American Journal of Analytical Chemistry
Vol. 3 No. 4 (2012) , Article ID: 18428 , 6 pages DOI:10.4236/ajac.2012.34041
Experiments and Considerations through the Phase Diagram in Open Tubular Capillary Chromatography Based on Tube Radial Distribution of Ternary Mixed Solvents Using a Fused-Silica Capillary Tube
Department of Chemical Engineering and Materials Science, Faculty of Science and Engineering, Doshisha University, Kyotanabe, Japan
Email: *ktsukago@mail.doshisha.ac.jp
Received February 22, 2012; revised March 23, 2012; accepted April 5, 2012
Keywords: Open Tubular Capillary Chromatography; Phase Diagram; Fused-Silica Capillary Tube; Water-Acetonitrile-Ethyl Acetate Mixture; Tube Radial Distribution Phenomenon (TRDP)
ABSTRACT
Capillary chromatography using an untreated open tubular capillary tube and a ternary solvent mixture consisting of water-hydrophilic/hydrophobic organic solvent as a carrier solution has been developed. The system is called tube radical distribution chromato-graphy (TRDC). Separation performance of the TRDC system using a fused-silica capillary tube was examined through the phase diagram for the ternary water-acetonitrile-ethyl acetate solvent mixture. The TRDC system required homogeneous carrier solutions with solvent component ratios around the boundary curve between homogeneous and heterogeneous solution in the phase diagram. The data obtained using the fused-silica capillary tube were compared with those obtained using a polytetrafluoroethylene capillary tube in our previous study.
1. Introduction
Capillary tubes with inner diameters less than several hundred micrometers that provide micro-flow are known to exhibit interesting and useful physical or hydrodynamic phenomena, such as electro-osmotic flow and laminar flow. The electro-osmotic flow in a capillary tube promotes capillary electrophoresis [1,2] and capillary electrochromatography [3], while laminar flow conditions enable hydrodynamic chromatography [4,5]. Recently, our group reported the tube radial distribution phenomenon of carrier solvents in a micro-flow [6-8], which we call the tube radial distribution phenomenon (TRDP).
The TRDP can be phenomenologically described as follows. When the ternary mixed solvents of water-hydrophilic/hydrophobic organic solvent mixtures are delivered into a micro-space, such as a micro-channel or a capillary tube under laminar flow conditions, the solvent molecules are radially distributed in the micro-space, generating inner and outer phases. The TRDP creates a phase interface or kinetic aqueous-organic interface in the micro-space. That is, in a water-rich solution we would observe a water-rich major inner phase and organic solvent-rich minor outer phase, while in an organic solvent-rich solution we would observe an organic solvent-rich major inner phase and water-rich minor outer phase in micro-flow under certain conditions [6,8].
An untreated open tubular capillary chromatography system where the outer phase functions as a pseudo-stationary phase under laminar flow conditions has been developed based on the TRDP, which we call tube radial distribution chromatography (TRDC) [9-11]. According to the inner and outer phase formation based on the TRDP, with a water-rich carrier solution, the hydrophilic analyte, which is dispersed in the water-rich major phase (around the middle of the capillary tube), is eluted with almost average linear velocity. The hydrophobic analyte, which is dispersed in the organic solvent-rich minor phase near the inner wall of the tube (pseudo-stationary phase), is eluted with a smaller velocity than the average linear velocity. In contrast, with an organic solvent-rich carrier solution, the hydrophobic analyte dispersed in the organic solvent-rich major phase is eluted with almost average linear velocity. The hydrophilic analyte dispersed in the water-rich minor phase near the inner wall of the tube is eluted with a lower velocity. Therefore, the elution times of the analytes can be reversed by altering the component ratio of the solvents in the carrier solution.
Separation performance in the TRDC system was examined from the viewpoint of pressure in a capillary tube under laminar flow conditions [8] and the phase diagram of the ternary mixed solvents of water-acetonitrile-ethyl acetate mixture [7]. The component ratios of the solvents that were positioned near the boundary between homogeneous and heterogeneous solution in the diagram were required for TRDC separation. The data suggested that the phase transformation from homogeneous to heterogeneous solution in the capillary tube under pressure in micro-flow was important for TRDP and TRDC [7,8]. That is, experiments and considerations based on the phase diagram are useful for TRDP or TRPC study.
