Open Journal of Medical Microbiology
Vol.2 No.3(2012), Article ID:22818,8 pages DOI:10.4236/ojmm.2012.23010
Influence of Ethanol on Probiotic and Culture Bacteria Lactobacillus bulgaricus and Streptococcus thermophilus within a Therapeutic Product
1School of Animal Sciences, Louisiana State University Agricultural Center, Baton Rouge, USA
2Department of Food Science, Louisiana State University Agricultural Center, Baton Rouge, USA
Email: karyana@agcenter.lsu.edu
Received May 28, 2012; revised June 30, 2012; accepted July 10, 2012
Keywords: Therapeutic Yogurt; Streptococcus thermophilus; Lactobacillus bulgaricus; Titratable Acidity; Viscosity
ABSTRACT
Probiotic bacteria in plain yogurt namely of Lactobacillus ssp. have been reported to treat thrush, diarrhea, athlete’s foot, jock itch and vaginal yeast infections. Lactobacillus delbrueckii ssp. bulgarius (LB-12) and Streptococcus thermophilus (ST-M5) are lactic acid bacteria widely used in the manufacture of yogurt. Alcohol is used in manufacture of some medications such as cough syrups and some products such as eggnog and rum-raisin ice cream. The objectives were to study the effect of food grade ethanol on the growth of yogurt culture bacteria and the physico-chemical characteristics of therapeutic yogurt. The treatments were 0% (control), 2.5%, 5%, and 7.5% v/v ethanol in plain yogurt. The ethanol was incorporated by stirring it into one day old plain yogurt. Product characteristics were studied weekly for a month of refrigerated (4˚C) storage. Data were analyzed using Proc Mixed model of Statistical Analysis System. The ethanol amount × storage period interaction effect was significant for Lactobacillus bulgaricus counts while the ethanol amount × storage period effect was not significant for Streptococcus thermophilus counts, viscosity, pH and titratable acidity (TA). Therapeutic yogurts with ethanol, at these concentrations, can successfully be manufactured without adversely influencing counts of its probiotic bacteria over product shelf life.
1. Introduction
Plain yogurt has been referred to as a therapeutic product and has been used to treat fungal infections [1]. Probiotic bacteria in plain yogurt namely of Lactobacillus ssp. have been reported to treat thrush, diarrhea, athlete’s foot, jock itch and vaginal yeast infections [1]. Consumers are becoming more demanding when considering a food product purchase. One of the factors that influence their buying behavior is the nutritional value of the product. The increasing costs of health care, the steady increase in life expectancy, and the desire of the elderly for improved quality in their lives, are driving forces for extensive research into the area of therapeutic foods [2]. Shah [3] affirm that the most extensively used organisms, for human gut health, in probiotic preparations are lactic acid bacteria (LAB), particularly the species of Lactobacillus ssp., Bifidobacterium ssp. and Streptococcus ssp. Yeasts and filamentous fungi are the organisms which, besides LAB, also are currently used during the probiotic preparation. Guarner et al. [4] reported that probiotics are live microorganisms that when administered in adequate amounts confer a health benefit on the human host. Most probiotics are normally consumed in the form of yogurt, kefir, koumiss, cheese and other fermented dairy and food products. Parvez et al. [5] most probiotics fall into the group of organisms known as lactic acid-producing bacteria. Some of the beneficial effects of lactic acid bacteria consumption include: 1) improving intestinal tract health; 2) enhancing the immune system, synthesizing and enhancing the bioavailability of nutrients; 3) reducing symptoms of lactose intolerance, decreasing the prevalence of allergy in susceptible individuals; and 4) reducing risk of certain cancers [5].
Consumption of yogurt has been shown to induce measurable health benefits linked to the presence of live bacteria as previously mentioned. Many studies have clearly demonstrated that yogurt containing viable bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus) improves lactose digestion and eliminates symptoms of lactose intolerance. These beneficial effects are also due to microbial beta-galactosidase in the fermented milk product [6]. In addition, yogurt has an adequate amount of nutrients to improve the health of the consumer. McKinley [7] stated that in terms of its nutritional profile, yogurt has a similar composition to the milk from which it is made but will vary somewhat if fruit, cereal or other components are added. Its nutritional similarity with milk means that yogurt is an excellent source of protein, calcium, phosphorus, riboflavin (vitamin B2), thiamin (vitamin B1) and vitamin B12, and a valuable source of folate, niacin, magnesium and zinc [7]. The protein it provides is of high biological value (i.e. it contains all the amino acids essential to heath), and the vitamins and minerals found in milk and dairy foods are bioavailable (i.e. available for absorption and use by the body) [7]. To achieve those benefits it is recommendable to consume 113 - 170 grams of yogurt per day.
Alcohol is consumed on a daily basis by a large fraction of the population, and in many countries. Light-tomoderate alcohol consumption is considered as an integral part of some diets, such as Italian diet. In moderate dosage (about 10 g of alcohol per day), alcohol may have some advantages through psychobiological mechanisms (e.g. it suppresses fat oxidation and reduces pain and anxiety [8]. Mattes [9] reported that while the adverse consequences of alcohol abuse are well established, recent evidence that moderate consumption may provide protection against heart disease has prompted increased interest in the positive roles of ethanol in the diet. Alcohol is commonly used in the preparation of cough syrups and some other medications. Ethanol has an effective olfactory and trigeminal stimulus and is used as an ingredient in several dairy products such as eggnog and rum-raisin ice cream. International Dairy Foods Association (IDFA) [10] the sales of eggnog have increased drastically in the last 50 years (1960-2010): from 46 Million lbs. of product sold up to 127 million lbs. Ethanol is a volatile compound which enhances flavor in foods. Ethanol was found as an important flavor component in aged Parmesan cheese [11]. The most extensively studied properties of ethanol that pertain to its level of ingestion include its psychoactive effects and energy value. Suter and Schutz [8] state that the energy derived from alcohol is a usable source for ATP production. This alcohol is also an important product of fermentation that contributes with other organoleptic attributes of a product, and functions as a preservative over time. Notermans et al. [12] reported that after 3 weeks of incubation at 22˚C the numbers of Salmonella spp., S. aureus and L. monocytogenes decreased more than 3 log10 units in an egg-noglike product containing 7.0% v/v ethanol. An egg-noglike product was made adding different concentrations of ethanol 0%, 3%, 5%, 7% and 10% v/v. The 3% and 5% of ethanol were highly effective in preventing growth of the Salmonella spp. and S. aureus, respectively [12]. Aroma compounds, such as 2-Phenylethanol, represents an important tool in the production of natural flavors for food and beverage industries [13].
