Journal of Sustainable Bioenergy Systems
Vol.04 No.04(2014), Article ID:52205,5 pages
10.4236/jsbs.2014.44022
Bacterial Lysis of Microalgal Cells
Meng Wang, Wenqiao Yuan*
Department of Biological and Agricultural Engineering, North Carolina State University, Raleigh, USA
Email: *wyuan2@ncsu.edu
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 September 2014; revised 22 October 2014; accepted 24 November 2014
ABSTRACT
This short communication reports a pioneering research of using bacteria for simultaneous algal cell disruption and cell wall/membrane utilization. Microalgae are regarded as one of the most promising feedstock that can potentially address the twin challenges of energy security and environmental protection due to their fast growth rate, high lipid content and CO2 biofixation capabilities. However, different from their terrestrial oil crops, the extracellular coverings of algae vary significantly, ranging from multiple layers of elaborate scales to highly mineralized coats to complex cell walls consisting of structural fibrils enmeshed in complex matrices. These strong cellular walls and membranes are resistant to disintegration, which makes lipid extraction from microalgae difficult. A bacteria-assisted algal cell disruption and lipid extraction method was studied here. The bacteria Sagittula stellata showed strong algicidal activity against two microalgae, Nannochloropsis oculata and Dunaliella salina. The algicidal rate reached 64.7% on N. oculata and 52.4% on D. salina in six days. A decrease in chlorophyll-a fluorescence density of both algae and bacteria addition was also observed. After 6-day treatment by S. stellata, hexane-extracted crude lipid contents increased from 32.9% to 45.7% and from 19.6% to 36.4% for N. oculata and D. salina, respectively, when compared with no bacterial addition. The preliminary results concluded that S. stellata was effective in the lysis of microalgal cells for effective lipid recovery.
Keywords:
Microalgae, Cell Lysis, Lipid Extraction

1. Introduction
In the last two decades, bacterial species that are capable of lysing algae have been identified and isolated from water bodies where harmful algal blooms have often occurred [1] [2] . These bacteria targeting specific phyto- planktons play important roles in preventing the proliferation of harmful algae in water ecosystems, therefore they have been considered as potentially important regulators of algal growth and toxin production [2] - [4] . However, to the best of our knowledge, using algicidal bacteria for algal cell lysis and lipid recovery has never been studied elsewhere. The strong cell walls and/or membranes of microalgae are resistant to disintegration, which makes cost-effective and scalable lipid extraction and cell wall/membrane utilization of algae a big challenge [5] - [7] .
To address this challenge, Sagittula stellata was studied for the purpose of microalgal cell lysis. Among various algicidal bacteria, S. stellata has been mainly studied in biosynthesis or environmental protection. For example, Boden et al. [8] found that S. stellata could be used to oxidize dimethylsulfide to dimethylsulfoxide. In addition, nanomolar concentrations (20 μg∙l−1) of hydrophobic exopolymer released by S. stellata could induce dissolved organic matters self-assembly and formation of marine microgels in seawater [9] . Another application of S. stellata was to hydrolyze cellulose and solubilize/mineralize lignin in lignin-rich and pulp mill effluent [10] . The authors reported that S. stellata could utilize a variety of monosaccharides, disaccharides and amino acids to accumulate the following major fatty acids: 16:0, 18:0, 12:l and 19:0. However, using S. stellata in microalgae cell lysis has never been reported. We hypothesized that S. stellata could lyse algal cell wall/membranes to facilitate lipid extraction. This short communication reports our efforts and preliminary results of testing this hypothesis.
2. Materials and Methods
2.1. Algal Sample Preparation
Nannochloropsis oculata (UTEX 2164) and Dunaliella salina (UTEX LB200) were obtained from the Culture Collection of Algae at the University of Texas at Austin (Austin, TX). N. oculata was grown in modified artificial seawater medium [11] . D. salina was cultivated in an artificial hypersaline medium [12] . Cultures were carried out in 1-l Erlenmeyer flasks containing 600 ml growth media at 25˚C ± 1˚C under continuous shaking (150 rpm). Light (60 - 70 µmol photons m−2・s−1) was provided by cool white fluorescent lamps with 12 h:12 h light:dark cycles.