In our previous study [7], when using polytetrafluoroethylene (PTFE) capillary tubes, with the water-rich carrier solution, hydrophilic 2,6-naphthalenedisulfonic acid and hydrophobic 1-naphthal as model analytes were separated in this order, while with the organic solventrich carrier solution, the reverse elution order was observed—i.e., 1-naphthol was first detected, followed by 2,6-naphthalenesulfonic acid—although they showed splitseparation. The observed elution orders were reasonable because of the inner and outer phase formation based on the TRDP. In this study, separation performance of the analytes was examined by the TRDC in a similar way to the previous study through phase diagrams [7], but using a fused-silica capillary tube instead of a PTFE capillary tube.
2. Experimental
2.1. Reagents and Capillary Tubes
Water was purified with an Elix 3 UV system (Millipore Co., Billerica, MA). All reagents used were commercially available and of analytical grade. 1-Naphthol, 2,6-naphthalenedisulfonic acid, acetonitrile, and ethyl acetate were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Fused-silica capillary tubes with an inner diameter of 50 μm were purchased from Yasaka Industries, Inc. (Tokyo, Japan).
2.2. Apparatus and Procedures
A schematic diagram of the present TRDC system comprised of a fused-silica capillary tube (120 cm total length and 100 cm effective length), micro-syringe pump (MF- 9090; Bioanalytical Systems, Inc., West Lafayette, IN), and absorption detector (modified SPD-10AV spectrophotometric detector; Shimadzu Co., Kyoto, Japan) is shown in Figure 1. The tube temperature was controlled by dipping the capillary tube (ca. 60 cm) in water maintained at a definite temperature (0˚C or 20˚C) in a beaker with stirring. The ternary mixed solvents of water-hydrophilic/hydrophobic organic solvent mixture (water-acetonitrile-ethyl acetate) solutions with various volume ratios were used as carrier solutions. Analyte solutions containing 1-naphthol and 2,6-naphthalenedisulfonic acid (1 mM each) as models were prepared with the carrier solutions.
The analyte solution was introduced directly into the capillary inlet side by the gravity method (20 cm height for 30 s). After analyte injection, the capillary inlet was connected through a joint to a micro-syringe. The syringe was placed on the micro-syringe pump. The carrier solution was fed into the capillary tube at 0.2 μL∙min–1 flow rate under laminar flow conditions. On-capillary absorption detection (254 nm) was performed with the detector.
3. Results and Discussion
3.1. Phase Diagram for Water-Acetonitrile-Ethyl Acetate Mixture
The phase diagram for the ternary mixed solvents of water-acetonitrile (hydrophilic organic solvent)-ethyl acetate (hydrophobic organic solvent) in a vessel at a temperature of 20˚C was constructed. The obtained phase diagram is shown in Figure 2. The dotted curve in the
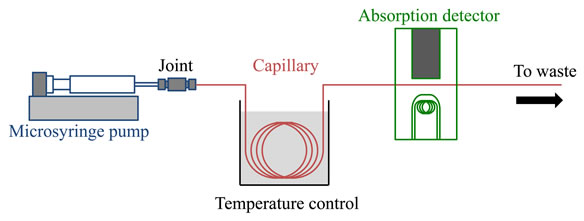
Figure 1. Schematic diagram of the present TRDC system.
