Journal of Sustainable Bioenergy Systems
Vol.05 No.03(2015), Article ID:59764,9 pages
10.4236/jsbs.2015.53010
Physicochemical Characterization of Jatropha curcas Linn Oil for Biodiesel Production in Nebbi and Mokono Districts in Uganda
Yonah K. Turinayo1*, Fred Kalanzi1, Jude M. Mudoma1, Peter Kiwuso1, Godwin M. Asiimwe2, John F. O. Esegu1, Paul Balitta1, Christine Mwanja1
1National Forestry Resources Research Institute (NaFORRI), National Agricultural Research Organisation (NARO), Kampala, Uganda
2College of Engineering, Design, Art and Technology (CEDAT), Makerere University, Kampala, Uganda
Email: *t2rinayonah@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 July 2015; accepted 18 September 2015; published 21 September 2015
ABSTRACT
Jatropha curcas Linn has been identified worldwide as one of the sources of biodiesel. Biodiesel has energy properties close to fossil diesel and can be a potential energy alternative. However, these properties may vary based on soils, plant genetics and agro-climatic conditions in a given geographical location. Several studies on biodiesel production under such conditions have been done elsewhere, but few have been done on J. curcas oil in Uganda. This study analysed the physicochemical properties of J. curcas L. oil for biodiesel production in Nebbi and Mukono districts using American Standards and Testing Methods (ASTM D6751) and European Standards (EN 14214). J. curcas seed kernel contained 51% w/w and 48% w/w of oil with high levels of Free Fatty Acids (1.52% and 1.93%) and acid values (35 and 36 mg KOH/g) for Nebbi and Mukono, respectively; the difference was significant (p ≤ 0.05). Generally, the quality and quantity of the oil from Nebbi were better than those of Mukono, based on the biodiesel standard values. Nevertheless, kinematic viscosity, acidity, potassium and phosphorus content values were found abnormally high (31.46 - 33.23 mm2/s, 35.23 - 36.66 mg KOH/g, 16.50 - 20.52 mg/100g and 16.13 - 26.02 mg/kg, respectively) for both regions as compared to the standard values (3.5 - 5.0 mm2/s, 2 mg KOH/g, <5 mg/100g and <10 mg/kg, respectively) of biodiesel for diesel engine. Such properties are very important for engine fuels and if not considered well, may affect engine performance negatively. Therefore adequate treatment of the oil by degumming, etherification and transesterification before use in a diesel engine could avert this difficulty.
Keywords:
J. curcas, Oil, Biodiesel, Engine, Energy

1. Introduction
The use of fossil diesel as engine fuel has been criticized worldwide for its negative effects on the environment as well as its exhaustibility. Use of biodiesel as an alternative has emerged as a prospect for abating this criticism and Jatropha curcas Linn has been identified as one of the potential sources of biodiesel. J. curcas L. is a tropical tree species believed to have originated from Mexico and Central America [1] , though it is widely distributed in Africa, Latin America, India and South-East Asia [1] . Studies have found that the plant is drought- resistant, pest-tolerant, well adapted to degraded, arid and semi-arid lands and its flowering starts after 90 to 120 days of seeding [1] - [5] . Its optimal productivity level is believed to be after 4 to 5 years [6] . In America, the seeds harvested from a 3-year plantation of 640 J. curcas L. seedlings can yield substantial quantity of oil estimated at 1260 gallons (≈4770 liters) per year per acre, based on the ideal scenario [7] . Whereas in sub-Saharan Africa and Asia, experience with J. curcas L. production as a cash crop has found that yields are marginal in a range of 1 to 1.6 tonnes per ha [8] , equivalent to 400 - 650 liters of oil per acre per year. Besides being a fuel substitute, J. curcas L. oil has been used as medicine, insecticide and raw material for soap making. J. curcas L. can be intercropped with various crops on marginal sites to improve on land productivity [9] [10] . Furthermore, the plant has a phytoprotective action and can be used to protect crops against pests, pathogens and grazing animals [1] . J. curcas L. oil is not edible due to its toxin phorbol ester, but can best be used in a diesel engine after being converted to biodiesel (transesterification) [11] [12] . This toxicity has made J. curcas L. oil advantageous over edible oils in biodiesel production and therefore does not compete directly with the food markets [11] .
