American Journal of Analytical Chemistry
Vol.08 No.04(2017), Article ID:75799,14 pages
10.4236/ajac.2017.84021
Co-Combustion Characteristics and Kinetics of Cotton Stalk and Polypropylene Blends
Yeliz Durak Çetin1, Tülay Durusoy2
1TÜBİTAK, Energy Institute, Marmara Research Center, Kocaeli, Turkey
2Chemical Engineering Department, Hacettepe University, Ankara, Turkey

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 17, 2016; Accepted: April 27, 2017; Published: April 30, 2017
ABSTRACT
The combustion kinetics of biomass-cotton stalk (CS), polymer-polypro-py- lene (PP) and blend of polymer/biomass-polypropylene/cotton stalk blends were examined through thermo gravimetric analysis in this study. The experiments were performed under non-isothermal conditions in the 298 - 873 K temperature interval. The heating rate of this research realized under the air atmosphere was designated as 5 K・min−1. The particle size effect on the com- bustion behavior of cotton stalk was also studied. A decrease in the maximum rate of decomposition and an increase in the temperature of maximum decomposition with increasing particle size were obtained. Three different mo- dels based on the Arrhenius method were used to analyze differential thermo gravimetric data. Blending ratio effects of biomass-cotton stalk and polymer- polypropylene on the combustion kinetics were further explored. Additionally, factors and kinetic parameters were also discussed. Activation energies obtained through the Arrhenius method (n = 1) were much lower than that of polypropylene for all blends. As a result of the research, as the weight percentage of polypropylene in the mixture rises, an increase in activation energy values was observed. The minimum value of the activation energy was cal- culated with PP/CS with 2/3 blending ratio as 35.8 kJ·mol−1.
Keywords:
Cotton Stalk. Polypropylene, Combustion, Thermogravimetry

1. Introduction
Processing of biomass for energy production is presently being considered as an alternative means of reducing CO2 emission and replacement of fossil fuels. Cot- ton is one of the Turkey’s most important agricultural crops, since Turkey is one of the main producer countries of world’s cotton. Cotton universally used as a raw textile material. In addition cottonseed is an important source of vegetable oil.
Cotton straw and stalk pyrolysis under different conditions was investigated by Pütün [1] . The highest yield 39.51% was obtained at 550˚C final temperature. Pütün et al. [2] have studied pyrolysis of cotton stalk for determining the main characteristics and quantities of liquid and solid products. Six inorganic com- pounds have been investigated by Jun et al. [3] with regard to their catalytic effects on pyrolysis of three biomass species by thermal analysis experiments.
Co-combustion of coal and non-recyclable paper and plastic waste in a flui- dised bed reactor was investigated by Boavida et al. [4] . Results obtained indicate that the feeling of waste materials plays an important role to achieve conditions for a stable combustion. Excess air and temperature are found to be important parameters. Halogen emissions are found to be very low.
The possibility of collecting cotton stalks and using them for energy pro- duction was investigated by Gemtos and Tsiricoglou [5] . It is proved that the feasibility of harvesting cotton stalks using conventional machinery giving the possibility to collect energy material with a total energy content of 500,000 tons of oil equivalent at national level. A generalized pyrolysis model for the oxidative pyrolysis of wood was investigated by Lautenberger and Fernandez-Pello [6] . It was found that the specific heat capacity for wet wood, dry wood and char falls between literature values for generic wood and specific data for white pine.
Thermogravimetric analysis (TGA) is one of the most commonly used tech- niques to study the primary reactions of decomposition of solids. Oxidation of carbonaceous solid fuel is dependent on the concentration of oxygen. [7] [8] [9] . The thermal decomposition kinetics of biomass samples were investigated by several researchers [10] [11] [12] [13] .
At the present, plastics compose approximately 10% of the total waste stream in terms of mass. One of the methods to overcome this type of waste that is not biologically degradable is thermal decomposition. Yields of this process which can all be used for various purposes are not only energy but also gaseous and liquid products [14] [15] [16] .
There has been a recent and growing interest in polymer combustion with materials such as coal, oil shale, biomass and peat [17] [18] [19] [20] [21] . Com- bined combustion realized with a mix of polymers and biomass can enhance the efficiency of the processing. This method can further ensure an alternative so- lution to handle the waste problem.