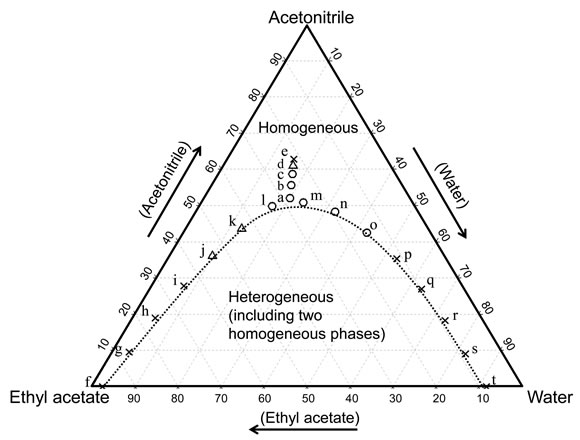
Figure 2. Phase diagram for water-acetonitrile-ethyl acetate mixture (20˚C) and the component ratios of the solvents for the chromatograms obtained by the TRDC system with tube temperature 20˚C. The component ratios of the carrier solutions a-e related to the experiments shown in Figure 3 as well as the carrier solutions f-t related to the experiments in Figure 4 are plotted in the diagram with the symbols ○, Δ, and ×, indicting baseline-separation, split-separation, and non-separation, respectively, for the analytes.
diagram indicates the boundary between homogeneous and heterogeneous phases. The phase diagram showed that each component ratio of the solvents formed a homogeneous (one homogeneous phase) or heterogeneous (two homogeneous phases) solution. The solutions with component ratios of a-t were used as carrier solutions in the TRDC system in the following sections. The component ratios of the solvents or the carrier solutions a-t are plotted in the diagram based on separation performance of analytes described below, where circles (○), triangles (Δ), and crosses (×) indicated baseline-separation, split-separation (not baseline-separation), and non-separation for the analytes on the chromatograms, respectively.
3.2. Chromatograms Obtained at Various Component Ratios of Water-Acetonitrile-Ethyl Acetate Mixture Carrier Solution
To a solution with a constant water-ethyl acetate volume ratio of 5:7 was added acetonitrile in a vessel at a temperature of 20˚C to prepare carrier solutions (organic solvent-rich carrier solutions) with the following compositions: a) water-acetonitrile-ethyl acetate 5:13:7 volume ratio; b) 5:15:7 volume ratio; c) 5:17:7 volume ratio; d) 5:19:7 volume ratio; and e) 5:20:7 volume ratio. The five carrier solutions a)-e) were all homogeneous solutions. The analyte mixtures of 1-naphthol and 2,6-naphthalenedisulfonic acid were examined with the present TRDC system using carrier solutions a)-e).
The obtained chromatograms are shown in Figure 3. Carrier solutions a)-c) could separate the analytes in the mixture. 1-Naphthol (hydrophobic) was eluted first with near the average linear velocity in the capillary tube under laminar flow conditions and 2,6-naphthalenedisulfonic acid (hydrophilic) was eluted second with lower velocity than the average linear velocity. The elution order was reasonable in the TRDC system with the organic solvent-rich carrier solution as mentioned in the Introduction. The other homogeneous carrier solutions d) and e) of water-acetonitrile-ethyl acetate showed split-separation and non-separation of the analytes, respectively, under the present analytical conditions. The separation performances obtained with carrier solutions a)-e) are plotted with the following symbols: circles (○), triangles (Δ), and crosses (×) in the phase diagram in Figure 2. The separation performance decreased from carrier solution a) to e) as shown in Figure 3. However, even carrier solutions b) and c) in which the compositions were a little far from the boundary in the diagram also showed baseline-separation for the analytes. TRDP must occur with such allowance for the solvents compositions around the boundary in this area of the diagram.
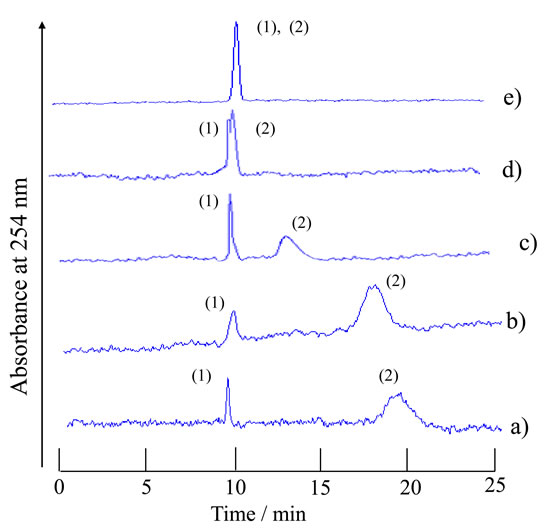
Figure 3. Chromatograms of a mixture of 1-naphthol and 2,6-naphthalenedisulfonic acid by the TRDC system using various component ratios of water-acetonitrile-ethyl acetate mixture as carrier solutions. Tube temperature 20˚C. (1) 1- Naphthol and (2) 2,6-naphthalenedisulfonic acid were eluted in this order with carrier solutions a)-d).
