Advances in Microbiology
Vol.4 No.6(2014), Article ID:45816,7 pages DOI:10.4236/aim.2014.46038
Detection of β-Glucuronidase Activity within Actinomadura madurae Grains of Human Actinomycetoma
Alejandro Palma-Ramos1, Samantha Reyes-Mayén1, Laura Estela Castrillón-Rivera1, Silvia Elena Fernández-López1, Carmen Padilla-Desgarennes2, María Elisa Vega-Memije3, Roberto Arenas-Guzmán4, María Elisa Drago-Serrano1, Teresita Sainz-Espuñes1*
1Department of Biological Systems, Universidad Autónoma Metropolitana-Xochimilco, Mexico City, México
2Dermatology Center “Dr. Ladislao de la Pascua”, Mycology Service, Mexico City Health Secretary, Mexico City, México
3General Hospital “Manuel Gea González”, Dermatology Service, Mexico City Health Secretary, Mexico City, México
4General Hospital “Manuel Gea González”, Mycology Service, Mexico City Health Secretary, Mexico City, México
Email: *trsainz@correo.xoc.uam.mx
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 24 September 2013; revised 30 October 2013; accepted 30 November 2013
ABSTRACT
Actinomycetoma syndrome by Actinomadura (A.) madurae is characterized by a subcutaneous chronic lesion that affects fascia, muscle and bone. A. madurae produces colonies that form grains of less than 1 mm in diameter. Grains are surrounded and infiltrated by neutrophils involved in the grain disruption by enzymes like β-glucuronidase released after the neutrophil degranulation. The aim of this work was to evaluate the polysaccharide degradation of grains treated with β-glucuronidase and to detect the presence and activity of β-glucuronidase within the A. madurae grains. Actinomadura madura grains from patients infected were processed to quantify the total content of polysaccharide with the phenol-sulfuric acid reaction. Grains were treated with β-glucuronidase at different conditions to evaluate the optimal polysaccharide degradation. Grains were analyzed to detect the enzyme by using anti-human β-glucuronidase antibody while enzymatic activity was assessed by evaluating the release of reduced sugars and by in situ enzymatic activity. Optimal degradation of polysaccharide in the grains treated with β-glucuronidase was found with 300 units/ml of enzyme and 24 hr of incubation at 37˚C. Presence and activity of β-glucuronidase enzyme within the grains were detected. Results suggested that β-glucuronidase present within A. madurae grain resulted from degranulated neutrophils surrounding and/or infiltrated within the grain.
Keywords:Actinomycetoma, A. madurae, β-Glucuronidase

1. Introduction
Actinomycetoma is a subcutaneous chronic infection from bacterial origin caused by Actinomadura (A.) madurae present among the population of some areas of developing countries like Mexico. The total number of actinomycetoma cases recorded at Mycology laboratory of Ladislao De La Pascua dermatological Center of Mexico City in 42 years (1956-1998) was 732. Seventy one (9.6%) corresponded to A. madurae affecting mainly females as the predominant gender. In México the distribution of actinomycetoma cases is located in three main areas: the Central Western, Southern Center and West of Hidalgo state [1] .
Actinomycetoma is a syndrome characterized by disfiguring, painless inflammatory lesions and fistulas that affect the cutaneous and subcutaneous tissue, fascia and bone. Actinomycetoma is a suppurative infection which usually affects the lower extremities, but it can occur in almost any region of the body. Actinomycetoma evolution is very slow and therefore the inability condition is lately achieved (months or years) [2] [3] .
Actinomadura madurae produces white and soft grains with a diameter size of 1 - 10 mm and by direct examination it can be observed that the grains are surrounded by swaths of long fringes, consisting of filaments, often bifurcated and uneven edges. Histopathological observation of tissue samples stained with hematoxylin-eosine shows multilobular grains with irregular or uneven edges and with a blue peripheral band intensely stained, while the center is often devoid of color [1] -[3] .
Hematoxylin-eosin staining of A. madurae actinomycetoma has evidenced neutrophil infiltration, as well as disruption of the surface of A. madurae grain [4] . This may reflect the presence of enzymes released by the neutrophil degranulation.
Neutrophils are endowed with azurophil (primary) granules containing polysaccharide degrading enzymes like β-glucuronidase released in surrounding environment after degranulation [5] .
Polysaccharides are commonly components of a matrix where the bacteria are embedded to generate biofilms which functions as a shield of protection that decreases the antimicrobial efficiency of antibiotics [6] [7] .
