in 5 patients, egg collection procedure in 1 patient and laparoscopic myomectomy in 1 patient (Table 4). Table 5 summarizes the clinical course of each patient. The duration from surgery to symptom onset, such as fever, ranged from 3 to 56 days. Cases 5 and 6 had undergone surgery for gynecologic cancer and subsequently received chemotherapy and pelvic abscess occurred at 56 and 37 days after surgery, respectively. Re-operations were performed to treat the abscess in both cases. The mean time from surgery to symptom onset was 27.4 ± 19.8 days. WBC and CRP levels were high in all 7 patients. Remission was achieved in all by abscess removal or drainage.
Among the total 20 patients, a positive bacterial culture
Table 1. Background data of patients with spontaneous pelvic abscess.

IUD: intrautrine device.
Table 2. Post-admission clinical course of patients with spontaneous pelvic abscess.

Table 3. Surgical treatment for spontaneous pelvic abscess.
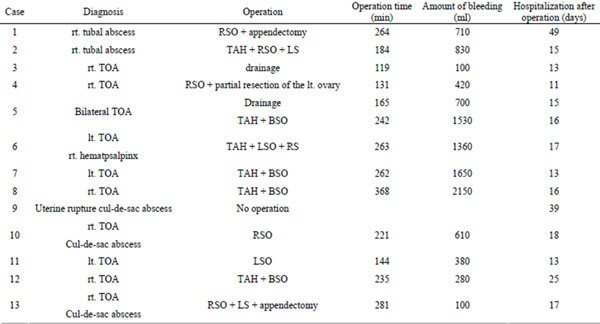
TOA: tubo-ovarian abscess; SO: salpingo-oophorectomy; S: salpingorectomy; TAH: total hysterectomy; BSO: bilateral salpingo-oohorectomy; RSO: right SO; LSO: left SO; RS: right S; LS: left S.
Table 4. Background data of patients with pelvic abscess after surgical manipulation.
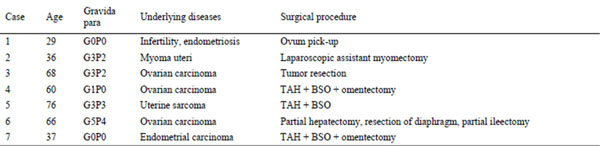
Table 5. Post-admission clinical course of patients with pelvic abscess after surgical manipulation.

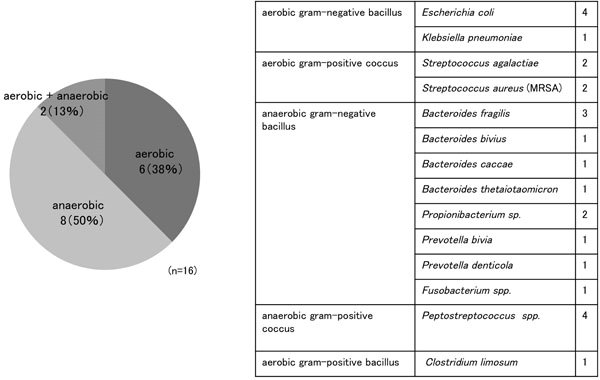
from the abscess was obtained in 16 of 19 (84.2%) patients. One patient who did not undergo surgery was excluded as no abscess sample was submitted for bacterial culture. Causative bacteria included 4 aerobic bacterial species detected in 7 patients, including Escherichia coli from 5 patients and Streptococci from 3 patients, and 11 anaerobic bacterial species detected in 10 patients, including Bacteroides spp. from 5 patients and Peptostreptococci from 4 patients (Figure 2).