Yogurt is an important product to work with because of its high nutritional quality, consumer preference and health benefits of its culture bacteria. Yogurt attributes such as viscosity, pH, flavor and presence of live active cultures are important characteristics that influence the purchase decision. Zajsek and Gorsek [14] stated that the acidic attributes, pH and titratable acidity, are important factors that can strongly affect the quality of a fermented product, such as yogurt. Padan et al. [15] stated that the ability of lactic acid bacteria to regulate their cytoplasmic or intracellular pH is one of the most important physiological requirements of the cells. Growth of Streptococcus thermophilus in milk occurs until the pH drops to approximately 4.5 [15].
The objectives of this study were to determine the influence of ethanol on yogurt culture bacteria: Lactobacillus delbrueckii ssp. bulgarius (LB-12) and Streptococcus thermophilus (ST-M5), and to elucidate the physicochemical characteristics namely pH, titratable acidity and viscosity of this therapeutic product over its shelf life.
2. Materials and Methods
2.1. Therapeutic Product/Yogurt Manufacture
Plain yogurt was manufactured according to standard procedure at the Louisiana State University Dairy Processing Plant. The milk was preheat at 60˚C then homogenized at 13.8 MPa at the first stage and 3.45 MPa at the second stage in the homogenizer and later pasteurized at 85˚C for 30 mins. Milk was warmed to 40˚C using a water bath and inoculated. Freshly thawed frozen yogurt starter culture concentrate of Streptococcus thermophilus and Lactobacillus bulgaricus (CH-3, yogurt culture, Chr. Hansen’s Laboratory, Milwaukee, WI, USA) was added at 0.75 ml per 3.78 L of milk. Inoculated milk was incubated until pH reached 4.65 ± 0.1, and then transferred to the cooler at 4˚C. Next morning ethanol was added at 0%, 2.5%, 5%and 7.5% v/v to the yogurts. After mixing the milk and the ethanol, the plain yogurt was poured into 228 g plastic labeled cups and refrigerated at 4˚C until further analysis. Yogurt manufacture was replicated 3 times.
2.2. Microbial Counts
2.2.1. Lactobacillus bulgaricus Counts
Counts of L. bulgaricus were enumerated using the pour plate technique with serial dilutions of yogurt samples. Difco Lactobacilli MRS agar was prepared according to manufacturer’s directions. The 70 g of powder was weighed and suspended into 1 L of distilled water. A few drops of 1 N HCL were added to reduce pH to 5.2 ± 0.1 then autoclaved at 121˚C for 15 mins. Yogurts were sampled at weeks 1, 2, 3 and 4 of storage period. The yogurt in the cup was briefly agitated and 1 g of yogurt was pipetted from the center of the yogurt cup into a sterile bottle containing 99 ml of sterile 0.1% peptone water (Difco, Detroit, MI, USA). Contents were agitated and several dilutions were prepared. Plate counts were determined by plating serial dilutions of yogurt in MRS agar. Pour plates were incubated anaerobically at 43˚C for 72 hrs. The Lactobacillus bulgaricus and Streptococcus thermophilus counts were converted to log10 scale before the data were analyzed by SAS.
2.2.2. Streptococcus thermophilus Counts
Counts of S. thermophilus were determined using pour plate technique. The S. thermophilus agar was prepared according to Dave and Shah [16]. To 12 g, 6 ml of 0.5% Bromocresol Purple solution was added as an indicator and also a few drops of 1 N HCL were added to reduce pH to 6.8 ± 0.1. This mixture was autoclaved at 121˚C for 15 mins. Yogurts were sampled at weeks 1, 2, 3 and 4 of storage period. Using a sterile pipette, the yogurt was briefly agitated in the cup and 1 g of yogurt was pipetted from the center of the yogurt cup into a sterile bottle containing 99 mL of sterile 0.1% peptone water (Difco, Detroit, MI, USA). Contents were agitated and several dilutions were prepared. Plate counts were determined by plating serial dilutions of yogurt in S. thermophilus agar. Pour plates were incubated aerobically at 37˚C for 24 hrs.
2.3. Analytical Procedures
2.3.1. pH
The pH of the yogurts at 11˚C was determined using an Orion pH meter model 250A/610 (Fisher Scientific, Instruments, Pittsburgh, PA) calibrated using commercial pH 4.00 and 7.00 buffers (Fisher Scientific).
2.3.2. Titratable Acidity (TA)
The titratable acidity was determined by weighing 9 g of yogurt and adding about 6 drops of phenolphthalein solution and titrating with 0.1 N NaOH as until color changed to rose pink.
2.3.3. Apparent Viscosity
The apparent viscosities were determined according to Farooq and Haque [17]. Apparent viscosities of yogurts at 8˚C were measured using a Brookfield DV II + viscometer (Brookfield Engineering Lab Inc., Stoughton, MA, USA) with a helipath stand. An R-V #4 spindle was set to 5 rpm. The data were acquired using the Wingather® software (Brookfield Engineering Lab Inc., Stoughton, MA, USA). The viscosity measurements were continuous over 33 seconds required to collect one hundred data points averaged per sample per replication.
2.4. Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) using PROC GLM (SAS, 2004). Significant differences between means were determined using LSD means separation and Bonferroni test. Significant differences were determined at α = 0.05.