Since β-glucuronidase enzyme released by neutrophils may degrade polysaccharide components of biofilm within A. madurae grain the aim of this work was 1) to evaluate polysaccharide degradation of A. mandurae grain in presence of β-glucuronidase enzyme, 2) to detect the presence and 3) the enzymatic activity of β-glucuronidase released by neutrophils within the grain. This approach may impact on the developing of an auxiliary treatment toward A. madurae actinomicetoma due to the enzymatic degradation of polysaccharide biofilm by β- glucuronidase that eventually would allow the entry of antibiotics to eliminate the A. madurae inside the grain.
2. Materials and Methods
2.1. Actinomycetoma Grain Sampling
Actinomycetoma grains from two different patients with positive diagnostic for actinomycetoma by Actinomadura madurae were obtained either at the Mycology laboratory of “Ladislao De La Pascua” Dermatological Centre or at Mycology laboratory of “Manuel Gea González” General Hospital. Patients decided voluntarily participate therefore they signed a written inform consent form approved by the ethic commission for biomedical research in human according to the guidelines from the Secretary of Health, prior to the onset of the study.
2.2. Polysaccharides Quantification in Actinomadura madurae Grains
Prior to treatment with β-glucuronidase, total polysaccharide content in the actinomycetoma grains was quantified using the method of phenol-sulphuric acid reaction [8] . Two samples with 4 beads each, were dried, weighed and grounded. Afterwards, samples were suspended in 1 ml of distilled water and then 3 ml of sulfuric acid (H2SO4, 95.5% w/v, specific gravity 1.84) and 50 µl of phenol (80% w/v) were added. The absorbance value of the colorful compounds produced were measured spectrophotometrically at 485 nm to quantify the total content of polysaccharide based on a standard curve by plotting the concentration of glucose (2 to 20 µg/ml) vs. the corresponding absorbance values.
2.3. “In Vitro” Determination of the Polysaccharide Degradation of Actinomadura madurae Grain with the β-Glucuronidase Enzyme
In order to determine the optimal time of incubation, 3 grains were used for this purpose. Each whole grain was suspended in 1 ml of sodium acetate buffer (100 mM, pH 3.8), inside a well from a 24 well culture plate and 100 μl of commercial β-glucuronidase (EC 3.2.1.31 Sigma-Aldrich G025) solution at a concentration of 900 units/ml was added. Each whole sample grain was then incubated for 24, 48 or 72 hours in a humid chamber at 37˚C and 150 rpm agitation.
To test the optimal concentration of the enzymatic activity, 3 sample grains were treated each with 300, 600 or 900 units/ml of β-glucuronidase for 24 h under the same conditions of incubation in a humid chamber at 37˚C and 150 rpm agitation [9] [10] .
After incubation, samples were centrifuged and the supernatants were collected to estimate the content of the total degraded polysaccharides according to the method of Dubois described earlier [8] .
An additional assay was done to visualize the enzymatic degradation of the grain by using a concentration of 300 units/ml β-glucuronidase and incubation time of 24 h. One untreated grain was used as control.
2.4. In Vitro Determination of β-Glucuronidase Activity within Grains
The activity of the β-glucuronidase secreted by PMN infiltrated within the grain was analyzed. The in vitro enzyme activity of β-glucuronidase on A. madurae grains, was performed by using phenolphthalein-glucuronic acid as the substrate, so when the reaction takes place the enzyme frees phenolphthalein, which is then quantified by spectrophotometry at 540 nm [11] .
To do so, the grain was finely sectioned with a scalpel and the obtained fragments were suspended in 700 μl of 100 mM sodium acetate buffer, pH 3.8 and pre-warmed at 37˚C. After that, 700 μl of 3 mM phenolphthalein-glucuronic acid substrate was added and the mixture was incubated 30 min with agitation at 150 rpm. The reaction was stopped by addition of 5 ml of 200 mM glycine buffer pH 10.4, and the absorbance at 540 nm was determined.
In order to quantify the amount of phenolphthalein released in the above reaction, a phenolphthalein standard curve was assayed using the following concentrations: 12.3, 30.7, 61.4, 92.1, 153.6 and 307.2 µg/ml and the absorbance at 540 nm were determined.
2.5. In Situ Detection of β-Glucuronidase in A. madurae Grains
Presence of β-glucuronidase was detected by using an anti-human β-glucuronidase antibody the antigen-antibody interaction was reveled with a commercial kit (Cell and Tissue Staining Kit, Goat Kit, HRP-AEC System by R&D (Minneapolis, Mn, USA Cat No. CTS009).
