American Journal of Analytical Chemistry
Vol.4 No.3(2013), Article ID:29052,7 pages DOI:10.4236/ajac.2013.43018
Synthesis, Spectral and Electrochemical Studies of Complex of Uranium(IV) with Pyridine-3-Carboxylic Acid
1Department of Chemistry, Federal Urdu University of Arts, Science and Technology, Gulshan Campus, Karachi, Pakistan
2National Core Group in Chemistry, H.E.J Research Institute of Chemistry, University of Karachi, Karachi, Pakistan
Email: *nazir_misbah@yahoo.com
Received February 4, 2013; revised March 5, 2013; accepted March 17, 2013
Keywords: Uranium(IV); Pyridine-3-Carboxylic Acid; Spectroscopic Techniques; Magnetic Susceptibility; Electrochemical Studies
ABSTRACT
The investigation of complexation of uranium with biological active ligands is vital for understanding uranium speciation in biosystems. A number of studies have been undertaken for investigating the complexation of uranium in its (VI) oxidation states but similar investigations pertaining to the interaction of uranium, in lower oxidation states, with biological ligands is scarce. The aim of the work is to bridge this gap and studies have been carried out to determine the coordination pattern of pyridine-3-carboxylic acid with uranium(IV). Semi-micro analysis, spectro-analytical techniques, magnetic susceptibility and cyclic voltammetry have been employed for the characterization of the synthesized complex.
1. Introduction
The presence of uranium in the crust of the earth is about 2 parts per million and among the elements, ranks about 48th in natural abundance in crust’s rocks. It is also present in seawater, though in very small concentrations of about 3.3 ng/mL, but distributed uniformly all over the world [1,2]. We cannot ignore the importance of research on behavior of this metal in biosystems. A score of publications have been surfaced on the complexation tendencies of uranium(VI) with different biological ligands [3, 4]. The aim of this work is to examine the interaction of uranium, in its (IV) oxidation state, with a ligand of biological significance i.e. pyridine-3-carboxylic acid (Figure 1). Pyridine-3-carboxylic acid (also known as Nicotinic acid) is an essential vitamin. It possesses two potential ligating groups; pyridine ring nitrogen and carboxyl oxygen. But when it acts as a monodentate ligand, bonding may occur through either of these sites [5]. Different techniques have been employed to characterize the newly synthesized complex of uranium(IV) with pyridine-3-carboxylic acid.
2. Experimental
2.1. Materials and Method
Uranium(VI) stock solution, prepared by dissolving ura-
Figure 1. Pyridine 3-carboxylic acid.
nyl nitrate hexahydrate, (B.D.H) (1.255 g, 2.5 mmol) in 0.2 M HCl (25 ml), was reduced to uranium(IV) by catalytic hydrogenation, in the presence of platinized alumina (Aldrich) as a catalyst [6]. The complex was prepared by mixing pyridine-3-carboxylic acid (0.6155 g, 5 mmol) to that of metal ion solution whose concentration was predetermined. Small volume of NaOH (1 M) solution was then added to the reacting mixture to adjust the pH between 4.5 and 5. It is the pH range where precipitation of the desired complex occurs. The precipitates of the complex thus formed were filtered through whatmann 542 filter paper and washed with deionized water and 99% absolute alcohol. The residue was dried over silica gel.
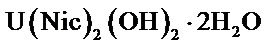 mol.wt. 552.22 g/mol. Found: C26.09; H, 2.48; N, 5.55; U, 42.99; Anal. Calcd: C, 26.07; H, 2.53; N, 5.07; U, 43.09, Yield (75.39%).
mol.wt. 552.22 g/mol. Found: C26.09; H, 2.48; N, 5.55; U, 42.99; Anal. Calcd: C, 26.07; H, 2.53; N, 5.07; U, 43.09, Yield (75.39%).