Studies show that J. curcas L. oil has the potential to replace fossil diesel as an engine fuel; [12] provided that the oil is free from contamination, and its acid value, viscosity, phosphorus, ash and water content are kept at low levels [8] . Crude J. curcas L. oil is quite viscous but has a high cetane (ignition quality) rating. The typical low levels of free fatty acids in J. curcas L. oil improve on its storability, though susceptible to oxidation due to the presents of high unsaturated oleic and linoleic acids [8] . The occurrence of unsaturated fatty acids (high iodine value) permits it to stay in liquid state during lower temperatures. Crude J. curcas L. oil has low sulphur content compared to fossil oil. This indicates less harmful exhaust emissions of sulphur dioxide (SO2) gas when used as a fuel in an engine. These qualities make J. curcas L. oil a potential alternative to fossil diesel fuel, even in its raw form [12] , and can be used in any diesel engine without modification [13] .
Quite a number of studies on the physicochemical properties of J. curcas L. seed oil have been done in different parts of the world [4] [9] [14] - [17] . Ginwal, et al. [14] found that oil quantity ranged from 33% to 39% in whole seeds and 47% to 58% in kernel from various areas of Central India, which varied across the seed sources. According to Brittaine and Lutaladio [8] , knowledge on J. curcas L. oil quality is essential for biodiesel production, owing to the extreme variability in its physicochemical characteristics. Such variations may be due to differences in environmental and geographical factors [15] e.g. rainfall distribution, temperature, soil quality and genetic interaction [8] [14] . Research has found that optimal growing conditions are in regions of 1000 to 1500 mm annual rainfall, 20˚C to 28˚C temperature with no frost, and free-draining sand and loam soils with no risk of water logging [8] . The maturity of the fruits, seed size and weight also can affect the fatty acid composition of the oil. J. curcas L. seed-oil processing and storage further affect oil quality [18] . Therefore, more studies are necessary to determine the quality and quantity of oil that can realistically be attained across different agro- ecological conditions.
In Uganda, J. curcas L. got incorporated within the agricultural landscapes as a support plant for Vanilla planiforia especially in Mukono district. Since the last decade, there have been efforts to promote Jatropha growing as an energy crop. To date, Jatropha growing has spread in Nebbi, Masaka, Karamoja, Lira, Katakwi, Hoima and Masindi districts. NEMA [19] reports that J. curcas L. can thrive in the Ugandan agro-ecological setting with about 60% of the arable land area suited to its cultivation. However, most of the potential private sector entrepreneurs lack scientific knowledge to justify their investments. Moreover, due to variations in agro-ecological conditions, differences in quality and quantity of J. curcas L. oil may arise. Knowing these variations is important in the promotion of J. curcas L. for biodiesel production in Uganda. This paper is a step forward towards filling this knowledge gap. The study therefore aims at evaluating the quality and quantity of J. curcas L. oil from Nebbi and Mukono districts in Uganda.
2. Methods
2.1. Description of the Study Area
The study was conducted in Nebbi and Mukono districts in the West-Nile and L. Victoria Crescent agro-eco- logical zones (AEZs) of Uganda, respectively (Figure 1).
Nebbi and Mukono districts were selected because they are the main hotspots for J. curcas L. growing in Uganda. Besides, the two districts have differences in agro-ecological conditions (Table 1) which could cause variations in J. curcas L. oil production.
Table 1. Agro-ecological conditions of Mukono and Nebbi districts.
Figure 1. Study area (Nebbi and Mukono districts).
2.2. Seed Collection and Preparation for Oil Analysis
J. curcas L. seeds were collected and bulked from randomly selected plants from farmers’ plantations in Nebbi and Mukono districts. This was done in three phases (June 2014, November 2014 and March 2015) according to the region in order to get a composite sample. The collected seeds were cleaned thoroughly, sun dried, kept in muslin bags and stored at room temperature until analysis for oil quality and quantity. Prior to oil extraction and analysis, three samples per region, each weighing 5 kg, were then dehulled to remove seed coats by using a small stick and later the hulls and the seeds were separated by winnowing.
2.3. Oil Extraction
The oil contained in 3 kg of J. curcas L. seeds was extracted in a Soxtec apparatus for 4 hours, using petroleum ether (boiling point of 40˚C - 60˚C) as an extraction solvent. The extracted oil was recovered by solvent evaporation using a Soxhlet apparatus to remove the majority of the solvent; followed by rotatory evaporation at 40˚C under reduced pressure. The extracted seed oil was weighed and stored in amber glass flasks, at 18˚C, for subsequent analysis. The amount of oil in seeds was calculated by dividing the mass of extracted oil by the mass of crushed Jatropha seed kernels. For each accession, the oil content of three samples was determined in triplicate tests in order to enhance accurate statistical inference.