The effect of lignin on the thermal degradation of isotactic polypropylene was studied by Canetti et al. [22] . An increase in the thermal degradation tempera- ture of the blends was observed as a function of lignin content. Maldhure et al. [23] examined the influence of lignin in different amount on the thermal degra- dation behavior of polypropylene/lignin blends in nonoxidative atmospheres [23] .
In this research, the effect of particle size on the combustion behavior of cotton stalk and combustion behavior of polypropylene (PP)/cotton stalk (CS) mixtures were studied by using a thermo gravimetric analyzer. Within this con- text, the study aims to obtain an overall understanding on cotton stalk and polypropylene interaction. As a consequence, the kinetic data were obtained to fit thermo gravimetric data with the help of three different models.
2. Experimental Section
2.1. Properties of Cotton Stalk
The cotton stalk studied for this study was taken from Şanlıurfa located in the South-Eastern Anatolia. The ash content of cotton stalk and elemental analysis are shown in Table 1. Original cotton stalk dried at 106˚C was ground in a ball mill and sieved to four different particle size ranges:(+88-100), (+210-250), (+250-420), (+420-500) µm. Afterwards, carbon, hydrogen, sulphur and nitrogen contents were identified with the help of LECOCHNS-932 instrument. Following this stage, the amount of oxygen was specified from the difference. The resulting data obtained are given in Table 1. Before, during and after the experiment, samples were stored within sealed containers in room temperature.
2.2. Properties of Polypropylene
Polypropylene was used for the combined combustion with cotton stalk. The melting point of (444 K) polypropylene was determined by using differential calorimetry apparatus (“Perkin Elmer Diamond DSC”). The resulting thermo- gram is shown in Figure 1.
Table 1. Ultimate analysis of the cotton stalk.
Figure 1. The melting point of polypropylene.
2.3. Experimental Methods
Experiments were done by using a Setaram TG DTA92 thermobalance in which the sample mass loss (thermogravimetric (TG) signal) and rate of mass loss (DTG) signal as functions of time or temperature were recorded continuously under dynamic conditions. The combustion reactions were carried out under an air atmosphere. In order to keep the effect of mass transfer at minimum, an air flow rate of 42 mL・min?1 was used. Other conditions provided throughout the experiment are as follows: interval of the combustion temperature was fixed at 298 - 873 K and the heating rate was selected as 5 K·min?1. Blending ratios of polypropylene to (+88-100) µm particle size cotton stalk of 0/1, 2/3, 1/2, and 3/2, 1/0 were performed by weight ratio.
Starting sample weight was approximately 20 mg during the experiments. Changes of maximum devolatilization rate (DTGm) and the temperature of maximum devolatilization rate (Tm) were found from the data. Total conversion (TC) values were calculated based on the weight of the moisture-free sample combusted.Experiments were performed twice for repeatability. The results given in Table 2 are the average of at least two experimental runs.
3. Results and Discussions
3.1. Combustion of Cotton Stalk
In order to identify the combustion kinetics of cotton stalk, thermo gravimetric analysis was used. The effect of particle size is showed by TG and DTG data obtained from the experiments performed with (+88 - 100), (+210 - 250), (+250 - 420), (+420 - 500) µm particle size Figure 2 and Figure 3 respectively. There are three temperature ranges to be considered for all particle sizes. Below 520 K, the cotton stalk loses moisture and the crystalline structured components of H2O decomposes. Hemicellulose contains different kinds of saccharides. It is rich of branches which are not difficult to remove from the main stem as well as to degrade to volatiles evolves at low-temperatures [24] . Hemicellolose, on the
Table 2. The variation in DTGm, Tm, and TC values relative to the polypropylene (PP)/ cotton stalk (CS) blending ratios.
Figure 2. TG curves of different particle sizes of cotton stalk.
Figure 3. DTG curves of different particle sizes of cotton stalk.
other hand, started its decomposition easily, by with the experienced weight lossparticularly realized between the temperatures: 520 - 620 K. This interval is known as the “Primary Combustion Region”. In contrast to hemicellulose, the thermal stability value of cellulose is much more higher [24] . In depth, cellulose degradation was happened at a higher temperature range, within the interval of 620 - 760 K (“Secondary Combustion Region”). It should be underlined that when the rate of temperature was higher than 760 K, almost all cellulose were degraded by leaving a solid residue in very small quantities behind. The degra- dation of lignin, on the other hand, occurs in 373 - 873 K which means a much wider temperature range compared with other materials experimented.In order to exemplify, the relative mass loss and corresponding combustion rate curves of the (+88-100) m cotton stalk particles are demonstrate in Figure 4.