3. Results and Discussion
3.1. Microbiological analysis
3.1.1. Lactobacillus bulgaricus Counts
The Lactobacillus bulgaricus counts are reported in Figure 1(a). The Probability > F value for ethanol amount *storage period interaction was significant (P < 0.0001) (Table 1). The ethanol amount effect did not significantly influence (P = 0.0728) the counts (Tables 1 and 2). However, the storage period effect significantly influenced the Lactobacillus bulgaricus counts (P < 0.0001) (Table 1). The counts in week one were higher compared to weeks 2, 3 and 4 (Table 3). Somkuti et al. [18] reported that the magnitude of cell death resulting from ethanol treatment increased with solvent concentration. They reported that bactericidal properties of ethanol take effect at around 40% (v/v). They further reported that survivors could not be detected on MRS agar plates after a 20 min. exposure of cells to 30% (v/v) or higher aqueous ethanol solutions. There was about a 3 log decrease in Lactobacillus bulgaricus counts after the first week (Figure 1(a), Table 3). From the second week the counts stabilized to 6.7 log, over a million cells/ml. Damin et al. [19] reported that it is normally recommended that yogurt or fermented milk should contain at least one million viable cells per gram at the time of consumption for its health benefits.
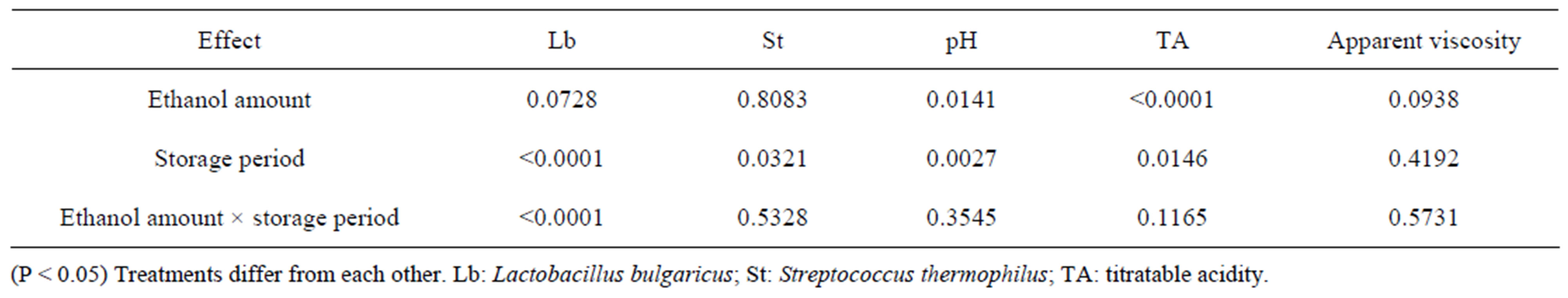
Table 1. Probability > F value (Pr > F) for L. bulgaricus counts, S. thermophilus counts, pH, titratable acidity and apparent viscosity in the yogurts containing 0%, 2.5%, 5% and 7.5% of ethanol over the storage period of 4 weeks.
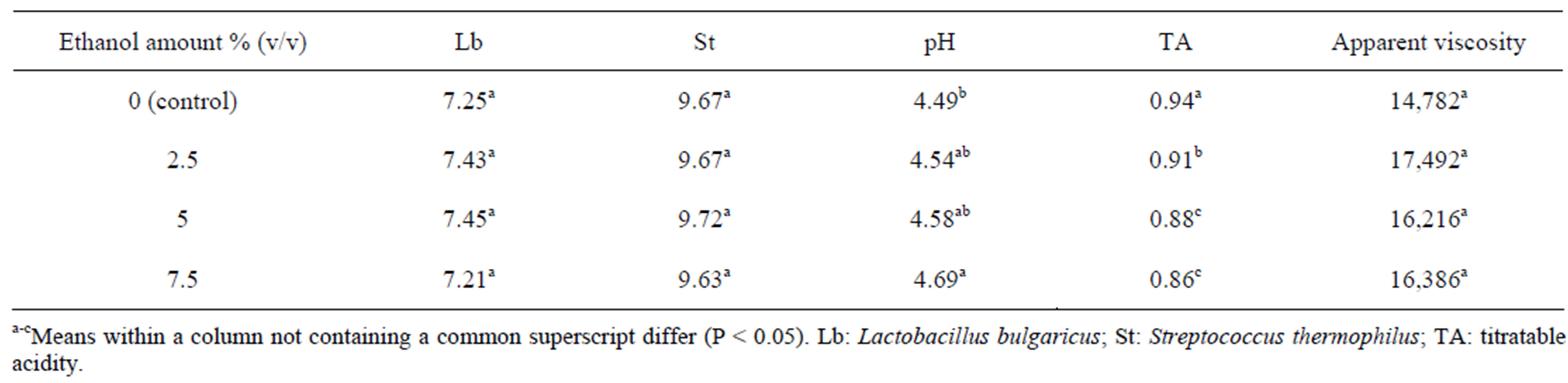
Table 2. Means of L. bulgaricus counts, S. thermophilus counts, pH, titratable acidity and apparent viscosity at various ethanol concentrations.

Table 3. Means of L. bulgaricus counts, S. thermophilus counts, pH, titratable acidity and apparent viscosity over a storage period.
3.1.2. Streptococcus thermophilus counts
Streptococcus thermophilus counts are shown in Figure 1(b). The ethanol amount × storage period interaction was not significant (P = 0.5328) (Table 1). Ethanol amount did not have a significant effect in Streptococcus thermophilus counts (P = 0.8083) (Table 1). The storage period effect was significant (P = 0.0321) (Table 1). Counts at week two were higher than week four (Table 3). Muyanja et al. [20] worked with bushera (a Ugandan fermented beverage) and reported that LAB counts increased from 5.5 to 9.0 log cfu/ml during the 48hrs laboratory fermentation which released 0.27% of ethanol which was the major volatile organic compound. Those results suggested that LAB, including Streptococcus thermophilus, are able to grow in a low amount of ethanol. For weeks 2, 3, and 4 the Streptococcus thermophilus counts (Figure 1(b)) were higher than Lactobacillus bulgaricus counts (Figure 1(a)), which is the normal behavior of these bacteria as has been previously reported by [21,22].