2.2. Bacterial Seed Cultivation
The bacterium Sagittula stellata (ATCC 700073) was obtained from Dr. Mary Moran’s research group at University of Georgia. The strain was cultivated in Marine Broth 2216medium [10] . After 24 h of growth at 30˚C ± 2˚C in 250-ml Erlenmeyer flasks containing 150-ml growth media on a shaker at 150 rpm in the dark, the culture was used in the following experiments.
2.3. Algal Cell Lysis
Bacterial and algal cells in their mid-exponential growth phases were harvested by centrifugation at 2020 g for 5 min. The harvested bacterial and algae cell pellets were washed twice by salt water (19.45 g/l NaCl), and then incubated together (with final volume ratio of bacteria:algae = 1:2) in modified Marine Broth 2216 medium. The modified Marine Broth 2216 medium was prepared by excluding any peptone or yeast extract in the standard Marine Broth 2216 medium while keeping all other compounds the same. The purpose of not adding peptone and yeast extract was to remove carbon and nitrogen sources in the medium so to force the bacteria to feed on algae. Algae in the modified Marine Broth medium without bacteria addition was used as the control. All treatments and the control were cultivated with three replications under the same conditions as the bacteria seed preparation.
Algal cell number was enumerated daily by direct counting method using a bright field Epifluorescence microscopy (Nikon Eclipse microscope, Model E600) with the aid of a hemocytometer, and the algicidal rate was calculated according to the following formula:

where Nc represents the number of algae cells in the control group (without bacteria addition), and Ne re- presents the number of cells in the experimental group (with bacteria addition). The fluorescence density of the samples was measured by a Synergy Mxmonochromator-based multi-mode microplate reader (Synergy Mx, Winooski, Vermont) at the excitation wave-length of 450 nm and the emission wavelength of 680 nm. Results were analyzed by one-way ANOVA and Tukey’s tests.
2.4. Lipid Extraction
Before and after the six-day treatments, crude lipids were extracted according to the following procedures. First, 10-ml sample was filtered through a pre-dried (75˚C for five hours in an oven) and weighed (w0) glass-fiber filter paper (55 mm, nominal pore size 1.2 μm) under vacuum. The filter paper was dried again in the same oven (75˚C for five hours) and kept in a vacuum desiccator overnight before weighing (w1). Algae biomass DW (per 10 ml) was obtained by subtracting w0 from w1. Then, 112.5-ml sample in each treatment was transferred to five 50-ml centrifuge tubes (22.5 ml sample per tube). Hexane was then added to the samples to make the total volume 45-ml in each tube (hexane:sample = 1:1, V/V). The tube containing algal/bacterial cells and solvent was shaken on a reciprocating shaker (150 r/min) overnight. After that, the tube was centrifuged at 2020 g for 15 minutes to remove cell solids. The supernatant was carefully collected and evaporated and then dried in an oven at 95˚C for 1.5 h. Lipids left in the flask without solvent were weighed to calculate recoverable crude lipid content by the following equation:
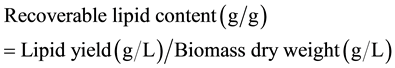
3. Results and Discussion
The effect of S. stellata treatment on algicidal rate and algal cell number can be seen from Figure 1. The cell number of both algal strains in the bacterial addition groups reduced quickly, but the control groups did not show significant changes in cell number or there were even slight cell number increases in the first 3 or 4 days. The algicidal rate reached 59.6% on N. oculata and 44.5% on D. salina in only 2 days, indicating that S. stellata was effective in the lysis of N. oculataand D. salina. In addition, it should be noted that the algicidal rate on N. oculata was always higher than that on D. salinaas shown in Figure 1, which can probably be explained by their differences in cell motility. N. oculata is non-motile while D. salina is motile [13] [14] . A static cell may be easier for an algicidal bacterium to attack [2] [15] .
When a chlorophyll-containing cell is dead or killed by predators, chlorophyll synthesis of the cell is inhibited progressively, and chlorophyll content may reduce rapidly, which results in weaker fluorescence, thus, chlorophyll-a fluorescence density (CAFD) can be considered as an indirect measure of cell viability or cell lysis [16] . As can be seen from Figure 2, larger reductions in CAFD were observed in all treatments (with bacterial additions) compared to the control. On day 1 for N. oculata, the treatment exhibited a fluorescence signal of approximately 21.0 % weaker than the control (Figure 2(a)), while on day 3 for D. salina the treatment had 11.3% weaker CAFD than the control (Figure 2(b)). A cell culture is considered to be destroyed seriously when relative fluorescence density declines more than 10% of the control [3] .