2.2. Characterization
The complex was analyzed for its uranium(IV), C, H, N and S contents. uranium(IV) analysis was performed by means of a volumetric titration with K2Cr2O7, using barium salt of diphenylamine sulphonic acid as an indicator [6]. UV-Visible spectra of free metal and metal plus ligand solutions were taken using Shimadzu UV-160A UVVis Spectrophotometer. IR spectra of complex and the ligand were recorded for the wavenumber range of 4000 - 400 cm−1 using IR Prestige-21 FTIR spectrophotometer. IR assignments of pure ligand were also confirmed from literature. The spectra were recorded in association with KBr through the discs, prepared for this purpose. Sherewood Scientific Magnetic Susceptibility Balance (M.S.B) was used to measure magnetic susceptibility values of the complex in solid state. Cyclic voltammetric measurements were carried out on Electrochemical Analyzer/workstation, CHI 660C, under nitrogen atmosphere at 302 K. A platinum working electrode, a platinum-wire counter electrode and an Ag/AgCl reference electrode were used in a single three electrode cell.
3. Results and Discussion
3.1. UV-Visible Measurements
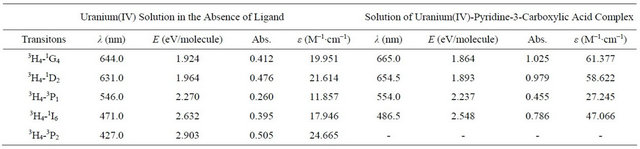
Figure 2 depicts a comparative illustration of UV-Visible absorption spectra of free uranium(IV) solution and a solution of complexed uranium(IV) with pyridine-3-carboxylic acid. The pattern of absorption of visible radiations remains same with the slight bathochromic shift. However, the absorption intensity of metal in the presence of the ligand increases. The value of molar absorptivity coefficient for the synthesized complex, calculated from the data of UV-Visible absorption of the complex is tabulated in Table 1. The mole ratio plot at different wave lengths (Figure 3) gives an evidence of formation of the complex with 1:2 metal to ligand ratio.
The bathochromic shift for the bands at 644.0 nm and 631.0 nm is almost equal but the increase in the intesity of 644.0 nm band is stronger than that for 631.0 nm. This
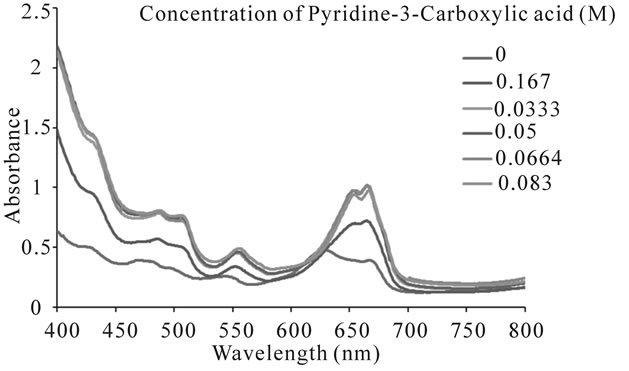
Figure 2. UV visible absorption spectrum of uranium(IV)- pyridine-3-carboxylic acid complex with constant metal concentration of 0.0167 M and varying ligand concentration.
indicates that in the complex the largest decrease in transition energy occurs for the transition between the ground level 3H4 and 1G4. The bathochromic shift and an increase in the absorption intensity indicate a more uniform distribution of electronic charge over the whole compound and that around the central ion [7,8].
3.2. IR Spectra
The infrared spectrum of uranium(IV) complex with pyridine-3-carboxylic acid is shown in Figure 4. The characteristic infrared absorption frequencies of the ligand and complex are summed up in Table 2. In pyridine-3-carboxylic acid the major IR bands, due to the carboxylic group at 1703.0 and 1415.7 cm−1, remain almost uninterrupted in the spectra of its complex with uranium(IV). In contrast, IR spectra of these complexes exhibit considerable perturbations related to the significant vibrational frequency of the pyridine moiety of the ligand. Absorptions at 1589.2 and 1490.9 cm−1 due to v (CC) and v (CN) modes respectively and that of the pyridine ring vibrations at 1031.8 and 950.8 cm−1 of the uncoordinated ligand undergo significant positive shifts. These vibrational fre-
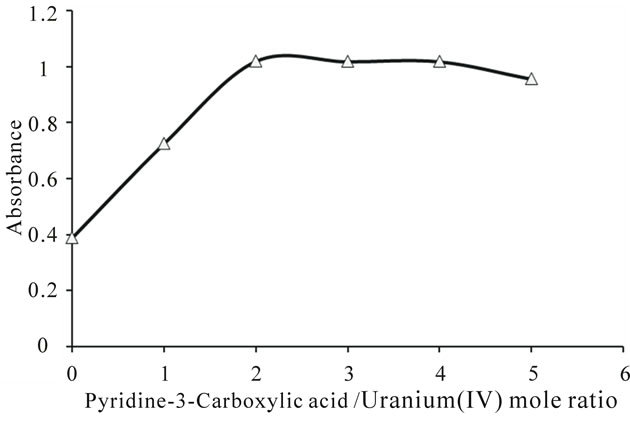
Figure 3. Mole ratio plot of uranium(IV)-pyridine-3-carboxylic acid complex at 664 nm wavelength.