3.2. Physico-Chemical Characteristics
3.2.1. pH
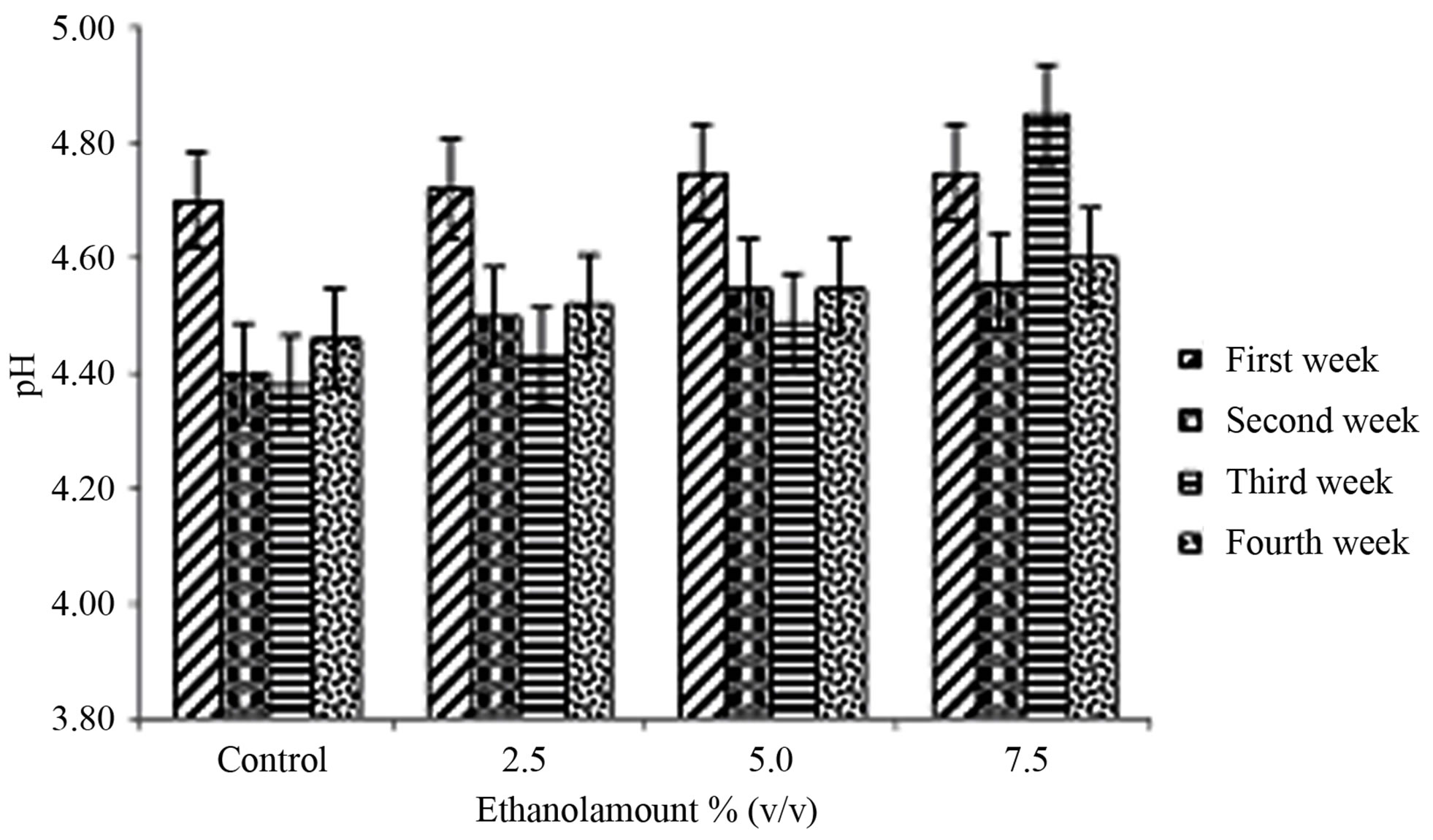
The pH values are illustrated in Figure 2(a). The ethanol amount × storage period interaction was not significant (P = 0.3545) (Table 1). Ethanol amount had a significant effect (P = 0.0141) (Table 1). The pH for yogurt containing 7.5% ethanol was higher compared to control but not with the other yogurts (Table 2). The storage period effect was significant (P = 0.0027) (Table 1). The pH at week one was higher compared to weeks 2, 3 and 4 (Table 3). Damin et al. [19] reported that post-acidification occurred independently of the level of supplementation or the ingredient used. A decrease in pH during storage is expected as result of the activity of Lactobacillus bulgaricus. This has been observed in lactic beverages [22] and also due to the presence of Streptococcus thermophilus that produces large amounts of acids [23].
3.2.2. Titratable Acidity
The titratable acidity vales are reported in Figure 2(b). The ethanol amount × storage period interaction was not significant (P = 0.1165) (Table 1). The ethanol amount had a significant effect (P < 0.0001) (Table 1). The storage period effect was also significant (P = 0.0146) as shown in Table 1. A dilution effect occurs as the ethanol amount increased; hence 7.5% ethanol had the lowest amount of titratable acidity. Control had a higher TA compared to 2.5%, 5% and 7.5% of ethanol (Table 2). The TA at weeks one and two were lower than that at week 3 (Table 3). Fan et al. [24] reported that hot water treatments immediately induced an increase in ethanol concentration (log10 μmol/m3) and reduced titratable acidity and soluble solids content of blueberries, but had no significant effect on fruit firmness, pH, or most flavor volatile concentrations. When an alcohol reacts with an acid, it forms an ester, which has more volatility, giving aroma to food products [25]. Obenland et al. [26] studied that ethanol levels increased in the oranges as a result of storage.
3.2.3. Apparent Viscosity
The apparent viscosity values are reported in Figure 3. The interaction of ethanol amount × storage period was not significant (P = 5731) (Table 1). Amount of ethanol had no effect on apparent viscosity (P = 0.0938) (Table 1). Storage period effect was also not significant (P = 0.4192) (Table 1). This phenomenon may be explained because the concentration levels, (2.5%, 5% and 7.5%), used in this experiment were apparently not high enough to influence apparent viscosity.