The chromatograms of the analytes, the mixture of 1- naphthol and 2,6-naphthalenedisulfonic acid, were examined with the homogeneous carrier solutions f)-t). The compositions for the solutions f)-t) were positioned near the boundary in the phase diagram; f) water-acetonitrile-ethyl acetate of 0.25:0:10 volume ratio, g) 0.4:1:9 volume ratio, h) 0.55:2:8 volume ratio, i) 0.8:3:7 volume ratio, j) 1.1:4:6 volume ratio, k) 1.5:5:5 volume ratio, l) 2.05:6:4 volume ratio, m) 14:30:15 volume ratio, n) 4:6:2.4 volume ratio, o) 5:5:1.75 volume ratio, p) 6:4:1.3 volume ratio, q) 7:3:1.1 volume ratio, r) 8:2:0.95 volume ratio, s) 9:1:0.95 volume ratio, and t) 10:0:0.9 volume ratio. The carrier solutions f)-i) and p)-t) showed no separation behavior and presented just single peaks on the chromatograms with near the average linear velocity (data not shown). The carrier solutions j)-o) showed separation behavior as indicated in Figure 4. The component ratios of the solvents for solutions f)-t) were plotted in the phase diagram in Figure 2 with the symbols based on the chromatographic data.
As shown in Figure 2, the TRDC system was used with several homogeneous carrier solutions with component ratios of the solvents around the boundary curve in the phase diagram. Such specific carrier solutions caused tube radial distribution of the carrier solvents in the capillary tube under laminar flow conditions, i.e., the TRDP. The pressure imposed on the carrier solution in the capillary tube under laminar flow conditions might alter the carrier solution from homogeneous in the batch vessel to heterogeneous in the tube, affecting the tube radial distribution of the solvents in the capillary tube.
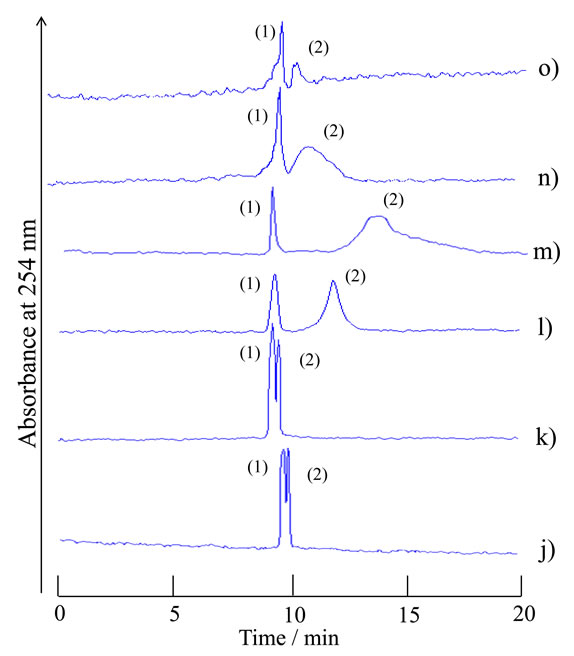
Figure 4. Chromatograms of a mixture of 1-naphthol and 2,6-naphthalenedisulfonic acid by the TRDC system using various component ratios of water-acetonitrile-ethyl acetate mixture as carrier solutions that were positioned near the boundary curve in the phase diagram. Tube temperature 20˚C. (1) 1-Naphthol and (2) 2,6-naphthalenedisulfonic acid were eluted in this order with the carrier solutions j)-o).