Figure 1. The algicidal rate and cell number change of N. oculata (a) and D. salina (b) in the control and experiment groups.
Because the main purpose of S. stellata treatment on algae in this study was to assist with lipid extraction, it would be interesting to see how much lipid could be extracted after the treatment. The recoverable lipid contents of N. oculata and D. salina before and after S. stellata treatment are shown in Figure 3. After 6-day treatment, crude lipid content of algae increased from 32.9% to 45.7% (of total biomass dry weight) for N. oculata (Figure 3(a)) and from 19.6% to 36.4% (of total biomass dry weight) for D. salina (Figure 3(b)). In comparison, Chiu et al. [17] concluded that lipid content of N. oculata cells ranged from 30.8% to 50.4% at different growth phases. In the case of D. salina, Weldy and Huesemann [18] found that D. salina contained cellular lipids ranging from 16% to 44% (wt). It must be noted that part of the increased crude lipids in the treated algal samples may be from S. stellata because it is known that S. stellata could also accumulate lipids. However, because we were not able to separate S. stellata from algae in lipid extraction, we cannot estimate how much lipid was actually from the bacteria and how much was from the algae.
4. Conclusions
The algicidal bacteria Sagittula stellata (E-37) was found effective in the lysis of microalgae N. oculata and D. salina for lipid recovery. The algicidal rate reached 64.7% on N. oculata and 52.4% on D. salina in six days, suggesting that cell lysis was significant and algal species dependent. Recoverable crude lipid contents of bacte-

Figure 2. Chlorophyll-a fluorescence density change of N. oculata (a) and D. salina (b).

Figure 3. Recoverable crude lipid contents of N. oculata (a) and D. salina (b).
ria treated algae also increased significantly. After 6-day treatment by S. stellata, hexane-extracted crude lipid contents increased from 32.9% to 45.7% and from 19.6% to 36.4% for N. oculata and D. salina, respectively, when compared with no bacterial addition. It was therefore concluded that S. stellata could not only lyse algal cells, but might also increase lipid recovery.
Future research should focus on understanding bacteria-algae interactions and specific mechanisms of algal cell lysis. It will also be important to understand lipid accumulation and characterization of the bacteria. Scale- up of the process and other algal species should also be considered.
Acknowledgements
This research was financially supported by the U.S. National Science Foundation (Award # CMMI-1239078) and the startup fund of North Carolina State University. We thank Dr. Mary Ann Moran and Ms. Christa Smith at University of Georgia for providing the bacteria and assisting with its cultivation.
References
- Manage, P.M., Kawabata, Z. and Nakano, S. (2000) Algicidal Effect of the Bacterium Alcaligenes denitrificanson on Microcystis spp. Aquatic Microbial Ecology, 22, 111-117. http://dx.doi.org/10.3354/ame022111
- Mayali, X. and Azam, F. (2004) Algicidal Bacteria in the Sea and Their Impact on Algal Blooms. Journal of Eukaryotic Microbiology, 51, 139-144. http://dx.doi.org/10.1111/j.1550-7408.2004.tb00538.x
- Roth, P.B., Twiner, M.J., Mikulski, C.M., Barnhorst, A.B. and Doucette, G.J. (2008) Comparative Analysis of Two Algicidal Bacteria Active against the Red Tide Dinoflagellate Karenia brevis. Harmful Algae, 7, 682-691. http://dx.doi.org/10.1016/j.hal.2008.02.002
- Bai, S.J., Huang, L.P., Su, J.Q., Tian, Y. and Zheng, T.L. (2011) Algicidal Effects of a Novel Marine Actinomycete on the Toxic Dinoflagellate Alexandrium tamarense. Current Microbiology, 62, 1774-1781. http://dx.doi.org/10.1007/s00284-011-9927-z