In addition, Figure 5 and Figure 6 represent the maximum rate of decomposition (DTGm) and the temperature of maximum decomposition (Tm) for all particle size ranges in the primary combustion region, respectively. A decrease in the maximum rate of decomposition and an increase in the temperature of maximum decomposition with increasing particle sizes were obtained. Considering the difference between the initial and final weight of the sample after the combustion, the values of total conversion (TC) were identified with respect to the weight of the moisture free solid fed to the system. These values were also shown in Figure 7 for in four particle size ranges. It is observed that total conversion values exhibit a peak with increasing cotton stalk particle size. The reason for this peak is that the mass transfer limitation for the degradation of small cotton stalk particles is not as effective as they are for larger particles.
Figure 4. TG and DTG curves of+88-100 μm cotton stalk particle size.
Figure 5. Change of maximum devolatilization rate with particle sizes of cotton stalk.
Figure 6.Change of temperature of the maximum devolatilization rate with particle sizes of cotton stalk.
Figure 7. Change of total conversion percentage with particle sizes of cotton stalk.
3.2. Combustion of Polypropylene
TG and DTG curves of the process of polypropylenecombustion are shown in Figure 8. As identified from the figure, likewise the cotton stalk, polypropylene degrades in a double-staged process (520 - 660 K and 660 - 760 K). The DTGm peak, Tm values, and the total conversion, TC, values for polypropylene com- bustion for primary and secondary combustion sections are presented in Table 2.
3.3. Combustion of Blends
Blends of (+88 - 100) µm particle size cotton stalk with polypropylene were analyzed under the same conditions of combustion. 2/3, 1/2, and 3/2 blending ratios of polypropylene to cotton stalk were implemented by weight ratio. The TG curves of the blends can be followed from the Figure 9. The curves of the components as well as the mixture were compared to study the catalytic effect of polypropylene on the degradation process of cotton stalk,. The DTGm peak, Tm and TC values for all blends are also shown in Table 2.
As it can be followed from the Figure 9, the resulting data obtained from the study presents that polypropylene-cotton stalk blends degrade in a double-sta- ged process as cotton stalk and polypropylene. When blended, degradation of polymer and cotton stalk is compensated. Compensation effect emerges where thermal degradation is complex and occurs in two parallel competitive reactions, compensation effect emerges [25] [26] . For the case of the mixtures, the radicals
Figure 8. TG and DTG curves of polypropylene.
Figure 9. TG curves of blends with different blending ratios of polypropylene to cotton stalk.
are believed to form during the decomposition stage of polymer to its monomers. These radicals further react with the organic content of cotton stalk. These findings mediate for the acceleration of the degradation. Eventually, the quantity of organic content, which can react with polymer in the mixture, decreases because of the decay of the amount of cotton stalk. During the process, the sample mass remained approximately constant below 520 K. Above 520 K, the mass started to decrease sharply and this tendancy continued for 520 - 620 K interval for all blending ratios of cotton stalk. As shown in Table 2, an increase in the value of DTGm with increasing percentage of polypropylene in the blends was examined, when a blend in any ratio of polypropylene to cotton stalk was reacted. The DTGm value of polypropylene is higher than that of cotton stalk. For the case of all blend ratios, an increase in the values of maximum peak temperature with polypropylene addition was identified for the primary com- bustion region. It was determined that blends have lower Tm values compared to polypropylene (Table 2). The lignin present in the blend is able to produce a high char yield that is responsible for the increase in the blend degradation temperature. The increase is more pronounced for the experiments carried out in air atmosphere, where, the interactions between the polypropylene and the charring lignin lead to the formation of a protective surface shield to reduce the oxygen diffusion towards the bulk polymer [22] . As a result under the experimental conditions, the reaction rate is believed to be controlled by gas diffusion. Also an increase in the TC values with increasing mass amount of polypropylene of the mixtures was examined. Maximum total conversion value was obtained with the combustion of 3/2 polypropylene/cotton stalk blending ratio as 97%.