2.6. In Situ β-Glucuronidase Activity Detection in A. madurae Grains
In addition, histological sections where used in order to detect the activity in situ of β-glucuronidase within the grains according to instructions of the commercial kit of lymphocyte enzymes (Sigma Aldrich 181C-1KT, Lot.060M4351, Germany). The kit used naphtol AS-BI, glucuronic β-D-acid as the substrate and pararosaniline as an indicator. When the substrate takes contact with the enzyme (β-glucuronidase) present in the histological cuts forms a red chromogenic complex with the naphtol-pararosaniline and it may be seen under the microscope. Methylene blue was used as contrast dye. The test was done in 4 histological sections [12] .
3. Results
3.1. “In Vitro” Determination of the Enzyme Required for the Polysaccharide Degradation
Enzymatic activity of β-glucuronidase was tested at different times of incubation in order to assess the optimal time required for the enzymatic degradation of polysaccharide on the grain. Table 1 showed that 24 h was optimal time for the polysaccharide degradation by the β-glucuronidase enzyme using 900 units/ml.
Once the optimal incubation time was determined, the optimal concentration of the enzyme was assessed. Table 2 showed that 300 units/ml rendered the maximum sugar release.
3.2. Enzymatic Activity of Commercial β-Glucuronidase Added to the Grains
This analysis using 300 units /ml of the enzyme incubated during 24 h, was made to visualize the effect of β-glucuronidase in the actimomycetome treated grains. By comparison with the untreated grain (Figure 1(a)) a substantial degradation of the exopolysaccharide in the grain surface was observed (Figure 1(b)). Enzymatic degradation was observed also in supernatants obtained from the grains (Figure 1(c)). These results showed the ability of β-glucuronidase for the exopolysaccharide degradation.
3.3. Quantitative Evaluation of the Effect of β-Glucuronidase Released by Polymorphonuclear Neutrophils (PMN) Infiltrated into Actinomadura madurae Grain
Results in Table 3 showed the standard curve of phenophthalein for the quantification of the activity of the enzyme within the grain using a commercial enzyme. It also showed the value of the activity of the enzyme within the grain sample. The sample presented an absorbance of 0.030 which correspond to the 51.34 μg/mL of phenolphthalein released. According to the reaction between the enzyme and the substrate the calculated activity was 30.78 units/mL of β-glucuronidase released by PMN infiltrated in the grain sample.
3.4. In Situ Detection of β-Glucuronidase Enzyme within A. madurae Grain
As shown in Figure 2, the presence of the enzyme was detected by the appearance of dots of red color within the A. madurae grain using anti-human β-glucuronidase antibody.
Table 1. Enzymatic degradation of polysaccharide present in the grains of Actinomadura madurae at different incubation times with 900 units/ml of β-glucuronidase.
Table 2. Enzymatic degradation of the polysaccharide present in the grain of Actinomadura madurae at different concentrations of β-glucuronidase.
Table 3. Standard curve of phenolphthalein using a commercial enzyme for the analysis of the enzymatic activity of the β- glucuronidase from PMN infiltrated into Actinomadura madurae grain using 3 mM phenolphthalein-glucuronic acid substrate.
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. (a) Untreated Actinomadura madurae grain; (b) Actinomadura madurae grain treated with β-glucuronidase; (c) Supernatant with residues from the treatment of grain with β-glucuronidase.
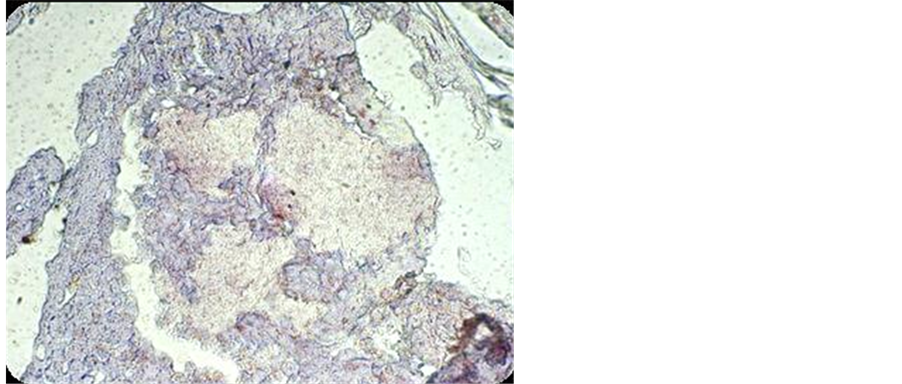
Figure 2. In situ detection of β-glucuronidase enzyme within A. madurae grain visualized by the presence of red-brown dots.