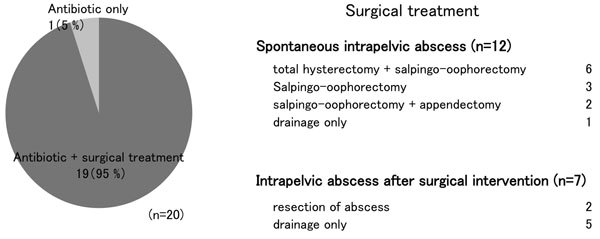
Although all of our patients were treated with multiple antibiotics, 19 of the 20 (95%) eventually required surgical intervention: total hysterectomy plus adnexectomy in 6 patients, adnexectomy alone in 5 patients, drainage under laparotomy in 7 patients and drainage alone in 2 patients. Although intraoperative blood loss tended to be increased in the laparotomy cases, no serious complications were observed. Case 9, a patient with spontaneous pelvic abscess, was the only patient that required no surgical treatment of the abscess (Figure 3). This patient presented with purulent discharge and disturbed consciousness. Imaging examination revealed fluid accumulation in the uterine cavity and Douglas’ pouch, indicative of uterine rupture. Rupture of the pyometra was therefore suspected. Surgical treatment was not selected due to the patient’s poor general condition and advanced age, but symptoms and laboratory findings were improved by administration of antibiotics, with almost complete resolution of the abscess shown on imaging. In case 5, a patient with spontaneous pelvic abscess, re-operation was required after drainage by laparotomy. In the remaining 19 cases, pelvic abscess was totally cured by the primary treatment.
4. Discussion
Combined inflammation of the uterus, uterine appendages and pelvic connective tissue is referred to as pelvic inflammatory disease (PID). A pelvic abscess forms as a result of progression of PID, and the most common type of pelvic abscess is tubo-ovarian abscess (TOA).
Pelvic abscess is reported to commonly affect those in their 30s and 40s [1]. In a study conducted by Lander et al., symptoms observed in patients with pelvic abscess included abdominal and pelvic pain in 98% of patients, fever onset and chill in 50%, abnormal discharge in 28%, vomiting in 26%, and irregular vaginal bleeding in 21%. In addition, 35% of the patients in their study did not report fever onset [2]. Similarly, in the present study, fever onset was not observed in 4 of the 13 (30.8%) patients with spontaneous pelvic abscess.
Causes of pelvic abscess are roughly divided into ascending infection extending from cervix to endometrium
 (a) (b)
(a) (b)
Figure 2. Identified bacterial species in the 20 patients. (a): Ratio of aerobic to anaerobic bacteria. (b): Breakdown of identified bacterial species.
 (a) (b)
(a) (b)
Figure 3. Summary of treatment. (a): Treatment contents. (b): Breakdown of surgical treatment.
and fallopian tube, infection following pelvic surgery, and extension of infection from adjacent infection foci, such as appendicitis and diverticulitis. In the present study, there were 13 cases of spontaneous pelvic abscess likely to have been caused by ascending infection and 7 cases of pelvic abscess that occurred following surgical manipulation of the pelvic cavity. Risk factors for pelvic abscess include previous history of PID, multiple sexual partners, use of IUDs, intrauterine manipulation such as endometrial cytology and artificial insemination, and being immunocompromised. Osser et al. suggest that the use of IUDs doubles the risk for PID [3]. Another study reported that 20% - 54% of IUD users were diagnosed with TOA[4]. In the present study, 2 of 12 (16.7%) patients were also using IUDs.
Common bacterial species responsible for pelvic abscess formation include anaerobic bacteria (e.g. Bacteroides spp.), Gram-negative bacilli (e.g. E. coli) and Gram-positive cocci. In addition, mixed infection with several bacterial species is commonly observed. Causative bacteria for TOA identified in the Landers’ study included E. coli (37%), Bacteroides fragilis (22%), Bacteroides spp. (26%), Peptostreptococci (18%) and Peptococci (11%) [2]. Anaerobic bacteria were also frequently detected in the present study, and mixed infection with aerobic and anaerobic bacteria was detected in 2 of 16 (13%) patients, with E. coli and Bacteroides spp. frequently isolated.
The first step in treatment is to identify the causative bacteria and then administer antibiotics to which the identified bacteria are sensitive. However, in clinical practice, broad-spectrum antibiotics are usually administered before bacteriological examination results are available. The rate of response to intravenous broad-spectrum antibiotics in patients with pelvic abscess is reported to be 34% - 87.5% [5]. However, conservative therapies are often ineffective for reasons such as insufficient penetration of antibiotics into the abscess; therefore, most cases eventually require surgical treatment. In a study conducted by Mirhashemi et al., 30% - 40% of patients with TOA required surgical treatment [4]. This was also true in the present study, with cure by antibiotics alone achieved in only 1 of 20 patients. This patient had Douglas’ abscess resulting from rupture of pyometra and responded to antibiotic treatment most likely because the abscess was not fully encapsulated. In a study conducted by Mirhashemi et al., 30% - 40% of patients with TOA required surgical treatment [4].