- Adam, F., Abert-Vian, M., Peltier, G. and Chemat, F. (2012) “Solvent-Free” Ultrasound Assisted Extraction of Lipids from Fresh Microalgae Cells: A Green, Clean and Scalable Process. Bioresource Technology, 114, 457-465. http://dx.doi.org/10.1016/j.biortech.2012.02.096
- Lam, M.K. and Lee, K.T. (2012) Microalgae Biofuels: A Critical Review of Issues, Problems and the Way Forward. Biotechnology Advances, 30, 673-690. http://dx.doi.org/10.1016/j.biotechadv.2011.11.008
- Araujo, G.S., Matos, L.J.B.L., Fernandes, J.O., Cartaxo, S.J.M., Gonçalves, L.R.B., Fernandes, F.A.N. and Farias, W.R.L. (2013) Extraction of Lipids from Microalgae by Ultrasound Application: Prospection of the Optimal Extraction Method. Ultrasonics Sonochemistry, 20, 95-98. http://dx.doi.org/10.1016/j.ultsonch.2012.07.027
- Boden, R., Murrel, J.C. and Schäfer, H. (2011) Dimethylsulfide Is an Energy Source for the heterotrophic Marine Bacterium Sagittula stellata. FEMS Microbiology Letters, 322, 188-193. http://dx.doi.org/10.1111/j.1574-6968.2011.02349.x
- Ding, Y.X., Chin, W.C., Rodriguez, A., Hung, C.C., Santschi, P.H. and Verdugo, P. (2008) Amphiphilic Exopolymers from Sagittula stellata Induce DOM Self-Assembly and Formation of Marine Microgels. Marine Chemistry, 112, 11- 19. http://dx.doi.org/10.1016/j.marchem.2008.05.003
- González, J.M., Mayer, F., Moran, M.A., Hodson, R.E. and Whitman, W.B. (1997) Sagittula stellata gen. nov., sp. nov., a Lignin-Transforming Bacterium from a Coastal Environment. International Journal of Systematic and Evolutionary Microbiology, 47, 773-780. http://dx.doi.org/10.1099/00207713-47-3-773
- Shen, Y., Pei, Z.J., Yuan, W.Q. and Mao, E.R. (2009) Effect of Nitrogen and Extraction Method on Algae Lipid Yield. International Journal of Agricultural and Biological Engineering, 2, 51-57.
- Azachi, M., Sadka, A., Fisher, M., Goldshlag, P., Gokhman, I. and Zamir, A. (2002) Salt Induction of Fatty Acid Elongase and Membrane Lipid Modifications in the Extreme Halotolerant Alga Dunaliella salina. Plant Physiology, 129, 1320-1329. http://dx.doi.org/10.1104/pp.001909
- Converti, A., Casazza, A.A., Ortiz, E.Y., Perego, P. and Borghi, M.D. (2009) Effect of Temperature and Nitrogen Concentration on the Growth and Lipid Content of Nannochloropsis oculata and Chlorella vulgaris for Biodiesel Production. Chemical Engineering and Processing, 48, 1146-1151. http://dx.doi.org/10.1016/j.cep.2009.03.006
- Leach, G., Oliveira, G. and Morais, R. (1998) Spray-Drying of Dunaliella salina to Produce a β-Carotene Rich Powder. Journal of Industrial Microbiology and Biotechnology, 20, 82-85. http://dx.doi.org/10.1038/sj.jim.2900485
- Imai, I. (1997) Algicidal Ranges in Killer Bacteria of Direct Attack Type for Marine Phytoplankton. Bulletin of the Plankton Society of Japan, 44, 3-9.
- Wang, M., Yuan, W., Jiang, X., Jing, Y. and Wang, Z. (2014) Disruption of Microalgal Cells Using High-Frequency Focused Ultrasound. Bioresource Technology, 153, 315-321. http://dx.doi.org/10.1016/j.biortech.2013.11.054
- Chiu, S.Y., Kao, C.Y., Tsai, M.T., Onga, S.C., Chenb, C.H. and Lin, C.S. (2009) Lipid Accumulation and CO2 Utiliza- tion of Nannochloropsis oculata in Response to CO2 Aeration. Bioresource Technology, 100, 833-838. http://dx.doi.org/10.1016/j.biortech.2008.06.061
- Weldy, C.S. and Huesemann, M. (2007) Lipid Production by Dunaliella salina in Batch Culture: Effects of Nitrogen Limitation and Light Intensity. US DOE Journal of Undergraduate Research, 7, 115-122.
NOTES
*Corresponding author.