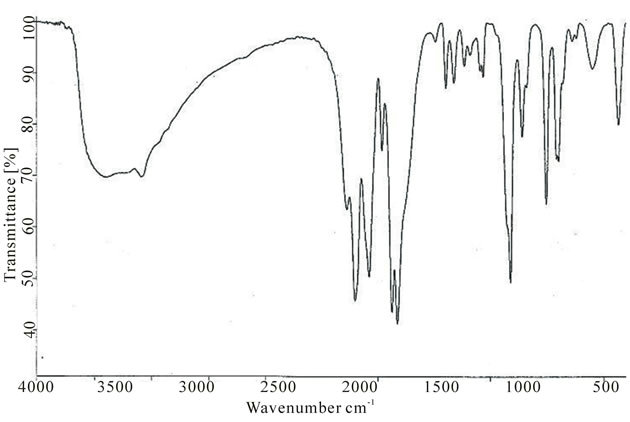
Figure 4. IR spectrum of uranium(IV) complex with pyridine-3-carboxylic acid.
Table 1. Energy levels assigned to the absorption spectra of free uranium(IV) and its complex with pyridine-3-carboxylic acid in aqueous media.
uency shifts (Table 2) show decisively that coordination of the ligand takes place via its pyridine ring nitrogen atom to the metal ion alone. It can be concluded that the ligand is acting as a monodentate entity [9-11].
3.3. Magnetic Susceptibility
The results of magnetic susceptibility measurements are summarized in Table 3. The μeff value, of the synthesized complex at room temperature, was estimated using Curie law. The difference in experimental value from the value calculated assuming spin-only magnetic moment, implying that the magnetic moments are not isolated. These do interact with the adjacent centers, as might be expected if magnetic dilutions are not adequate [12].
3.4. Electrochemical Studies
The cyclic voltammograms of uranium(IV)-pyri-dine-3- carboxylic acid complex (5e−3 M) in aqueous media (2 M HClO4) at different scan rates are shown in Figure 5. At 50 mV/s scan rate of the anodic and cathodic peaks appear at 1.299 V and 1.029 V respectively.
The complex exhibits a pair of cathodic and anodic waves. The anodic peak shifts somewhat towards positive side and cathodic peak moves towards negative side with the raise in scan rate (Table 4). The values of peak potential separation 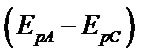 are greater than the theoretical value (0.059 V) for the reversible one electron transfer process. It was also observed that separations between peak potentials increases with the increase in scan rate (Figure 6) are the indicative of charge transfer kinetics [13]. The values for
are greater than the theoretical value (0.059 V) for the reversible one electron transfer process. It was also observed that separations between peak potentials increases with the increase in scan rate (Figure 6) are the indicative of charge transfer kinetics [13]. The values for  remain constant up to two decimal places, regardless of the scan rate with exceptions. In addition to that the anodic peak currents vary linearly with the square root of the scan rate (Figure 7). These results lead to the conclusion that the complexes of uranium(IV) oxidized quasi-reversibly to uranium(V) species at Platinum electrode [14].
remain constant up to two decimal places, regardless of the scan rate with exceptions. In addition to that the anodic peak currents vary linearly with the square root of the scan rate (Figure 7). These results lead to the conclusion that the complexes of uranium(IV) oxidized quasi-reversibly to uranium(V) species at Platinum electrode [14].