2.4. Oil Quality Analysis
The extracted oil was analysed for physicochemical properties (kinematic viscosity, cetane number, acid value, iodine value and free fatty acids (FFA)) using American Standards and Testing Methods (ASTM D6751). The calorific value and Density were determined by bomb calorimeter and digital density analyser, according to ASTM D240 and ASTM D5002-13, respectively; whereas fatty acids and ester contents were determined using high performance liquid chromatography (HPLC; Model: RF-20AXS) basing on EN 14103:2011 standards. The element content (P, Na and K) of the oil was determined by flame spectrometry using Spectro Arcos ICP-OES analyser according to procedures described in European standards (EN 14107, EN 14108 and EN 14109, respectively). Each parameter was determined thrice for each sample per geographical location.
2.5. Statistical Analysis
Means and standard deviations for the individual parameters in triplicate samples were determined to compare the quality and percent (w/w) quantity of J. curcas L. oil from Nebbi and Mukono districts. Data were statistically analyzed using paired-samples T-Test to study the difference between J. curcas L. oil quality and quantity from the study areas. All significance tests were set at P ≤ 0.05, and the statistics were analyzed using the statistical software package (SPSS version 16).
3. Results and Discussion
3.1. Oil Content in J. curcas L. Seeds from Nebbi and Mukono Districts
The percent (w/w) quantity of J. curcas L. oil recovered from seeds varied significantly (p < 0.05) between Nebbi and Mukono districts as shown in Figure 2.
Nebbi depicted high oil recovery (51.33% ± 0.58% w/w) from J. curcas seed kernel than Mukono (48.00 ± 1.00% w/w), though both values fall in a range (40% - 60% w/w) reported elsewhere [9] [10] [14] [24] - [28] . The difference in oil content could be attributed to variations in altitude, genetics, climatic and soil conditions between the two regions [25] [29] - [31] . Nebbi and Mukono districts have different soils, mean annual rainfall, temperature and altitudes, which could have contributed to the variance in seed oil content. Ovando-Medina, et al. [30] also found a negative correlation of the oil content with the altitude at which J. curcas L. plant is grown. In the same way, this conforms to the study by Pant, et al. [29] who determined seed oil content variation in J. curcas L. plant grown in two different soil conditions and three altitudinal ranges in India. They found higher oil content (43.19%) of seeds harvested from the plant grown in non-arable soils with low altitude (400 - 600 m) than arable soils with high altitude (38.66% and 800 - 1000 m). Temperature has also been found to have a significant effect towards oil content [16] and this has been supported by several authors [1] [8] [32] who found that J curcas L. performs very well in regions of high temperatures ranging from 20˚C to 40˚C mean annual.
Figure 2. J. curcas oil quantity (%) recovered from seeds harvested from Nebbi and Mukono districts (n = 3; p-value = 0.01).
Nevertheless, improper processing methods such as prolonged exposure of harvested seeds to direct sunlight can deteriorate the oil yield significantly [26] .
3.2. Physical Characteristics of J. curcas L. Oil
Analysis of J. curcas L. seed samples from Mukono and Nebbi revealed significant differences (p ≤ 0.05) in Free Fatty Acids (FFAs) and acid value (Table 2). However, the mean values of ester content (triglycerides), kinematic viscosity, calorific values, density, cetane number (CN) and iodine value of J. curcas L. oil were not significantly different (p > 0.05). The ester content of the extracted oil was in a range of 92.8% to 95.1% for the two districts, close to the values (96% to 98%) reported by other authors [13] [33] . Yet the acidity of the oils was found significantly higher (36.42 ± 0.24 mg KOH/g for Mukono and 35.43 ± 0.15 mg KOH/g for Nebbi) and deviated from biodiesel standard value (2 mg KOH/g [34] ) for diesel engine. The difference in FFAs and acid value in both regions could be due to variations in climatic conditions [8] , as well as the presences of high unsaturated oleic and linoleic acids which make J. curcus L. oil prone to oxidation in storage [8] .