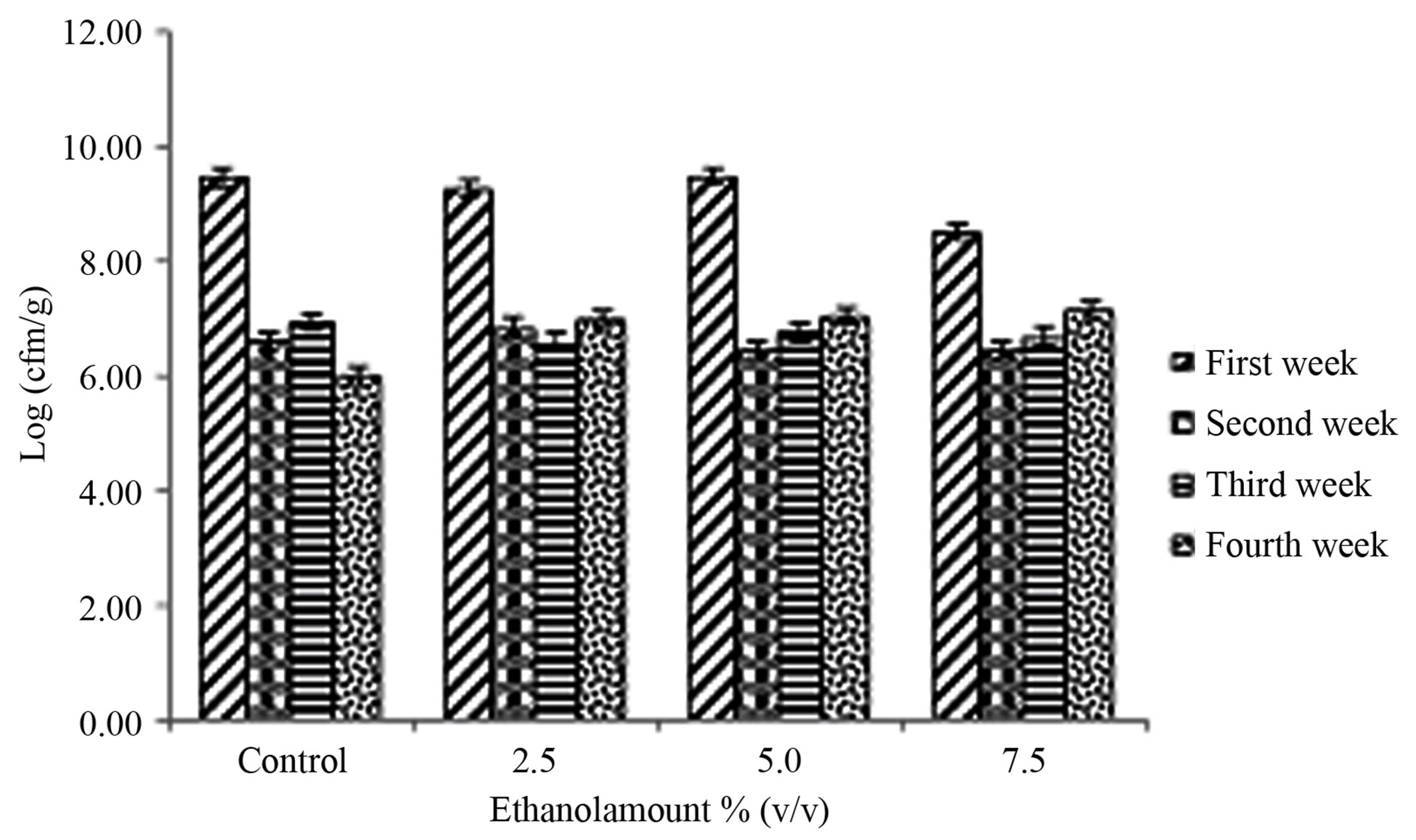 (a)
(a)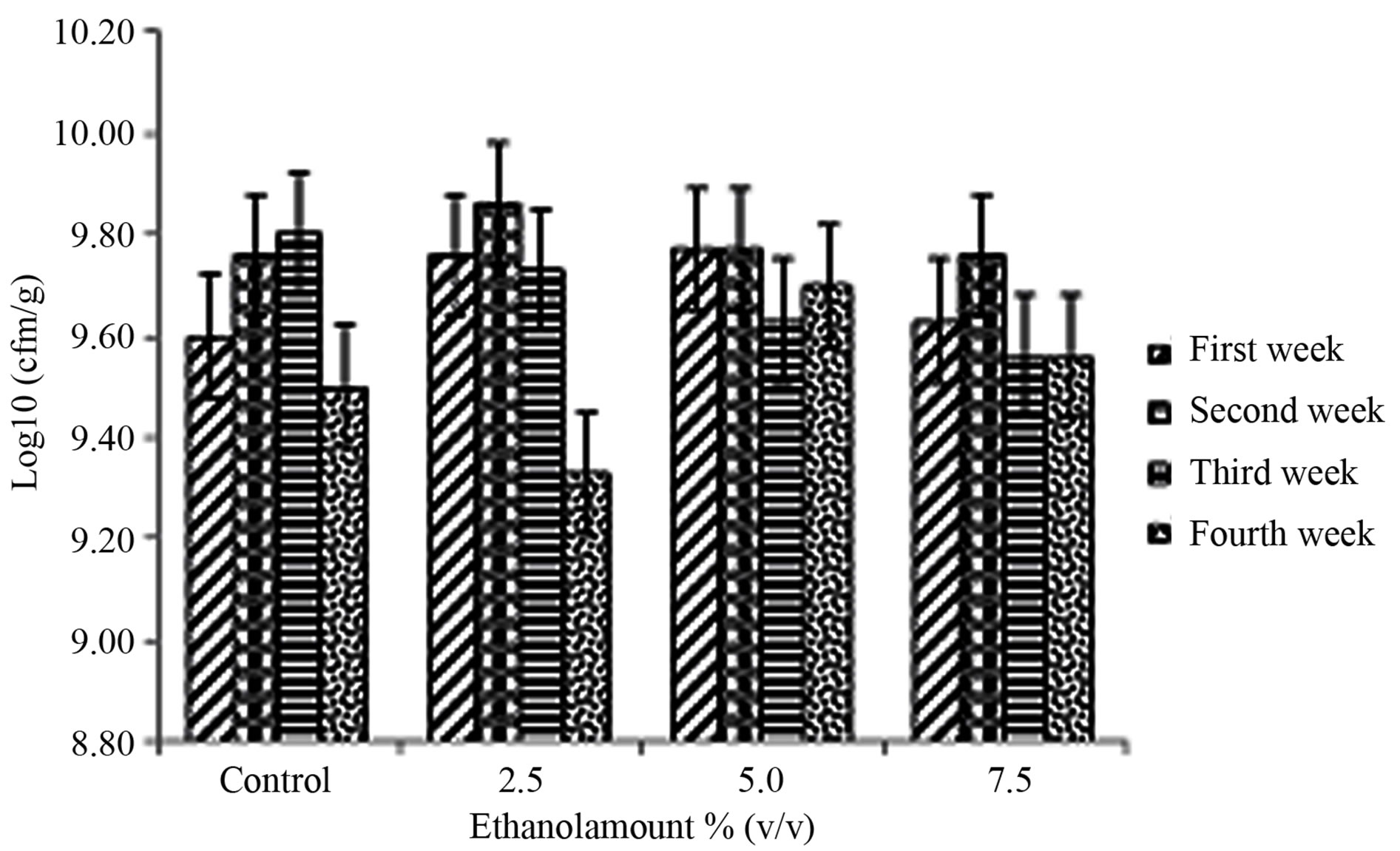 (b)
(b)
Figure 1. Mean ± SE of counts of (a) Lactobacillus bulgaricus and (b) Streptococcus thermophilus in the therapeutic product (yogurt) over the storage time, containing various amounts of ethanol (0% (control), 2.5%, 5% and 7.5% v/v) at first week , second week
, second week , third week
, third week  and fourth week
and fourth week .