The ratio of cathodic to the anodic peak current IpC/IpA
is less than 1 and the ratio 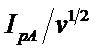 decreases with scan
decreases with scan
Table 2. IR assignments for uranium(IV) complex with pyridine-3-carboxylic acid.
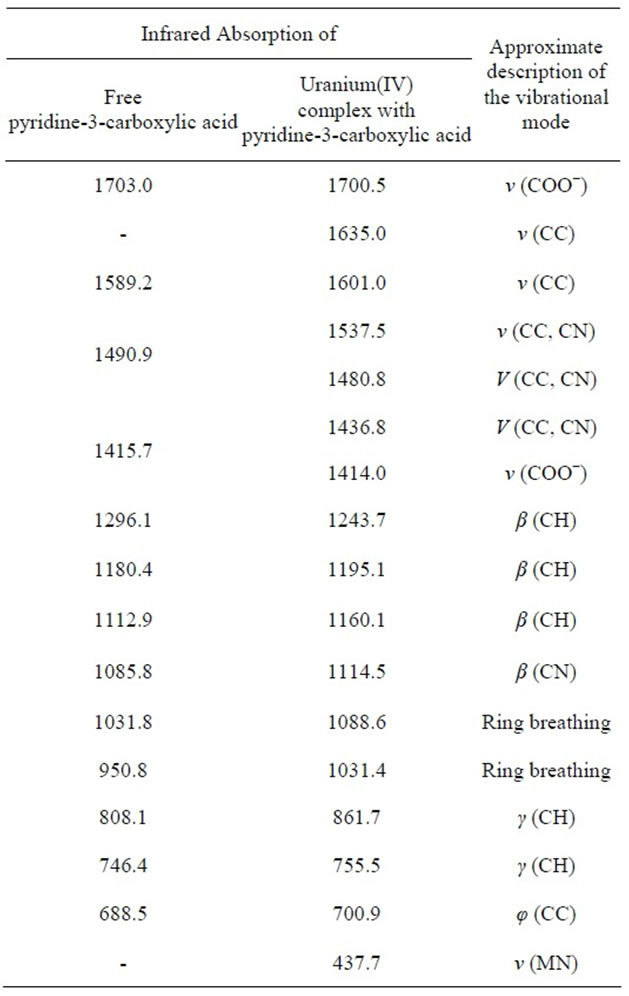
Table 3. Magnetic parameters for uranium(IV)-pyridine- 3-carboxylic acid complex.

aμso is spin-only magnetic moment. bμeff is an experimental effective magnetic moment calculated assuming curie behavior. cThe number of unpair electrons per uranium ion calculated from the values of μeff using μ = [n(n + 2)]1/2.
Table 4. Cyclic voltammetric data obtained at Pt electrode corresponding to the oxidation of uranium(IV)-pyridine-3- carboxylic acid complex in 2 M HClO4 and NaClO4 as an electrolyte.
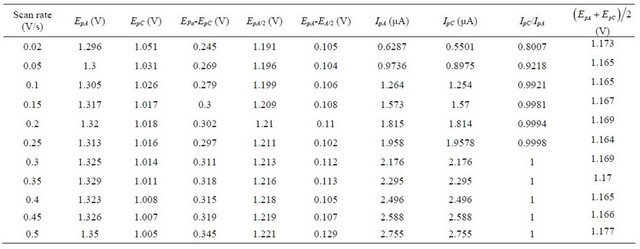
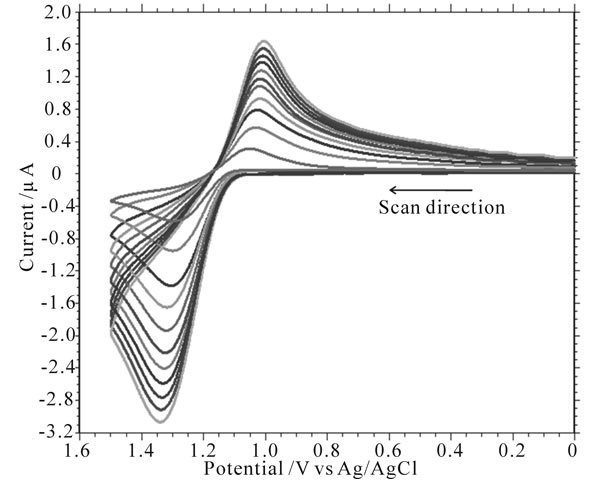
Figure 5. Cyclic voltammograms at Pt electrode corresponding to the oxidation of uranium(IV)-pyridine-3-carboxylic acid complex (Conc. = 5e−3 M) at scan rates from 20 mV/s to 500 mV/s in 2 M HClO4 and NaClO4 as an electrolyte; Temperature = 305 K.