The high acid values recorded in J. curcas L. oils from Mukono and Nebbi was generally due to the presence of high FFAs (1.93% ± 0.15% and 1.52% ± 0.10%, respectively, compared to 0.02% standard value for biodiesel engine), primarily resulting from hydrolysis of triglycerides [36] . This degradation reaction is supported by temperature and it mainly happens in damaged seeds by the action of lipases in the existence of water [36] . FFAs or triglycerides are usually transformed into alkyl-esters (biodiesel) by reaction with short-chain alcohols (e.g. methanol or ethanol) in the presence of a catalyst (Equation (1) and (2))―a process known as esterification and transesterification, respectively [13] . However, high amounts of FFA (mostly above 3%) would lead to formation of soap and emulsions, owing to excessive alkaline catalyst consumption, hence significantly reducing the biodiesel yield [32] [37] .
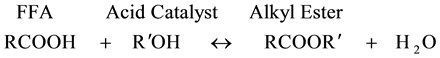 (1)
(1)
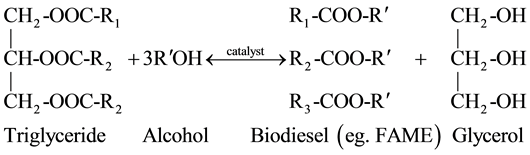 (2)
(2)
Despite the suitability of J. curcas L. oil for biodiesel production from Mukono and Nebbi in terms of ester content (93.30% ± 0.50% and 94.70% ± 0.40%, respectively; biodiesel standards: >96.5% [34] ), it has been
Table 2. Physical characteristics of J. curcas oil (n = 3; mean ± SD).
*Significant at p ≤ 0.05; otherwise not significant.
found that kinematic viscosity (32.27 ± 0.81 and 32.90 ± 0.35 mm2/s, respectively) deviated greatly from biodiesel standard value (3.5 - 5 mm2/s) for diesel engine [34] . In line with Ferrari, et al. [38] , high viscosity of J. curcas L. oil may affect its flow and ability to spray in the engine and at low temperatures may cause mechanical failure of injection pump drive systems. This may be reduced significantly through etherification and transesterification [38] [39] of the oil, by converting its high-molecular ester compounds (Triglycerides) into simpler Fatty Acid Methyl Esters (FAMEs). Biodiesel, a mixture of various kinds of esters such as FAMEs (esters of fatty acids), has been documented as having physical characteristics closer to those of petroleum diesel than pure vegetable oil, depending on the type of vegetable oil [16] [34] [37] [38] . Moreover, Drapcho, et al. [16] found that biodiesel contains about 13% lower energy density than petroleum diesel, though has greater lubricity and undergoes further complete combustion.
In this study, results presented in Table 2 show that calorific value of J. curcas L. oil from Mukono and Nebbi (37.10 ± 2.87 and 41.47 ± 0.76 MJ/kg, respectively) fall within what is recommended for biodiesel (>36 MJ/kg [34] ) and compares with petroleum diesel (43.35 MJ/kg [40] ). Notably [41] , the high calorific value could be attributed to the presence of high quantity of polyphenol and hydrocarbon in the oil. Yet according to Xiu, et al. [39] , addition of polar solvents such as methanol, ethanol, and furfural to the oil could further increase its calorific value, although it may have a substantial effect on cetane number (CN) [38] . As said by Ferrari, et al. [38] , cetane number is the tendency of a fuel to combust under certain conditions of pressure and temperature. Literature [38] suggest that high CN is associated with fast engine starting and smooth combustion, whereas low cetane deteriorates engine performance and leads to high exhaust emissions of hydrocarbon gases and particulate. In this work, results show that CN of J. curcas L. oil from Mukono and Nebbi was found to be 36.00 ± 0.00 and 37.00 ± 1.00, respectively―lower than fossil diesel (47, [40] ) and the norm (>51, according to EN ISO 5165 standard) for biodiesel in diesel engines (Table 2). However, it is important to note that CN value of biodiesel can be increased by increasing the length of hydrocarbons of fatty acid and ester groups, but can also be reduced significantly by increased number of double bonds [38] . It is therefore necessary to mention that the lower CN in the present work could be attributed to higher fractions of unsaturated fatty acids and esters (C18:1 and C18:2) in J. curcas L. oil than saturated acids and esters (C16:0 and C18:0), as depicted in Table 2.