Surgical treatments used for pelvic abscess are divided roughly into drainage and curative procedures, such as adnexectomy and hysterectomy. On the basis of a previous report where about one-third of patients with pelvic abscess whose uterus was preserved subsequently required hysterectomy [6], in case 5 with spontaneous pelvic abscess, we performed laparotomy drainage at first. However, bilateral adnexectomy with total hysterectomy was subsequently required due to abscess recurrence. There is general agreement that adnexectomy is required for the treatment of TOA. Because spontaneous pelvic abscess was likely to be caused by ascending infection through the uterine cavity, for patients who did not wish to preserve fertility, we chose to perform total hysterictomy to prevent recurrence; for patients who did wish to preserve fertility, we preserved the uterus and non-affected uterine appendages. In the latter cases, whether curative or conservative surgery was performed, the abdominal cavity was sufficiently washed and a drain tube was placed in the abdominal cavity. Many cases of secondary pelvic abscess occurred following surgery, including hysterectomy, for gynecologic malignancy. In some of these cases, only abscess removal or pus drainage via CT-guided puncture was performed and no recurrence was subsequently observed. Because laparotomy is often associated with prolonged operation time and increased bleeding volume due to infection-induced adhesion, adequate measures such as preparation for blood transfusion should be made prior to surgery.
The benefit of transvaginal puncture and laparoscopic surgery for the surgical management of pelvic abscess has recently been reported. Gjelland et al. reported the efficiency of transvaginal ultrasound-guided aspiration of abscess, with 282 of 302 (93.4%) TOA patients cured by this surgical treatment [7] . In addition, Perez-Medina et al. compared antibiotics therapy alone with antibiotics plus ultrasound-guided transvaginal needle aspiration in a prospective randomized study and showed the benefits of concurrent transvaginal aspiration with antibiotics therapy on the curative rate and hospitalization time [8] .
As for laparoscopic surgery, Henry-Suchet et al. reported that among 80 cases of pelvic abscess, laparoscopic drainage were successful in 72 (90%) cases [9]. Yang et al. also reported that drainage under laparoscopy was associated with a shorter hospital stay, shorter period of postoperative fever and fewer operative complications compared with drainage under laparotomy [10]. In our series, 1 patient who developed pelvic abscess after surgical manipulation was successfully cured by CT-guided puncture. In addition, transvaginal drainage successfully cured the patient with TOA that occurred after ovum pick-up.
As described above, we have surgically treated most cases of pelvic abscess we have encountered by both adnexectomy and hysterectomy. However, considering the fact that patients must undergo surgery under conditions of inflammation, such surgical treatment might be highly invasive or readily cause bleeding. Given the findings reported in previous studies, we have re-considered our therapeutic strategy: if antibiotics administration does not improve the pelvic abscess, we should first try transvaginal drainage, and if this is not effective, we should then try enucleation of the affected adnexa by laparotomy. However, in cases of TOA, the differential diagnosis must include malignant tumor, as there is one report that 8 of 17 postmenopausal women with TOA were diagnosed postoperatively with malignant tumor, such as endometrial cancer, cervical cancer or ovarian cancer [11] . Surgeons must rule out malignant disease before surgically treating the pelvic abscess.
Ovarian endometrial cyst was found in 6 of the 20 (30%) patients evaluated in the present study. Grammatikakis et al. reported that 21 of 720 (2.9%) patients undergoing surgery for ovarian endometrial cyst were found to have PID [12] . Kubota et al. reported that among 6557 gynecologic inpatients, TOA was found in 5 of 218 (2.3%) patients with ovarian endometrial cyst but in only 11 of 6339 (0.2%) patients without ovarian endometrial cyst [13] . It is speculated that ascending infection or transvaginal puncture of an ovarian endometrial cyst may cause TOA with the cyst serving as a bacterial culture medium. Endometriosis is therefore considered a risk factor for pelvic abscess.
5. Conclusion
We retrospectively reviewed 20 cases of pelvic abscess treated at our hospital. Many of the patients with spontaneous abscess also had concomitant endometriosis. Anaerobic bacteria were frequently detected as the causative bacteria. As treatment with multiple antibiotics alone was ineffective in almost all cases, surgical treatment was required. Drainage might be the first-choice treatment for pelvic abscess because surgery under conditions of inflammation is invasive.