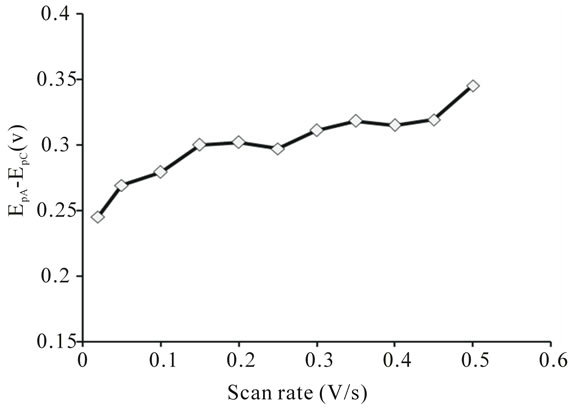
Figure 6. Variation of peak potential separation of uranium(IV)-pyridine-3-carboxylic acid complex with the scan rate.
rate (Figures 8 and 9). These observations lead to an assumption of an ECE mechanism. The U(V) species formed by the oxidation of U(IV) is unstable and disproportionates into U(IV) and U(VI). No reduction current due to U(VI) species was observed [15].
3.4.1. Determination of Diffusion Coefficient
The diffusion coefficient of the synthesized complex was calculated using Randles-Sevcik equation [16].
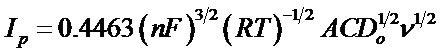 (1)
(1)
where Ip is the peak current (in amperes), n is the number of electrons transfer in the reaction, F, R and T have their usual meanings, A is the surface area of the electrode (0.0314 cm2 in this case), C is the concentration (in mole/ cm3), Do is the diffusion coefficient (in cm2/s) and ![]() is the scan rate (in V/s).
is the scan rate (in V/s).
The system seems not to be reversible but quasi-reversible. The above equation is verified in Figure 7, wherein the current was not only controlled by diffusion but also by charge transfer kinetics. The value of Do, calculated from the slope of plot  vs
vs 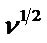 (Figure 7) is equal to 3.741e−6 cm2∙s−1.
(Figure 7) is equal to 3.741e−6 cm2∙s−1.
3.4.2. Determination of Heterogeneous Electron Transfer Rate Constant
The heterogeneous electron transfer rate constant “ks” was calculated by using the following equation [17].
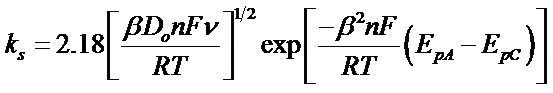 (2)
(2)
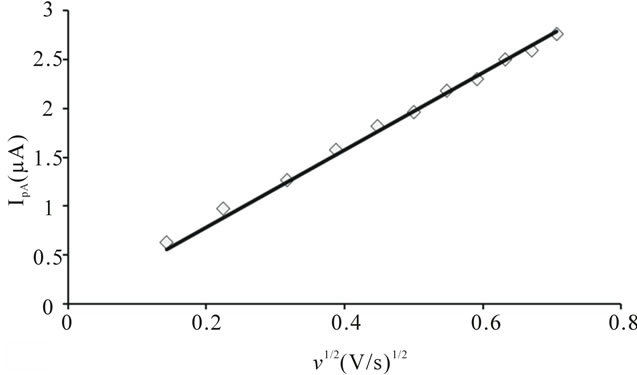
Figure 7. Variation of anodic peak current of uranium(IV)- pyridine-3-carboxylic acid complex with the square root of scan rate.