.
 (a)
(a)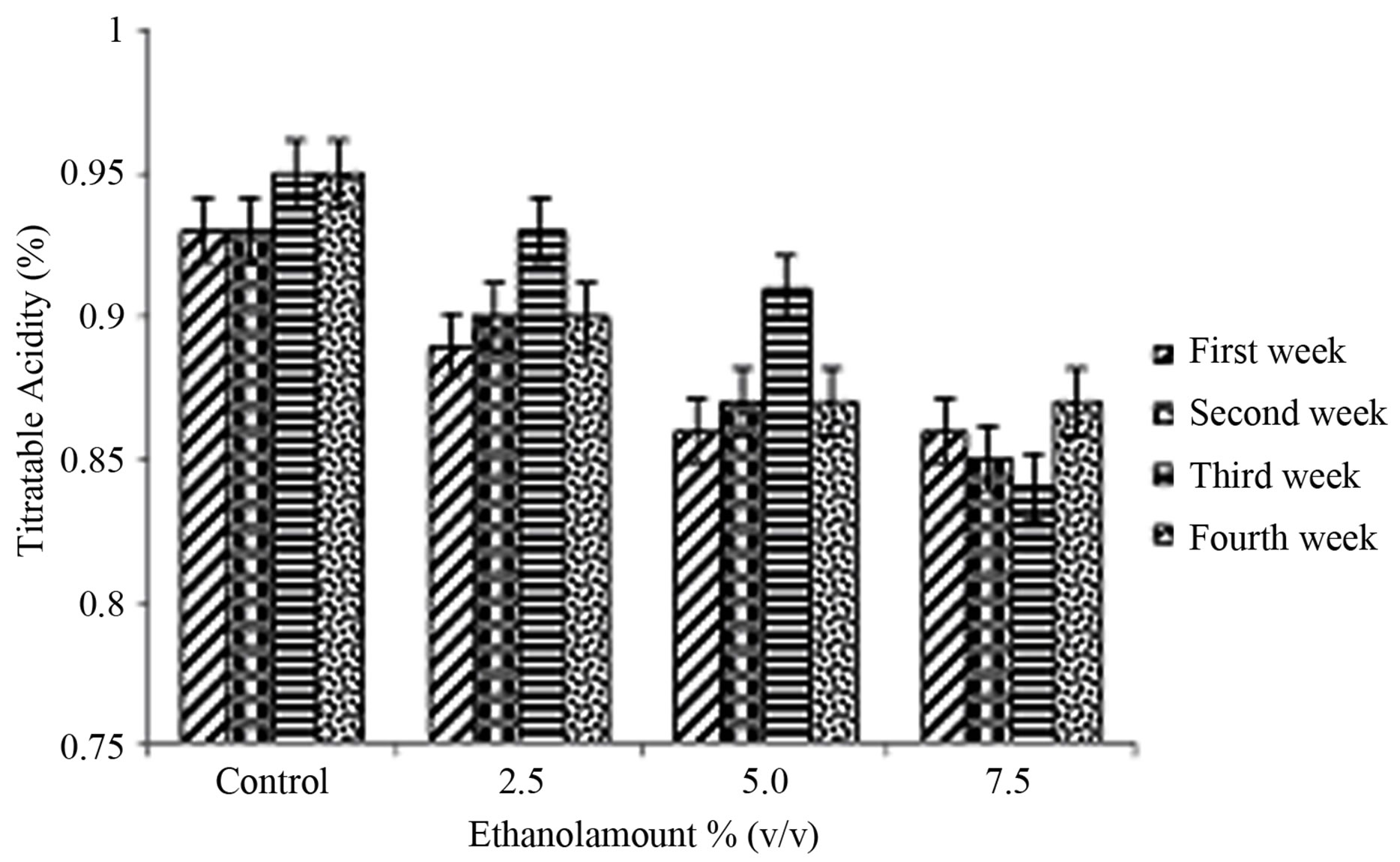 (b)
(b)
Figure 2. Mean ± SE of (a) pH and (b) titratable acidity in the therapeutic product (yogurt) over the storage time, containing various amounts of ethanol (0% control, 2.5%, 5% and 7.5% amounts v/v) at first week , second week
, second week , third week
, third week  and fourth week
and fourth week .
.