There was no significant difference between Iodine values of J. curcas L. oil from Mukono (108.97 ± 1.17 mg/g) and Nebbi (108.53 ± 1.05 mg/g) (Table 2). The Iodine value of J. curcas L. oil in both study sites was found within biodiesel standard value (<120) for diesel engine in reference to EN 14111 standards. This value, usually expressed in grams of iodine reacted with 100 grams of biodiesel, is a measure of the total unsaturation within a mixture of fatty acids [38] . A study by Prankl and Wörgetter [42] on an engine has found that fuels with higher iodine number have a tendency to polymerize and form deposits on piston rings and piston ring grooves when heated. Such high-molecular compounds, which to a large extent appear only in fatty acid esters with three or more double bonds, have a negative effect on the lubricating quality, thus resulting into engine damage [42] [43] . To avoid this difficulty, it has been recommended by some biodiesel experts [38] to limit the content of linolenic acid methyl esters and polyunsaturated biodiesel instead of the total degree of unsaturation as it is expressed by the iodine value.
3.3. Chemical Characteristics of the J. curcas L. Oil
Results on chemical characteristics (fatty acid, ester and element compositions) of J. curcas L. oil are presented in Table 3. The oil extract from Mukono and Nebbi consists of fractions of saturated (palmitic acid: 14.19% ± 0.14% and 14.60% ± 0.27%, respectively; and stearic acid: 3.91% ± 0.19% and 3.83% ± 0.12%, respectively) and unsaturated (oleic acid: 33.90% ± 0.40% and 34.90% ± 0.50 %, respectively) fatty acids, comparable with those reported by Augustus, et al. [41] . The difference in the mean values of oleic acid component between the two regions was significant; while for Palmitic and Stearic acids, the difference was insignificant. Linder [44] revealed that saturated fatty acids store additional energy per carbon than unsaturated fatty acids; though, unsaturated fatty acids have melting points much lower than saturated fatty acids. Furthermore, saturated fatty acids including Palmitic and Stearic acids influence a higher pour point temperature compared to unsaturated fatty acids in biodiesel [27] . According to Tremolieres, et al. [45] , there is also a strong correlation between oleic acid formation in the seed and temperature; whereby oleic acid increases under high temperature and decreases under lower temperature conditions during seed maturation. This probably explains the significant differences in oleic acid content between Mukono and Nebbi―where mean annual temperature is higher.
There were no significant differences in the content of the elements of Na, K and P in J. curcas L. oil between Nebbi and Mukono districts (Table 3). However, K and P values exceeded the standard value for biodiesel in the diesel engine (Table 3). According to literature [32] [34] , high levels of Na, K and P in biodiesel fuel may lead to end-use problems; Na and K forms ash within the engine and may lead to clogging of filters and fuel injection pumps, while P (in form of phospholipids) may lead to the impairment of the catalyst and hindrance of phase separation, during production process. Na and K (alkaline metals) may be introduced into biodiesel during production process, as a result of catalytic degradation, since they form a major constituent of the catalyst. Nevertheless, P in biodiesel may come from phospholipids and inorganic salts contained in the feedstock oil. As reported by Ferrari, et al. [38] P has a strong negative impact on the long term activity of exhaust emission catalytic systems and basing on this, its existence in biodiesel is limited by specification. On this note, oils with higher P content should first go through degumming before being processed into biodiesel [32] .
Table 3. Chemical characteristics of J. curcas oil (n = 3; mean ± SD) from Nebbi and Mukono districts.
*Significant at p ≤ 0.05; otherwise not significant.
4. Conclusion
Investigations on physicochemical properties of J. curcas L. oil for biodiesel production in Nebbi and Mukono districts have shown that the oil is suitable for biodiesel production and application in a diesel engine after going through pretreatment. The most favorable oil quality and quantity were from Nebbi; indicating high suitability for biodiesel production in the region compared to Mukono. However, both regions depict extremely high values of viscosity, acidity, potassium and phosphorus content. The control of such parameters is a major concern in engine fuels and if not considered well, may be detrimental to engine performance. Therefore adequate pretreatment such as degumming, etherification and transesterification could prevent this problem. Plant genetic variation, climatic conditions and soil quality appear to influence oil quality and quantity in the study districts. Therefore, further research on the influence of such factors on J. curcas L. oil would be of much interest to guide biodiesel production in Uganda.