4. Kinetic Analysis
Depending on the presence of numerous components as well as their parallel and consecutive reactions, non-isothermal kinetic study of mass loss under combustion processes is quite complex for cotton stalk. Within the scope of the research, three different models all based on “Arrhenius Kinetic Theory”; “Arrhenius”, “Arrhenius (n = 1)” and “Coats & Redfern”, were used for kinetic analysis of the data generated by the TG experiments. Combustion of cotton stalk and blends resulted in two main reaction regions, namely primary and secondary combustion. Since the highlighted regions are completely different from each other, “Arrhenius Kinetic Model” was adopted to the primary com- bustion region for calculating the energies of activation. For the application of Arrhenius Kinetics, at first, the temperature values were determined from the starting to the ending points of the peaks of maximum mass loss. This deter- mination was made for each experiment and first volatilization region in the DTG curves. Following the experiment, a computer program was used to evaluate the model and kinetic parameters identified.
4.1. Arrhenius Model
The expression of the “Arrhenius Model” was simply done with the help of the Equation (1):
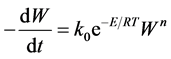 (1)
(1)
Here;
W:the mass of cotton stalk at any time in mg,
k0: the frequency factor in mg(1?n)·min?1,
E: the apparent activation energy in kJ·mol?1,
T: the absolute temperature in K,
R: the gas constant,
n: the total order of the combustion process, and
t: the time in minutes.
The logarithmic representation of Equation (1) is as follows:
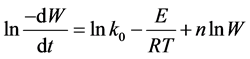 (2)
(2)
In order to determine the combustion kinetics of cotton stalk, polypropylene and polypropylene/cotton stalk blends, Equation (2) was applied to the mean of the measured TG and DTG data. Kinetic constants were determined by multiple regression analyses. These are given in Table 3.
4.2. Arrhenius Model (n = 1)
With regard to this model, it can easily be assumed that when the reaction order is assumed to be one (n = 1), the weight loss rate of the total sample depends only on the rate constant, the weight of the remaining sample and the tempe- rature (Equation (3)).
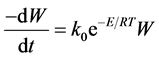 (3)
(3)
Table 3. Kinetic Parameters of combustion.
aArrhenius Method (n = 1), bArrhenius Method, cCoats and Redfern Method.
The logarithmic expression of Equation (3) yields:
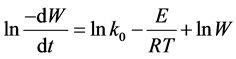 (4)
(4)
Kinetic constants and determination coefficients were determined by multiple regression analyses and are given in Table 3.
4.3. Coats & Redfern Model
Coats&Redfern [27] developed an integral method, which can be applied to TG data, assuming the order of reactions. The correct order is assumed to lead to the best linear plot, from which the activation energy is determined. The form of the equation, which is used for analysis, is
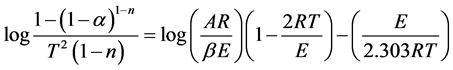 (5)
(5)
where α is the mass fraction and b is the linear heating rate. Thus a plot of, log k versus 1/T where
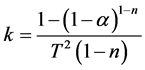 (6)
(6)
should result in a straight line of slope that equals ?E/2.303R for the corrected value of reaction order. In this study, reaction order was taken to be between 0.5 and 2. Determination coefficients of each case was calculated and listed in Table 3. Kinetic graph of polypropylene was given to explain the kinetic behavior, as an example (Figure 10).
Results showed that the values of the kinetic parameters changed with the particle size of cotton stalk and blending ratios of cotton stalk to polypropylene. A decrease was found in the activation energy values with the increasing cotton stalk particle size through both Arrhenius methods. Obtained activation energies through Arrhenius method (n = 1) were much lower than that of polypropylene
Figure 10. Coats and Redfern method result of polypropylene.
Table 4. Comparison of the activation energy and the reaction order determined in this study with those of the literature.
for all blends. An increase was observed in the activation energy values as the weight percentage of polypropylene in blends was increased. The minimum activation energy, 35.8 kJ・mol−1, was calculated with polypropylene/cotton stalk 2/3 mixing ratio. Calculated apparent activation energies by assuming the rea- ction order “1” in Arrhenius model were smaller than the valueswhich were calculated with no reaction order assumption.