3.5. Histological Analysis
A qualitative analysis to detect the activity of the β-glucuronidase was made on histological sections of actinomycetomas from patients affected with A. madurae using a lymphocyte enzyme commercial kit. Presence of the enzyme activity was detected by a dim red coloration. Decreased red coloration suggests a limited enzymatic activity on the polysaccharide by β-glucuronidase released by PMN infiltrated into the actinomycetoma grain (Figure 3).
4. Discussion
Under conditions of 900 units/ml of β-glucuronidase and 24 hr of incubation at 37˚C was revealed that 30.32% of polysaccharide was substrate for enzymatic degradation (Table 1).
According to the results, less degradation of polysaccharide grain was found with 600 and 900 units/ml of β-glucuroninase suggesting an effect of saturation of the enzyme (Table 2). Twenty eight percent of total polysaccharide of A. madurae grains was degraded in the presence of β-glucuronidase under optimal conditions of 24 hrs and 300 unit/ml of enzyme (Table 2). This may indicate that some substrates degraded by β-glucoronidase may include acidic sugars like β-glucuronosides although the bulk of polysaccharides were not degraded by this enzyme. Other acidic substrates for β-glucuronidase activity may include exopolysaccharides as in the case of sulfated acid mucopolysaccharides detected in the A. madurae grain [13] [14] . It has been proposed that sulfated acid mucopolysaccharide is a sticky material of the biofilm matrix which acts as a glue for joining the bacteria inside the grain [14] .
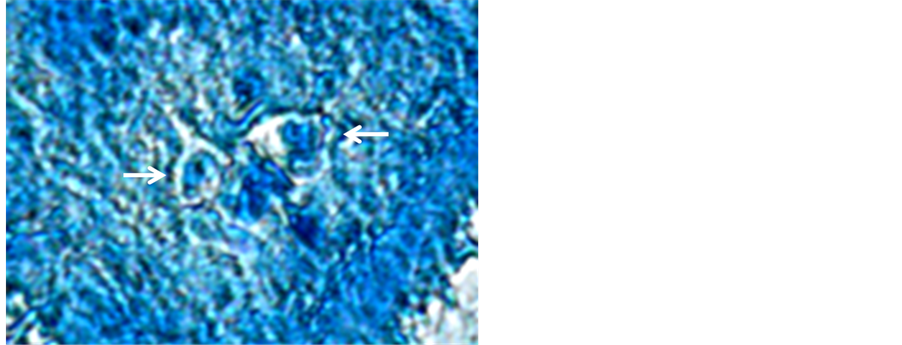
Figure 3. Positive activity of β-glucuronidase in histological sections, shown by the red coloration on the center of the grain of Actinomadura madurae.
In the current contribution we could detect the presence and the activity of β-glucuronidase inside A. madurae grain which probably contributed for 1) the disruption of the grain surface and for 2) the neutrophil infiltration inside the grain as evidenced previously by hematoxilin-eosin staining of A. madurae actinomycetoma from infected patients [4] .
Underlying mechanism of infiltration of neutrophils in the skin lesion involve a complex web of ligand-receptor interactions of addressins expressed in neutrophil surface with their corresponding receptors in endothelial blood vessels and neutrophil receptors for chemotatic factors released by skin cells from the focus of lesion [15] . In the case of A. madurae mechanisms which account neutrophil trafficking inside grains are not fully known but may involve the release of tumor necrosis factor (TNF)-α and interleukin (IL)-1β by keratinocytes as evidenced in biopsies of patients [4] . Proinflammatory cytokines like TNF-α and IL-1β have been ascribed to neutrophil activation which contributes for degranulation and infiltration of neutrophils inside A. madurae grain [4] .
In this study direct evaluation of enzymatic degradation of purified β-glucuronosides and/or sulfated acid polysaccharides by β-glucuronidase activity was not made, neither the characterization of other enzymes like heparanase from specific granules released by neutrophil. Evaluation of both events may provide a wide picture of the impact of neutrophil infiltration and degranulation within A. madura grain. Upon activation, neutrophils release granule proteins and chromatin that together form extracellular fibers known as extracellular traps that bind to the pathogen surface [16] . Neutrophil extracellular traps have a key role in the innate immunity for killing of pathogens including bacteria, yeasts and fungi [16] . In spite of these limitations the evidences presented in the current contribution suggest that under in vivo conditions β-glucuronidase may contribute, along with other enzymes released in situ after the neutrophil degranulation, in the disruption of A. madurae lesion.