Acknowledgements
This study was carried out under the project “Utilisation of biomass energy technologies and biofuels for domestic and industrial use in Uganda”. The funding was from Agricultural Technology and Agribusiness Advisory Services (ATAAS). Special thanks go to NaFORRI staff (George Niyibizi, Violet Namuyanja, Sarah Nabbuto, Rosemary Kakayi, Dan Kazigaba and Bernadette Kabonesa) and CHEMTEST (U) Ltd. for the support in data collection and analysis.
Cite this paper
Yonah K.Turinayo,FredKalanzi,Jude M.Mudoma,PeterKiwuso,Godwin M.Asiimwe,John F. O.Esegu,PaulBalitta,ChristineMwanja, (2015) Physicochemical Characterization of Jatropha curcas Linn Oil for Biodiesel Production in Nebbi and Mokono Districts in Uganda. Journal of Sustainable Bioenergy Systems,05,104-113. doi: 10.4236/jsbs.2015.53010
References
- 1. Pandey, V.C., Singh, K., Singh, J.S., Kumar, A., Singh, B. and Singh, R.P. (2012) Jatropha curcas: A Potential Biofuel Plant for Sustainable Environmental Development. Renewable and Sustainable Energy Reviews, 16, 2870-2883.
http://dx.doi.org/10.1016/j.rser.2012.02.004 - 2. Ahamad, S., Joshi, S.K., Mohommad, A. and Ahmed, Z. (2013) Performance of Jatropha curcas L. in Semi-Arid Zone: Seed Germination, Seedling Growth and Early Field Growth. Notulae Scientia Biologicae, 5, 169-174.
- 3. Singh, B., Singh, K., Shukla, G., Goel, V., Pathre, U.V., Rahi, T., et al. (2013) The Field Performance of Some Accessions of Jatropha curcas L. (Biodiesel Plant) on Degraded Sodic Land in North India. International Journal of Green Energy, 10, 1026-1040.
http://dx.doi.org/10.1080/15435075.2012.738336 - 4. Wilson, K., Maloney, K., Zulu, D., Mutamba, E. and Vermeylen, S. (2013) Can the Biofuel Crop, Jatropha curcas, Be Used as a Locally-Grown Botanical Pesticide?: A Lab and Field Study in Zambia. Proceedings of the First International Conference on Pesticidal Plants, 1, 124-127.
- 5. Silip, J.J., Tambunan, A.H., Hambali, H., Sutrisno, S. and Surahman, M. (2010) Lifecycle Duration and Maturity Heterogeneity of Jatropha curcas Linn. Journal of Sustainable Development, 3, 291.
http://dx.doi.org/10.5539/jsd.v3n2p291 - 6. Kyamuhangire, W. (2008) Perspective of Bioenergy and Jatropha in Uganda. International Consultation on Pro-Poor Jatropha Development, Casa Son Bernadu, via Lawrentina.
- 7. Beckford, R. (2009) Fundamentals of Producing Jatropha curcas. Agriculture. Natural Resources Agent University of Florida, IFAS, Lee County, 239, 533-7512.
- 8. Brittaine, R. and Lutaladio, N. (2010) Jatropha: A Smallholder Bioenergy Crop: The Potential for Pro-Poor Development, Vol. 8. Food and Agriculture Organization of the United Nations (FAO).
- 9. Nzikou, J., Matos, L., Mbemba, F., Ndangui, C., Pambou-Tobi, N., Kimbonguila, A., et al. (2009) Characteristics and Composition of Jatropha curcas Oils, Variety Congo-Brazzaville. Research Journal of Applied Sciences, Engineering and Technology, 1, 154-159.
- 10. Kumar, A. and Sharma, S. (2008) An Evaluation of Multipurpose Oil Seed Crop for Industrial Uses (Jatropha curcas L.): A Review. Industrial Crops and Products, 28, 1-10.
http://dx.doi.org/10.1016/j.indcrop.2008.01.001 - 11. Makkar, H.P. and Becker, K. (2009) Jatropha curcas, a Promising Crop for the Generation of Biodiesel and Value-Added Coproducts. European Journal of Lipid Science and Technology, 111, 773-787.
http://dx.doi.org/10.1002/ejlt.200800244 - 12. Bobade, S., Kumbhar, R. and Khyade, V. (2013) Preparation of Methyl Ester (Biodiesel) from Jatropha curcas Linn Oil. Research Journal of Agriculture and Forestry Sciences, Indore, índia, 1, 12-19.
- 13. Carels, N. (2011) The Challenge of Bioenergies: An Overview. INTECH Open Access Publisher.