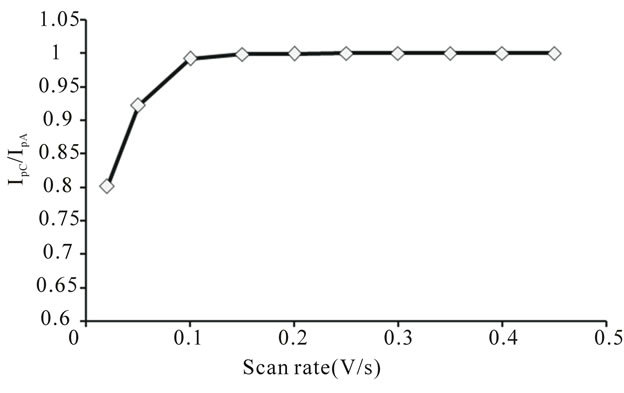
Figure 8. Variation of peak current ratio of uranium(IV)- pyridine-3-carboxylic acid complex with scan rate.
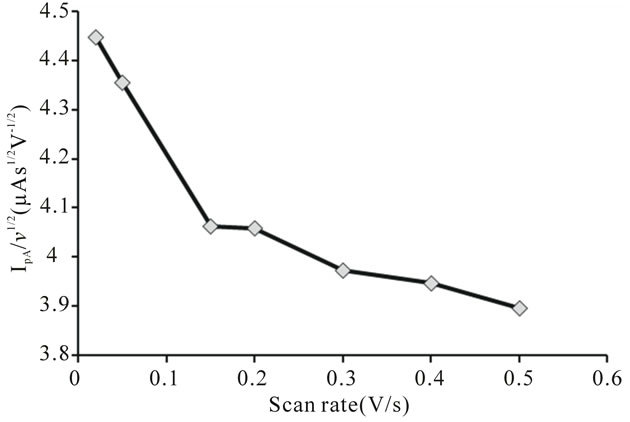
Figure 9. Variation of peak current function of uranium (IV)-pyridine-3-carboxylic acid complex with scan rate.
Where β is the dimensionless parameter known as electron transfer coefficient, Do the diffusion coefficient of the oxidized species in cm2/s, n the number of electrons transferred, ![]() the scan rate,
the scan rate, 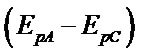 the peak separation and F, R and T have their usual meanings. Assuming the value of 0.5 for β the value of heterogeneous electron transfer rate constant “ks”, at 50 mV/s, was calculated to be 1.25e−5 cm∙s−1.
the peak separation and F, R and T have their usual meanings. Assuming the value of 0.5 for β the value of heterogeneous electron transfer rate constant “ks”, at 50 mV/s, was calculated to be 1.25e−5 cm∙s−1.
3.4.3. Estimation of Thermodynamic Parameters
Evaluation of thermodynamic parameters were made by calculating heterogeneous rate constants 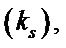 at various temperatures (Table 5). The increasing value of heterogeneous rate constant with increase in temperature, is an indication that oxidation of uranium(IV) at Platinum working electrode is an endothermic process. Following equations were used to estimate thermodynamic parameters.
at various temperatures (Table 5). The increasing value of heterogeneous rate constant with increase in temperature, is an indication that oxidation of uranium(IV) at Platinum working electrode is an endothermic process. Following equations were used to estimate thermodynamic parameters.
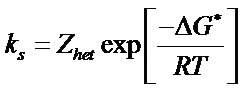
Above equation can be rewrite as
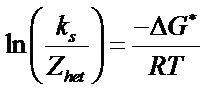
As 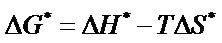
Hence, the expression can be modified as
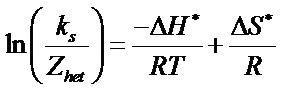
where 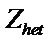 is the collision number for the heterogeneous electron transfer process and by using the equation
is the collision number for the heterogeneous electron transfer process and by using the equation
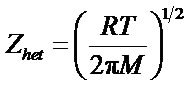 , its value can be determined at a particular temperature, where M is the molecular weight of the reacting species, R is the general gas constant and T is the temperature in Kelvin.