The highest activation energy through Arrhenius method obtained for cotton stalk was 81.2 kJ・mol−1. Besides activation energy, the frequency factor is also important in determining the combustion behavior. The same order of magnitude frequency factor values were obtained for all particle sizes with Arrhenius models. The obtained frequency factors of the mixtures were lower than that of polypropylene but higher than that of cotton stalk for both Arrhenius models. As a result of kinetic effects, the radicals formed during decomposition of the polypropylene react with the organic matter of the cotton stalk in the mixture and accelerate its degradation. An increase was observed in the frequency factor values as the weight percentage of polypropylene in blends was increased. Through the kinetic analysis it was observed that higher activation energies were obtained by the Coats and Redfern Method with polypropylene and all blends (Table 3). In addition, in terms of Coats and Redfern method higher activation energy values were obtained for all blends with respect to cotton stalk and propylene. Determination Coefficient of each reaction was calculated and the highest coefficient of determination was found to be 2 (reaction order) for all blends. The obtained reaction orders were almost the same for cotton stalk, polypropylene and all blends through Arrhenius and Coats and Redfern method. From the calculations done within each three models, it was seen that the increase of the cotton stalk ratio in blends was caused the decrease of the activation energies.
In most of the previous studies, the order of reaction was presumed and then activation energy and frequency factors were found subsequently. However, in the present study the order of reaction was determined by the proposed kinetic model. Due to the difference of the biomass and polymer, it is difficult to compare results of this study directly with those of the others. The minimum activation energy value obtained from the proposed reaction kinetics appears to be the lowest one when compared to those obtained from the literature (Table 4). However, it should be noted that the subject studies were not conducted under identical conditions.
5. Conclusions
The effect of particle size on the combustion behavior of cotton stalk and com- bustion behavior of polypropylene and polypropylene/cotton stalk blends were analyzed under air atmosphere by using the thermogravimetric analyzer. A maximum was observed in the total conversion values with increasing cotton stalk particle size. Polypropylene/cotton stalk blends decompose in a double- stage process as observed for cotton stalk and polypropylene. An increase in maximum decomposition rate value with increasing percentage of polypro- pylene in the blends was observed. It is concluded that polypropylene accelerates the combustion reaction of organic matter in the cotton stalk. Maximum total conversion value was observed with the combustion of 3/2 polypropylene/cot- ton stalk blending ratio as 97%.
In the present study, Arrhenius, Arrhenius (n = 1) and Coats and Redfern methods, all based on Arrhenius kinetic theory, were used for kinetic analysis of the data yielded by the TG experiments. It was seen an increase of the cotton stalk ratio in blends was resulted in a decrease on activation energies. The mini- mum activation energy, 35.8 kJ・mol−1, was calculated with polypropylene/cotton stalk 2/3 blending ratio through Arrhenius method by assuming the reaction order “1”. The calculated frequency factors of the mixtures were lower than that of polypropylene but higher than that of cotton stalk for both Arrhenius models. The obtained reaction orders were almost the same for all blends, cotton stalk and polypropylene through Arrhenius and Coats and Redfern method.
Cite this paper
Çetin, Y.D. and Durusoy, T. (2017) Co-Combustion Characteristics and Kinetics of Cotton Stalk and Polypropylene Blends. American Jour- nal of Analytical Chemistry, 8, 280-293. https://doi.org/10.4236/ajac.2017.84021
References
- 1. Pütün, A.E. (2002) Biomass to Bio-Oil via Fast Pyrolysis of Cotton Straw and Stalk. Energy Sources, 24, 275-285. https://doi.org/10.1080/009083102317243656
- 2. Pütün, A.E., Ozbay, N., Onal, E.P. and Pütün, E. (2005) Fixed-Bed Pyrolysis of Cotton Stalk for Liquid and Solid Products. Fuel Processing Technology, 86, 1207-1219.
https://doi.org/10.1016/j.fuproc.2004.12.006 - 3. Jun, W., Ming, X.Z., Ming, Q.C., Fan, F.M., Su, P.Z., Zheng, W.R. and Yong, J.Y. (2006) Catalytic Effects of Six Inorganic Compounds on Pyrolysis of Three Kinds of Biomass. Thermochimica Acta, 444, 110-114.
https://doi.org/10.1016/j.tca.2006.02.007 - 4. Boavida, D., Abelha, P., Gulyurtlu, I. and Cabrita, I. (2003) Co-Combustion of coal and Non-Recyclable Paper and Plastic Waste in a Fluidised Bed Reactor. Fuel, 82, 1931-1938.
https://doi.org/10.1016/S0016-2361(03)00151-0 - 5. Gemtos, T.A. and Tsiricoglou, T. (1999) Harvesting of Cotton Residue for Energy Production. Biomass and Bioenergy, 16, 51-59.