- 14. Ginwal, H., Rawat, P. and Srivastava, R. (2004) Seed Source Variation in Growth Performance and Oil Yield of Jatropha curcas Linn. in Central India. Silvae Genetica, 53, 186-191.
- 15. Abdullah, B.M., Yusop, R.M., Salimon, J., Yousif, E. and Salih, N. (2013) Physical and Chemical Properties Analysis of Jatropha curcas Seed Oil for Industrial Applications. International Journal of Chemical, Nuclear, Materials and Metallurgical Engineering, 7, 531-534.
- 16. Drapcho, C.M., Nhuan, N.P. and Walker, T.H. (2008) Biofuels Engineering Process Technology. McGraw-Hill, New York.
- 17. Tiwari, A.K., Kumar, A. and Raheman, H. (2007) Biodiesel Production from Jatropha Oil (Jatropha curcas) with High Free Fatty Acids: An Optimized Process. Biomass and Bioenergy, 31, 569-575.
http://dx.doi.org/10.1016/j.biombioe.2007.03.003 - 18. Achten, W., Verchot, L., Franken, Y.J., Mathijs, E., Singh, V.P., Aerts, R., et al. (2008) Jatropha Bio-Diesel Production and Use. Biomass and Bioenergy, 32, 1063-1084.
http://dx.doi.org/10.1016/j.biombioe.2008.03.003 - 19. NEMA (2010) The Potential of Bio-Fuel in Uganda: An Assessment of Land Resources for Bio-Fuel Feedstock Suitability. National Environment Management Authority, Kampala.
- 20. Camberlin, P. (2009) Nile Basin Climates. In: Dumont, H.J., Ed., The Nile, Springer, Berlin, 307-333.
http://dx.doi.org/10.1007/978-1-4020-9726-3_16 - 21. Ronner, E. and Giller, K.E. (2013) Background Information on Agronomy, Farming Systems and Ongoing Projects on Grain Legumes in Uganda. N2Africa Milestones. Accessed, 17, 9-13.
- 22. Monaghan, A.J., MacMillan, K., Moore, S.M., Mead, P.S., Hayden, M.H. and Eisen, R.J. (2012) A Regional Climatography of West Nile, Uganda, to Support Human Plague Modeling. Journal of Applied Meteorology and Climatology, 51, 1201-1221.
http://dx.doi.org/10.1175/JAMC-D-11-0195.1 - 23. FAO/UNEP (1992) A Suggested National Soils Policy for Uganda. FAO/UNEP Project FP/6101-88-01 Advisory Services to Syria and Uganda on the Formulation of National Soils Policies. FAO, Rome.
- 24. Derkyi, N.S.A., Sekyere, D. and Oduro, K.A. (2014) Variations in Oil Content and Biodiesel Yield of Jatropha curcas from Different Agro-Ecological Zones of Ghana. International Journal of Renewable and Sustainable Energy, 3, 76.
- 25. Herrera, J.M., Ayala, A.L.M., Makkar, H., Francis, G. and Becker, K. (2010) Agroclimatic Conditions, Chemical and Nutritional Characterization of Different Provenances of Jatropha curcas L. from Mexico. European Journal of Scientific Research, 39, 396-407.
- 26. Raja, S.A., Smart, D.R. and Lee, C.L.R. (2011) Biodiesel Production from Jatropha Oil and Its Characterization. Research Journal of Chemical Sciences, 1, 81-87.
- 27. Sanghamitra, K., Oramas, R.V. and Prasad, R.N. (2014) Comparative Yield and Oil Quality of Toxic and Non-Toxic Mexican Jatropha curcas Grown in the Same Agroclimatic Conditions. American Journal of Plant Sciences, 5, 230-234.
- 28. Wang, Z., Lin, J. and Xu, Z. (2008) Oil Contents and Fatty Acid Composition in Jatropha curcas Seeds Collected from Different Regions. Journal of Southern Medical University, 28, 1045-1046.
- 29. Pant, K., Khosla, V., Kumar, D. and Gairola, S. (2006) Seed Oil Content Variation in Jatropha curcas Linn. in Different Altitudinal Ranges and Site Conditions in HP India. Lyonia, 11, 31-34.