, its value can be determined at a particular temperature, where M is the molecular weight of the reacting species, R is the general gas constant and T is the temperature in Kelvin.
The value of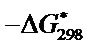 , an apparent free energy of activation, at 298 K were calculated using the equation.
, an apparent free energy of activation, at 298 K were calculated using the equation.
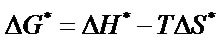
The value of ∆H* and ∆S* for each of the synthesized complex were evaluated from the slope and intercept of the plots of 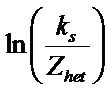 versus 1/T (Figure 10).
versus 1/T (Figure 10).
The values of 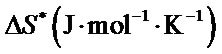
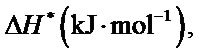
Table 5. Kinetic data for uranium(IV) ion at different temperatures when complexed with nicotinic acid.
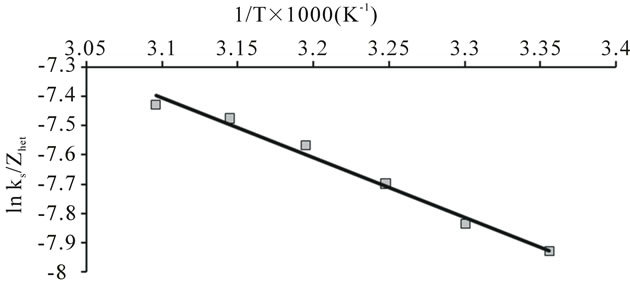
Figure 10. Plot of ln(ks/Zhet) vs 1/T for the evaluation of thermodynamic parameters for the oxidation of uranium (IV) when complexed with nicotinic acid at Pt electrode.
and 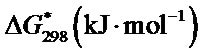 for the synthesized complex are found to 16.97, −60.71 and 35.06 respectively. The oxidation of uranium(IV) is a non-spontaneous and endothermic process, as specified by positive values of
for the synthesized complex are found to 16.97, −60.71 and 35.06 respectively. The oxidation of uranium(IV) is a non-spontaneous and endothermic process, as specified by positive values of 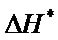 and
and 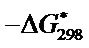 [18-20].
[18-20].
4. Conclusions
The study of uranium(IV) complex with pyridine-3-carboxylic acid shows that the ligand coordinates only via its pyridine ring nitrogen with metal to ligand ratio 1:2. Ahuja et al. also observed complexation of the same pattern while complexing this ligand with uranyl ion [5].
The oxidation of the synthesized complex of uranium (IV) leads to the formation of uranium(V) species. Following the oxidation process, disproportionation of uranium (V) takes place, representing an ECE mechanism. Moreover, the oxidation of uranium(IV) at platinum working electrode is an endothermic and non-spontaneous process.
REFERENCES
- M. Tamada, N. Seko and F. Yoshii, “Application of Radiation-Graft Material for Metal Adsorbent and Crosslinked Natural Polymer for Healthcare Product,” Radiation Physics and Chemistry, Vol. 71, No. 1-2, 2004, pp. 221-225. doi:10.1016/j.radphyschem.2004.03.044
- P. G. Barbano and L. Rigali, “Spectrophotometric Determination of Uranium in Sea Water after Extraction with Aliquat-336,” Analytica Chimica Acta, Vol. 96, No. 1, 1978, pp. 199-201. doi:10.1016/S0003-2670(01)93415-4
- T. A. Sullens, R. A. Jensen, T. Y. Shvareva and T. E. Albrecht-Schmitt, “Cation-Cation Interactions between Uranyl Cations in a Polar Open-Framework Uranyl Periodate,” Journal of the American Chemical Society, Vol. 126, No. 9, 2004, pp. 2676-2677. doi:10.1021/ja031695h
- M. J. Sarsfield and M. Helliwell, “Extending the Chemistry of the Uranyl Ion: Lewis Acid Coordination to a UO Oxygen,” Journal of the American Chemical Society, Vol. 126, No. 4, 2004, pp. 1036-1037. doi:10.1021/ja039101y
- S. A. Ishar, R. Singh and S. Rangarajalu, “Nicotinic Acid Complexes with Uranyl Salts,” Transition Metal Chemistry, Vol. 3, No. 1, 1978, pp. 193-195. doi:10.1007/BF01393544
- I. I. Naqvi, Journal of Pakistan Psychiatric Society, Vol. 2, 1980, p. 35.