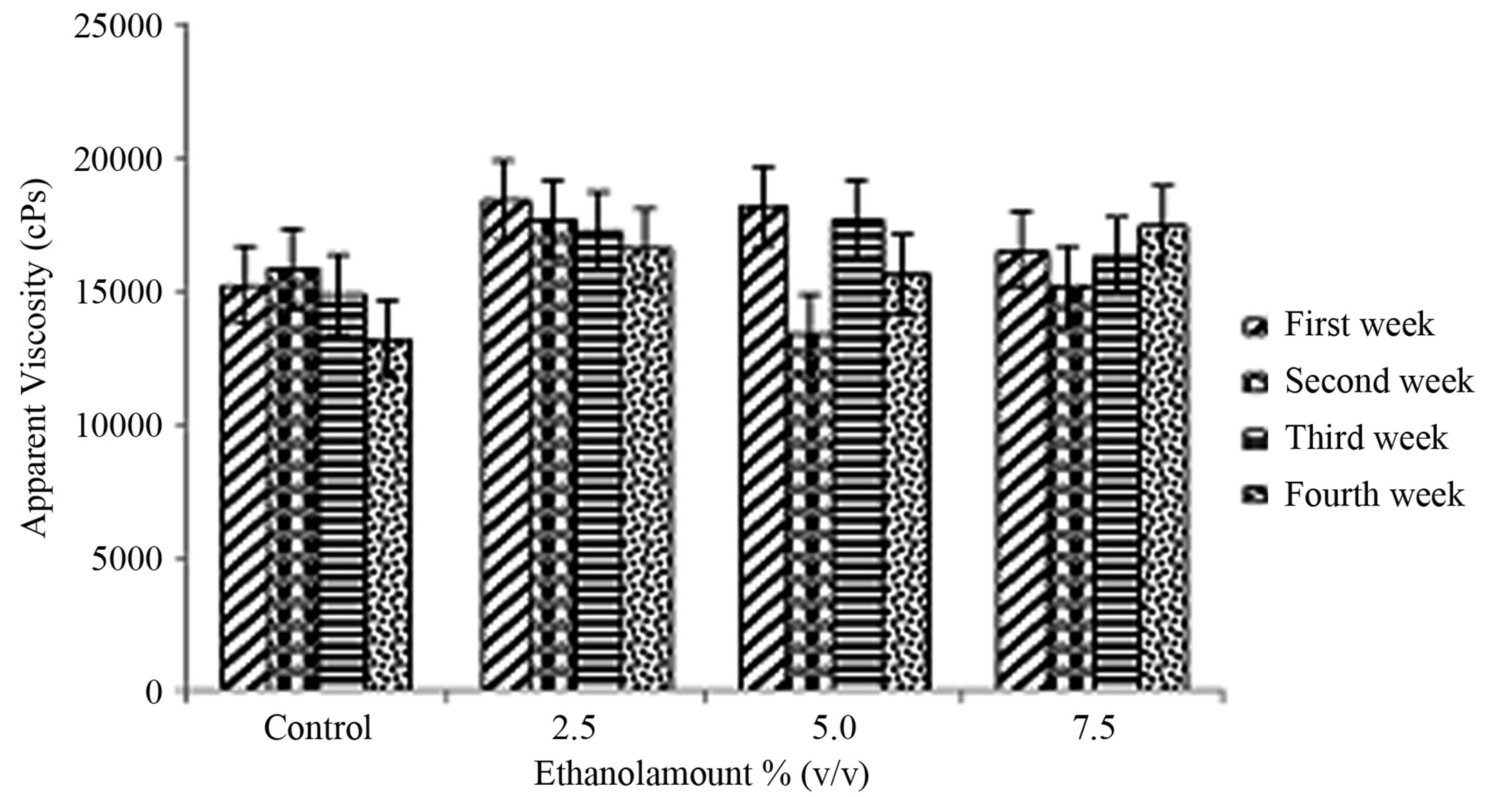
Figure 3. Mean ± SE of apparent viscosity of the therapeutic product (yogurt) over the storage time, containing various amounts of ethanol (0% (control), 2.5%, 5% and 7.5% v/v) at first week , second week
, second week , third week
, third week  and fourth week
and fourth week .
.
4. Conclusion
Ethanol incorporation in this therapeutic product (yogurt) significantly influenced pH and TA of the yogurts. The pH of the control yogurts was significantly lower than yogurts with 7.5% ethanol. Control yogurts had signifycantly the highest TA followed by yogurts with 2.5% ethanol while yogurts with 5% and 7.5% ethanol had significantly the lowest TA values not different from each other. Ethanol incorporation in yogurt did not influence the counts of Lactobacillus bulgaricus or the counts of Streptococcus thermophilus. Yogurt culture bacterial counts remained at levels sufficient to offer probiotic benefits. Neither did ethanol incorporation influence the apparent viscosity of the yogurts. Therapeutic yogurts with ethanol can successfully be manufactured without adversely influencing the yogurt culture bacterial counts. This could facilitate a novel therapeutic product with advantages of probiotic culture bacteria and ethanol.
5. Acknowledgements
The authors thank Tanuja Muramalla (School of Animal Sciences, Louisiana State University, Baton Rouge) and Damir Torrico (Department of Food Science, Louisiana State University, Baton Rouge) for their support with this study. Approved for publication by the Director of the Louisiana Agricultural Experiment Station as manuscript number 2012-230-7418.
REFERENCES
- M. Lind, “Natural Therapy: Using Yogurt to Treat Fungal Infections,” 2009. http://voices.yahoo.com/natural-thera-py-using-yogurt-treat-fungal-infections-32648848.html?cat=68
- T. Vasiljevic and N. P. Shah, “Probiotics—From Metchnikoff to Bioactives,” International Dairy Journal, Vol. 18, No. 7, 2008, pp. 714-728. doi:10.1016/j.idairyj.2008.03.004
- N. P. Shah, “Functional Cultures and Health Benefits,” International Dairy Journal, Vol. 17, No. 11, 2007, pp. 1262-1277. doi:10.1016/j.idairyj.2007.01.014
- F. Guarner, B. Koletzko, L. Morelli, S. Salminen, G. Perdigon and G. Corthier, “Should Yoghurt Cultures Be Considered Probiotic?” British Journal of Nutrition, Vol. 93, No. 6, 2005, pp. 783-786. doi:10.1079/BJN20051428
- S. Parvez, K. A. Malik, S. A. H. Kang and H.-Y. Kim, “Probiotics and Their Fermented Food Products Are Beneficial for Health,” Journal of Applied Microbiology, Vol. 100, No. 6, 2006, pp. 1171-1185. doi:10.1111/j.1365-2672.2006.02963.x
- M. D. Vrese, C. Laue, J. Schrezenmeir, S. Fenselau, A. Stegelmann and B. Richter, “Probiotics: Compensation for Lactase Insufficiency,” American Journal of Clinical Nutrition, Vol. 73, No. 2, 2001, pp. 421-429.