REFERENCES
- S. Granberg, K. Gjelland and E. Ekerhovd, “The Management of Pelvic Abscess,” Best Practice and Research Clinical Obstetrics and Gynaecology, Vol. 23, No. 5, 2009, pp. 667-678. doi:10.1016/j.bpobgyn.2009.01.010
- D. V. Landers and R. L. Sweet, “Tubo-Ovarian Abscess: Contemporary Approach to Management,” Reviews of Infectious Disesase, Vol. 5, No. 5, 1983, pp. 876-884. doi:10.1093/clinids/5.5.876
- S. Osser, B. Gullberg, P. Liedholm and N. O. Sjoberg, “Risk of Pelvic Inflammatory Disease among IntrauterineDevice Users Irrespective of Previous Pregnancy,” Lancet, Vol. 1, No. 8165, 1980, pp. 386-388. doi:S0140-6736(80)90942-3 [pii]
- R. Mirhashemi, W. M. Schoell, R. Estape, R. Angioli and H. E. Averette, “Trends in the Management of Pelvic Abscesses,” Journal of the American College Surgeons, Vol. 188, No. 5, 1999, pp. 567-572.
- S. G. McNeeley, S. L. Hendrix, M. M. Mazzoni, D. C. Kmak and S. B. Ransom, “Medically Sound, Cost-Effective Treatment for Pelvic Inflammatory Disease and Tuboovarian Abscess,” American Journal and Obstetrics and Gynecology, Vol. 178, No. 6, 1998, pp. 1272-1278.
- P. R. Rubenstein, D. R. Mishell Jr. and W. J. Ledger, “Colpotomy Drainage of Pelvic Abscess,” Obstetrics and Gynecology, Vol. 48, No. 2, 1976, pp. 142-145.
- K. Gjelland, E. Ekerhovd and S. Granberg, “Transvaginal Ultrasound-Guided Aspiration for Treatment of TuboOvarian Abscess: A Study of 302 Cases,” American Journal of Obstetrics and Gynecology, Vol. 193, No. 4, 2005, pp. 1323-1330. doi:10.1016/j.ajog.2005.06.019
- T. Perez-Medina, M. A. Huertas and J. M. Bajo, “Early Ultrasound-Guided Transvaginal Drainage of Tubo-Ovarian Abscesses: A Randomized Study,” Ultrasound in Obstetrics and Gynecology, Vol. 7, No. 6, 1996, pp. 435- 438. doi:10.1046/j.1469-0705.1996.07060435.x
- J. Henry-Suchet, “Laparoscopic Treatment of Tubo-Ovarian Abscess: Thirty Years’ Experience,” Journal of the American Association of Gynecologic Laparoscopists, Vol. 9, No. 3, 2002, pp. 235-237. doi:10.1016/S1074-3804(05)60395-7
- C. C. Yang, P. Chen, J. Y. Tseng and P. H. Wang, “Advantages of Open Laparoscopic Surgery over Exploratory Laparotomy in Patients with Tubo-Ovarian Abscess,” Journal of the American Association of Gynecologic Laparoscopists, Vol. 9, No. 3, 2002, pp. 327-332. doi:10.1016/S1074-3804(05)60412-4
- A. G. Protopapas, E. S. Diakomanolis, S. D. Milingos, A. J. Rodolakis, S. N. Markaki, G. D. Vlachos, D. E. Papadopoulos and S. P. Michalas, “Tubo-Ovarian Abscesses in Postmenopausal Women: Gynecological Malignancy until Proven Otherwise?” European Journal of Obstetrics, Gynecology, and Reproductive Biology, Vol. 114, No. 2, 2004, pp. 203-209. doi:10.1016/j.ejogrb.2003.10.032
- I. Grammatikakis, N. Evangelinakis, G. Salamalekis, V. Tziortzioti, C. Samaras, C. Chrelias and D. Kassanos, “Prevalence of Severe Pelvic Inflammatory Disease and Endometriotic Ovarian Cysts: A 7-Year Retrospective Study,” Clinical and Experimental Obstetrics and Gynecology, Vol. 36, No. 4, 2009, pp. 235-236.
- T. Kubota, K. Ishi and H. Takeuchi, “A Study of TuboOvarian and Ovarian Abscesses, with a Focus on Cases with Endometrioma,” Journal of Obstetrics and Gynaecology Researcl, Vol. 23, No. 5, 1997, pp. 421-426.
NOTES
*Corresponding author.