- 6. Lautenberger, C. and Fernandez-Pello, C. (2009) A Model for the Oxidative Pyrolysis of Wood. Combustion and Flame, 156, 1503-1513.
- 7. Liu, H., Zailani, R. and Gibbs, B.M. (2005) Comparisons of Pulverized Coal Combustion in Air and in Mixtures of O2/CO2. Fuel, 84, 833-840.
- 8. Tan, Y., Croiset, E., Douglas, M.A. and Thambimuthu, K.V. (2006) Combustion Characteristics of Coal in a Mixture of Oxygen and Recycled Flue Gas. Fuel, 85, 507-512.
https://doi.org/10.1016/j.fuel.2005.08.010 - 9. Borrego, A.G. and Alvarez, D. (2007) Comparison of Chars Obtained under Oxy-Fuel and Conventional Pulverized Coal Combustion Atmospheres. Energy and Fuels, 21, 3171-3179.
https://doi.org/10.1021/ef700353n - 10. Faravelli, T., Frassoldati, A., Migliavacca, G. and Ranzi, E. (2010) Detailed Kinetic Modeling of the Thermal Degradationof Lignins. Biomass and Bioenergy, 34, 290-301.
https://doi.org/10.1016/j.biombioe.2009.10.018 - 11. Jauhiainen, J.J., Conesa, J.A., Font, R. and Martin-Gullon, I. (2004) Kinetics of the Pyrolysis and Combustion of Olive Oil Solid Waste. Journal of Analytical and Applied Pyrolysis, 72, 9-15.
- 12. Daood, S.S., Munir, S., Nimmo, W. and Gibss, B.M. (2010) Char Oxidation Study of Sugar Cane Bagasse, Cotton Stalkand Pakistani Coal under 1% and 3% Oxygen Concentrations. Biomass and Bioenergy, 34, 263-271.
https://doi.org/10.1016/j.biombioe.2009.10.014 - 13. Shuang, N.X., Zhi, H.L., Bao, M.L, Wei, M. Y. and Xue, Y.B. (2006) Devolatilization Characteristics of Biomass at Flash Heating Rate. Fuel, 85, 664-670.
https://doi.org/10.1016/j.fuel.2005.08.044 - 14. Conesa, J.A. and Marcilla, A. (1996) Kinetic Study of the Thermogravimetric Behavior of Different Rubbers. Journal of Analytical and Applied Pyrolysis, 37, 95-110.
https://doi.org/10.1016/0165-2370(96)00942-4 - 15. Chen, F. and Qian, J. (2000) Studies on the Thermal Degradation of Polybutadiene. Fuel Processing Technology, 67, 53-60.
- 16. Manowski, A. and Siudyga, T. (2003) Thermal Analysis of Polyolefin and Liquid Paraffin Mixtures. Journal of Thermal Analysis and Calorimetry, 74, 623-630.
- 17. Zhou, L., Luo, T. and Huang, Q. (2009) Co-Pyrolysis Characteristics and Kinetics of Coal and Plastic Blends. Energy Conversion and Management, 50, 705-710.
https://doi.org/10.1016/j.enconman.2008.10.007 - 18. Aboulkas, A., El Harfi, K. and El Bouadili, A. (2008) Non-Isothermal Kinetic Studies on Co-Processing of Olive Residue and Polypropylene. Energy Conversion and Management, 49, 3666-3671.
- 19. Sharypov, V.I., Beregovtsova, N.G., Kuznetsov, B.N., Membrado, L., Cebolla, V.L., Marin, N. and Weber, J.V. (2003) Co-Pyrolysis of Wood Biomass and Synthetic Polymers Mixtures. Part III: Characterisation of Heavy Products. Journal of Analytical and Applied Pyrolysis, 67, 325-340.