- M. C. McKinley, “The Nutrition and Health Benefits of Yoghurt,” International Journal of Dairy Technology, Vol. 58, No. 1, 2005, pp. 1-12. doi:10.1111/j.1471-0307.2005.00180.x
- P. M. Suter and Y. Schutz, “The Effect of Exercise, Alcohol or Both Combined on Health and Physical Performance,” International Journal of Obesity, Vol. 32, No. 6, 2008, pp. S48-S52. doi:10.1038/ijo.2008.206
- R. D. Mattes, “Ethanol Perception and Ingestion,” Physiology & Behavior, Vol. 72, No. 1-2, 2000, pp. 217-229. doi:10.1016/S0031-9384(00)00397-8
- International Dairy Foods Association (IDFA), “Dairy Facts Manual,” 2011. http://www.idfa.org/files_willow/products/514_IDFA2011.pdf
- G. Barbieri, L. Bolzoni, M. Careri, A. Mangia, G. Parolari, S. Spagnoli and R. Virgili, “Study of the Volatile Fraction of Parmesan Cheese,” Journal of Agricultural and Food Chemistry, Vol. 42, No. 5, 1994, pp. 1170-1176. doi:10.1021/jf00041a023
- S. Notermans, P. S. S. Soentoro and E. H. M. DelfgouVan Asch, “Survival of Pathogenic Microorganisms in an Egg-Nog-Like Product Containing 7% Ethanol,” International Journal of Food Microbiology, Vol. 10, No. 3-4, 1990, pp. 209-218. doi:10.1016/0168-1605(90)90068-G
- E. Albertazzi, R. Cardillo, S. Servi and G. Zucchi, “Biogeneratoin of 2-Phenylethanol and 2-Phenylethylacetate Important Aroma Components,” Biotechnology Letters, Vol. 16, No. 5, 1994, pp. 491-496. doi:10.1007/BF01023331
- K. Zajsek and A. Gorsek, “Effect of Natural Starter Culture Activity on Ethanol Content in Fermented Dairy Products,” International Journal of Dairy Technology, Vol. 63, No. 1, 2010, pp. 113-118. doi:10.1111/j.1471-0307.2009.00549.x
- E. Padan, D. Zilberstein and S. Schuldiner, “pH Homeostasis in Bacteria,” Biochimica et Biophysica Acta, Vol. 650, No. 2-3, 1981, pp. 151-166. doi:10.1016/0304-4157(81)90004-6
- H. Farooq and Z. U. Haque, “Effect of Sugar Esters on the Textural Properties of Nonfat Low Calorie Yogurt,” Journal of Dairy Science, Vol. 75, No. 10, 1992, pp. 2676- 2680. doi:10.3168/jds.S0022-0302(92)78029-1
- R. I. Dave and N. P. Shah, “Viability of Yoghurt and Probiotic Bacteria in Yoghurts Made from Commercial Starter Cultures,” International Dairy Journal, Vol. 7, No. 1, 1997, pp. 31-41. doi:10.1016/S0958-6946(96)00046-5
- G. A. Somkuti, D. H. Steinberg and M. E. Dominiecki, “Permeabilization of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus with Ethanol,” Current Microbiology, Vol. 36, No. 4, 1998, pp. 202-206. doi:10.1007/s002849900294
- M. R. Damin, M. R. Alcântara, A. P. Nunes and M. N. Oliveira, “Effects of Milk Supplementation with Skim Milk Powder, Whey Protein Concentrate and Sodium Caseinate on Acidification Kinetics, Rheological Properties and Structure of Nonfat Stirred Yogurt,” LWT— Food Science and Technology, Vol. 42, No. 10, 2009, pp. 1744-1750.
- C. M. B. K. Muyanja, J. A. Narvhus, J. Treimo and T. Langsrud, “Isolation, Characterisation and Identification of Lactic Acid Bacteria from Bushera: A Ugandan Traditional Fermented Beverage,” International Journal of Food Microbiology, Vol. 80, No. 3, 2003, pp. 201-210. doi:10.1016/S0168-1605(02)00148-4
- G. A. Birollo, J. A. Reinheimer and C. G. Vinderola, “Viability of Lactic Acid Microfora in Different Types of Yoghurt,” Food Research International, Vol. 33, No. 9, 2000, pp. 799-805. doi:10.1016/S0963-9969(00)00101-0
- A. Y. Tamime and R. K. Robinson, “Yoghurt: Science and Technology,” 2nd Edition, CRC Press, Boca Raton, 1999.
- Y.-C. Wang, R.-C. Yu and C.-C. Chou, “Growth and Survival of Bifidobacteria and Lactic Acid Bacteria during the Fermentation and Storage of Cultured Soymilk Drinks,” Food Microbiology, Vol. 19, No. 5, 2002, pp. 501-508. doi:10.1006/yfmic.506
- L. Fan, C. F. Forney, J. Song, C. Doucette, M. A. Jordan, K. B. McRae and B. A. Walker, “Effect of Hot Water Treatments on Quality of Highbush Blueberries,” Journal of Food Science, Vol. 73, No. 6, 2008, pp. M292-M297. doi:10.1111/j.1750-3841.2008.00838.x
- B. Girard, P. Delaquis, A. G. Reynolds, D. Yuksel and M. A. Cliff, “Vinification Effects on the Sensory, Colour and GC Profiles of Pinot Noir Wines from British Columbia,” Food Research International, Vol. 34, No. 6, 2001, pp. 483-499. doi:10.1016/S0963-9969(00)00177-0
- D. Obenland, J. Doctor, M. L. Arpaia, K. Fjeld, S. Collin and J. Sievert, “Commercial Packing and Storage of Navel Oranges Alters Aroma Volatiles and Reduces Flavor Quality,” Postharvest Biology and Technology, Vol. 47, No. 2, 2008, pp. 159-167. doi:10.1016/j.postharvbio.2007.06.015