- 30. Ovando-Medina, I., Espinosa-García, F., Núñez-Farfán, J. and Salvador-Figueroa, M. (2011) Genetic Variation in Mexican Jatropha curcas L. Estimated with Seed Oil Fatty Acids. Journal of Oleo Science, 60, 301-311.
http://dx.doi.org/10.5650/jos.60.301 - 31. Kaushik, N., Kumar, K., Kumar, S., Kaushik, N. and Roy, S. (2007) Genetic Variability and Divergence Studies in Seed Traits and Oil Content of Jatropha (Jatropha curcas L.) Accessions. Biomass and Bioenergy, 31, 497-502.
http://dx.doi.org/10.1016/j.biombioe.2007.01.021 - 32. Rathbauer, J., Sonnleitner, A., Pirot, R., Zeller, R. and Bacovsky, D. (2012) Characterization of Jatropha curcas Seeds and Oil from Mali. Biomass and Bioenergy, 47, 201-210.
http://dx.doi.org/10.1016/j.biombioe.2012.09.040 - 33. Nakpong, P. and Wootthikanokkhan, S. (2010) Optimization of Biodiesel Production from Jatropha curcas L. Oil via Alkali-Catalyzed Methanolysis. Journal of Sustainable Energy & Environment, 1, 105-109.
- 34. Barabás, I. and Todoruţ, I.-A. (2011) Biodiesel Quality, Standards and Properties. In: Montero, G. and Stoytcheva, M., Eds., Biodiesel-Quality, Emissions and By-Products, InTech Publisher, Rijeka, 3-28.
http://dx.doi.org/10.5772/25370 - 35. Jongh, J. and van der Putten, E. (2010) The Jatropha Handbook: From Cultivation to Application. FACT Foundation, Eindhoven.
- 36. Rodrigues, J., Miranda, I., Gominho, J., Vasconcelos, M., Barradas, G., Pereira, H., et al. (2013) Variability in Oil Content and Composition and Storage Stability of Seeds from Jatropha curcas L. Grown in Mozambique. Industrial Crops and Products, 50, 828-837.
http://dx.doi.org/10.1016/j.indcrop.2013.08.038 - 37. de Oliveira, J.S., Leite, P.M., de Souza, L.B., Mello, V.M., Silva, E.C., Rubim, J.C., et al. (2009) Characteristics and Composition of Jatropha gossypiifolia and Jatropha curcas L. Oils and Application for Biodiesel Production. Biomass and Bioenergy, 33, 449-453.
http://dx.doi.org/10.1016/j.biombioe.2008.08.006 - 38. Ferrari, R.A., Pighinelli, A.L.M.T. and Park, K.J. (2011) Biodiesel Production and Quality. INTECH Open Access Publisher.
- 39. Xiu, S., Shahbazi, A. and Zhang, B. (2011) Biorefinery Processes for Biomass Conversion to Liquid Fuel. INTECH Open Access Publisher.
- 40. Baquero, G., Rius, A., Esteban, B., Riba, J.-R. and Puig, R. (2011) Use of Rapeseed Straight Vegetable Oil as Fuel Produced in Small-Scale Exploitations. INTECH Open Access Publisher.
- 41. Augustus, G., Jayabalan, M. and Seiler, G. (2002) Evaluation and Bioinduction of Energy Components of Jatropha curcas. Biomass and Bioenergy, 23, 161-164.
http://dx.doi.org/10.1016/S0961-9534(02)00044-2 - 42. Prankl, H. and Wörgetter, M. (1996) Influence of the Iodine Number of Biodiesel to the Engine Performance. Proceedings of the ASAE Conference “Liquid Fuels and Industrial Products from Renewable Resources”, Nashville, 15-17 September 1996, 191-196.
- 43. Heinrich, P., Manfred, W. and Josef, R. (1999) Technical Performance of Vegetable Oil Methyl Esters with a High Iodine Number. Proceedings of the 4th Biomass Conference of the Americas, Oakland, 29 August-2 September 1999.
- 44. Linder, C.R. (2000) Adaptive Evolution of Seed Oils in Plants: Accounting for the Biogeographic Distribution of Saturated and Unsaturated Fatty Acids in Seed Oils. The American Naturalist, 156, 442-458.
http://dx.doi.org/10.1086/303399 - 45. Tremolieres, A., Dubacq, J. and Drapier, D. (1982) Unsaturated Fatty Acids in Maturing Seeds of Sunflower and Rape: Regulation by Temperature and Light Intensity. Phytochemistry, 21, 41-45.
http://dx.doi.org/10.1016/0031-9422(82)80011-3
NOTES

*Corresponding author.