Host factors related with chronic infection of actinomycetoma have been ascribed to a depressed cellular immune response related with a decrease number of epidermal langerhans cells [17] . This chronic infection is aggravated because the presence of polysaccharides forming the biofilm matrix which shield and protect to the bacteria toward the action of antibiotics [18] . Along with other components present in primary and secondary neutrophil granules as lysozyme, lactoferrin and mieloperoxidase, β-glucuronidase may be used as an auxiliary for the antibiotic treatment.
References
- Lavalle, A.P., Padilla, D.M., Pérez, G.J., Rivera, I. and Reynoso, R.S. (2000) Micetomas por Actinomadura madurae en México. Revista del Centro Dermatologico Pascua, 9, 19-24.
- Welsh, O., Vera-Cabrera, L., Welsh, E. and Salinas, M.C. (2012) Actinomycetoma and Advances in Its Treatment. Clinics in Dermatology, 30, 372-381. http://dx.doi.org/10.1016/j.clindermatol.2011.06.027
- Venkatswami, S., Sankarasubramanian, A. and Subramanyam, S. (2012) The Madura Foot: Looking Deep. The International Journal of Lower Extremity Wounds, 11, 31-42. http://dx.doi.org/10.1177/1534734612438549
- Palma, R.A., Castrillón, L.R., Encinas, M.G., Padilla, C.D. and Guzmán, R.A. (2009) Participación De Los Queratinocitos En La Respuesta Inmunitaria Contra El Actinomicetoma. Dermatología Revista Mexicana, 53, 225-233.
- Mollinedo, F. (2003) Human Neutrophil Granules and Exocytosis Molecular Control. Immunología, 22, 340-358.
- Mah, T.F. (2012) Biofilm-Specific Antibiotic Resistance. Future Microbiology, 7, 1061-1072. http://dx.doi.org/10.2217/fmb.12.76
- Petrova, O.E. and Sauer, K. (2012) Sticky Situations: Key Components That Control Bacterial Surface Attachment. Journal of Bacteriology, 194, 2413-2425. http://dx.doi.org/10.1128/JB.00003-12
- Dubois, M., Gilles, K., Hamilton, J., Rebers, P. and Smith, F. (1956) Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry, 28, 350-356. http://dx.doi.org/10.1021/ac60111a017
- Goldstein, G. (1961) Serum Β-Glucuronidase Assay by the Phenolphthalein Mono-Β-Glucuronide Method. Clinical Chemistry, 7, 136-142.
- Fishman, W.H., Springer, B. and Brunettei, R. (1948) Application of an Improved Glucuronidase Assay Method to the Study of Human Blood Beta-Glucuronidase. Journal of Biological Chemistry, 173, 449-456.
- Talalay, P., Fishman, W.H. and Huggins, C. (1946) Chromogenic Substrates; Phenolphthalein Glucuronic Acid as Substrate for the Assay of Glucuronidase Activity. Journal of Biological Chemistry, 166, 757-772.
- Lorbacher, P., Yam, L. and Mitus, W. (1967) Cytochemical Demonstration of Β-Glucuronidase Activity in Blood and Bone Marrow Cells. Journal of Histochemistry & Cytochemistry, 15, 680-687.
- Palma, R.A., Castrillón, L.R., Padilla, D.M. and Reyes, F.F. (2005) Caracterización Histoquímica De Micetomas Por Actinomadura Madurae, Nocardia Brasiliensis Y Madurella Mycetomatis. Dermatología Revista Mexicana, 49, 51-58.
- Palma, R.A., Castrillón, R.L., Padilla, D.M., Rosas, H.L. and Márquez, C. (2006) Purificación Y Determinación De La Estructura De Los Polisacáridos Que Forman El Cemento De Unión En Granos De Actinomicetomas Ocasionados Por Actinomadura Madurae Y Nocardia Brasiliensis. Dermatología Revista Mexicana, 50, 165-173.
- Luster, A.D., Alon, R. and Von Andrian, U.H. (2005) Immune Cell Migration in Inflammation: Present and Future Therapeutic Targets. Nature Immunology, 6, 1182-1190. http://dx.doi.org/10.1038/ni1275
- Brinkmann, V. and Zychlinsky, A. (2012) Neutrophil Extracellular Traps: Is Immunity the Second Function of Chromatin? The Journal of Cell Biology, 198, 773-783. http://dx.doi.org/10.1083/jcb.201203170
- Guimarães, C.C., Castro, L.G. and Sotto, M.N. (2003) Lymphocyte Subsets, Macrophages and Langerhans Cells in Actinomycetoma and Eumycetoma Tissue Reaction. Acta Tropica, 87, 377-384. http://dx.doi.org/10.1016/S0001-706X(03)00139-6
NOTES

*Corresponding author.