- 20. Degirmenci, L. and Durusoy, T. (2005) Thermal Degradation Kinetics of Goynük Oil Shale with Polystyrene. Journal of Thermal Analysis and Calorimetry, 79, 663-668.
https://doi.org/10.1007/s10973-005-0593-x - 21. Yagmur, S. and Durusoy, T. (2009) Kinetics of Combustion of Oil Shale with Polystyrene. Journal of Thermal Analysis and Calorimetry, 96, 189-194.
- 22. Canetti, M., Bertini, F., Chirico, A.D. and Audisio, G. (2006) Thermal Degradation Behaviour of Isotactic Polypropylene Blended with Lignin. Polymer Degradation and Stability, 91, 494-498. https://doi.org/10.1016/j.polymdegradstab.2005.01.052
- 23. Maldhure, A.V., Chaudhari, A.R. and Ekhe, J.D. (2011) Thermal and Structural Studies of Polypropylene Blended with Esterified Industrial Waste Lignin. Journal of Thermal Analysis and Calorimetry, 103, 625-632.
- 24. Yang, H., Yan, R., Chen, H., Lee, D.H. and Zheng, C. (2007) Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel, 86, 1781-1788.
- 25. Budrugeac, P. (1997) On the Pseudo Compensation Effect Due to the Complexity of the Mechanism of Thermal Degradation of Polymeric Materials. Polymer Degradation and Stability, 58, 69-76. https://doi.org/10.1016/S0141-3910(97)00019-0
- 26. Budrugeac, P. (2000) On the Evaluation of the Thermal Lifetime of Polymeric Materials Which Exhibit a Complex Mechanism of Thermal Degradation Consisting of Two Successive Reactions. Polymer Degradation and Stability, 67, 271-278.
https://doi.org/10.1016/S0141-3910(99)00125-1 - 27. Coats, A.W. and Redfern, J.P. (1964) Kinetic Parameters from Thermogravimetric Data. Nature, 201, 68-69.
https://doi.org/10.1038/201068a0 - 28. Constante, A. and Pillay, S. (2017) Algae Fiber Polypropylene Composites: Modeling of the Degradation by Solid State Kinetics. Journal of Applied Polymer Science, 134, 44-62.
- 29. Cepeliogullar, O. and Pütün, A.E. (2013) Thermal and Kinetic Behaviors of Biomass and Plastic Wastes in Co-Pyrolysis. Energy Conversion and Management, 75, 263-270.
https://doi.org/10.1016/j.enconman.2013.06.036 - 30. Munir, S., Daood, S.S., Nimmo, W., Cunliffe, A.M. and Gibbs, B.M. (2009) Thermal Analysis and Devolatilization Kinetics of Cotton Stalk, Sugar Cane Bagasse and Shea Meal under Nitrogen and Air Atmospheres. Bioresource Technology, 100, 1413-1418.
- 31. Corradini, E., Teixeira, E.M., Paladin, P.D., Agnelli, J.A., Silva, O.R.R.F. and Mattoso, L.H.C. (2009) Thermal Stability and Degradation Kinetic Study of White and Colored Cotton Fibers by Thermogravimetric Analysis. Journal of Thermal Analysis and Calorimetry, 97, 415-419.
https://doi.org/10.1007/s10973-008-9693-8 - 32. Dahiya, J.B. and Kumar, K. (2009) Kinetic of Thermal Degradation of Intumescent Coated Cotton Fabric. Asian Journal of Chemistry, 21, 7254-7260.
- 33. Wang, X., Hu, M., Hu, W., Chen, Z., Liu, S., Hu, Z. and Xiao, B. (2016) Thermogravimetric Kinetic Study of Agricultural Residue Biomass Pyrolysis Based on Combined Kinetics. Bioresource Technology, 219, 510-520.
https://doi.org/10.1016/j.biortech.2016.07.136 - 34. Bhavanam, A. and Sastry, R.C. (2015) Kinetic Study of Solid Waste Pyrolysis Using Distributed Activation Energy Model. Bioresource Technology, 178, 126-131.
https://doi.org/10.1016/j.biortech.2014.10.028