- C. K. Jørgenson, “The Nephelauxetic Effect in Uranium (IV) Compounds,” Chemical Physics Letters, Vol. 87, No. 4, 1982, pp. 320-323. doi:10.1016/0009-2614(82)83594-X
- B. Jeżowska-Trazebiatowska and K. Bukietyńska, “The Electronic Structure of Uranium (IV) in Complex Compounds-I Comparison of the Absorption Spectra of Uranium (IV) and Praseodymium (III) Compounds,” Journal of Inorganic and Nuclear Chemistry, Vol. 19, No. 1-2, 1961, pp. 38-42. doi:10.1016/0022-1902(61)80043-2
- P. Moscibrode, H. Baranska, T. Drapala and W. Lewandowski, “Effect of Sodium and Lanthanides (III) on the Aromatic System of Nicotinic, Benzoic and Salicylic Acid,” Journal of Molecular Structure, Vol. 267, 1992, pp. 255-260. doi:10.1016/0022-2860(92)87041-S
- S. Chattopadhyay and S. K. Brahma, Spectrochimica Acta, Vol. 49A, No. 4, 1993, pp. 589-597.
- R. Singh and R. S. S. Rao, “Synthesis and Characterization of Divalent Manganese, Cobalt, Nickel, Copper and Zinc Complexes with Nicotinic Acid,” Journal of Molecular Structure, Vol. 71, 1981, pp. 23-30. doi:10.1016/0022-2860(81)85100-9
- Magnetic Susceptibility Balance Instructional Manual, Magway, England, 2001.
- M. S. Rahman, H. M. N. Akhtar, P. K. Bakshi and M. Q. Ehsan, Journal of Saudi Chemical Society, Vol. 11, No. 2, 2007, pp. 277-286.
- P. Giridhar, K. A. Venkatesan, T. G. Srinivasan and P. R. Vasudeeva Rao, “Electrochemical Behavior of Uranium (VI) in 1-Butyl-3-Methylimidazolium Chloride and Thermal Characterization of Uranium Oxide Deposit,” Electrochimica Acta, Vol. 52, No. 9, 2007, pp. 3006-3012. doi:10.1016/j.electacta.2006.09.038
- D. Hauchard, M. Cassir and J. Chivot, “Electrochemical Study of Uranium(IV) and Uranium(IV) Organometallic Compounds in Tetrahydrofuran by Means of Conventional Microelectrodes and Ultramicroelectrodes: Part I. Application to the Na(Hg) Reduction of Cp3UCl (Cp = η- C5H5),” Journal of Electroanalytical Chemistry, Vol. 313, No. 1-2, 1991, pp. 227-241. doi:10.1016/0022-0728(91)85182-O
- A. J. Bard and L. R. Faulkner, “Electrochemical Methods-Fundamentals and Applications,” Wiley, New York, 1980.
- R. J. Klingler and J. K. Kochi, “Electron-Transfer Kinetics from Cyclic Voltammetry. Quantitative Description of Electrochemical Reversibility,” Journal of Physical Chemistry, Vol. 85, No. 12, 1981, pp. 1731-1741. doi:10.1021/j150612a028
- A. S. A. Khan, R. Ahmed and M. L. Mirza, “Kinetics and Electrochemical Studies of Uranium in Acetate and Formate Media by Cyclic Voltammetry,” Radiochim Acta, Vol. 95, No. 12, 2007, pp. 693-699. doi:10.1524/ract.2007.95.12.693
- A. S. A. Khan, R. Ahmed and M. L. Mirza, Journal of the Chemical Society of Pakistan, Vol. 30, 2008, pp. 170- 177.
- R. A. Marcus, “On the Theory of Electron-Transfer Reactions. VI. Unified Treatment for Homogeneous and Electrode Reactions” Journal of Chemical Physics, Vol. 43, No. 2, 1965, pp. 679-701. doi:10.1063/1.1696792
NOTES
*Corresponding author.