3.3. Optimization of the Component Ratio of Solvents for Separation
As shown in Figure 4, high resolutions of the analytes in the TRDC system were observed around the carrier solutions of l) and m) in the phase diagram. The chromategrams were examined in more detail with carrier solutions u)-w) that had compositions between solutions l) and m) along the boundary curve in the phase diagram (Figure 2). They were u) water-acetonitrile-ethyl acetate with 5:14.5:8.5 volume ratio, v) 5:13:7 volume ratio, and w) 6:13.5:6.5 volume ratio. The obtained chromatograms are shown in Figure 5 together with their resolutions (Rs). Carrier solutions v) and w) showed the best resolutions, ca. 14, under the present analytical conditions.
3.4. Reversing of the Elution Order of the Analytes
As characteristic separation behavior of the TRDC system using the PTFE capillary tube, we reported previously that a water-rich carrier solution elutes the more hydrophilic analyte first and an organic solvent-rich carrier solution elutes the more hydrophobic analyte first [7]. However, the reverse elution behavior of the analytes was not observed in Figure 4 under these analytical conditions. The analytical conditions, such as tube temperature, tube length, and flow rate, were significant and critical for separation performance in the TRDC system. Tentatively, the chromatograms were examined by the TRDC system using carrier solutions f)-t) where the capillary tube was dipped in the beaker controlled at 0˚C instead of 20˚C. The separation performance with solutions f)-t) were plotted with the symbols in the diagram in Figure 6. The chromatograms obtained with carrier solutions l)-q) that show separation are presented in Figure 7. Water-rich carrier solutions p) and q) gave the reverse elution order, 2,6-naphthalenedisulfonic acid first and then 1-naphthol second, although they showed splitseparation.
Furthermore, the analyte solutions were subjected to the TRDC system with the water-rich carrier solution of water-acetonitrile-ethyl acetate in a 15:3:2 volume ratio (water-rich carrier solution) under the analytical conditions of tube temperature 0˚C instead of 20˚C, tube effective length of 200 cm instead of 100 cm, and flow rate of 0.8 μL∙min–1 instead of 0.2 μL∙min–1. The obtained chromatogram is shown in Figure 8. 2,6-Naphthalenedisulfonic acid and 1-naphthol were eluted in this order with baseline-separation. The individual peaks were identified with the chromatogram of each analyte.
3.5. Consideration of the Influence of the Tube Materials on Separation
Almost the same elution behavior or elution order in the TRDC system with the fused-silica capillary tube was observed as that with the PTFE capillary tube reported in
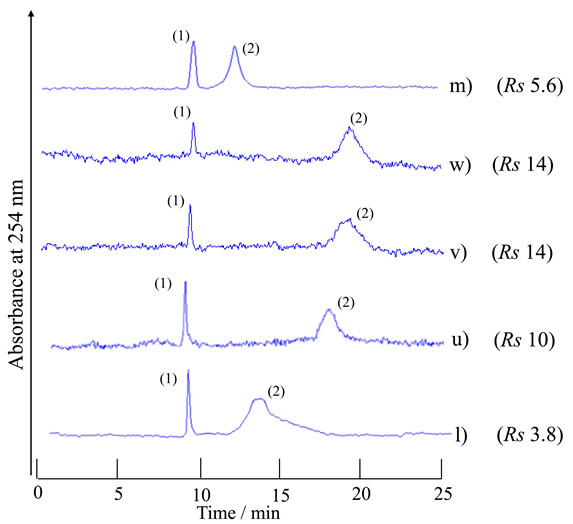
Figure 5. Chromatograms of a mixture of 1-naphthol and 2,6-naphthalenedisulfonic acid by the TRDC system using various component ratios of water-acetonitrile-ethyl acetate mixture as carrier solutions that were positioned between solutions l) and m) in Figure 4 and the resolutions (Rs) for the analyte peaks. Tube temperature 20˚C. (1) 1-Naphthol and (2) 2,6-naphthalenedisulfonic acid were eluted in this order with carrier solutions l)-m).
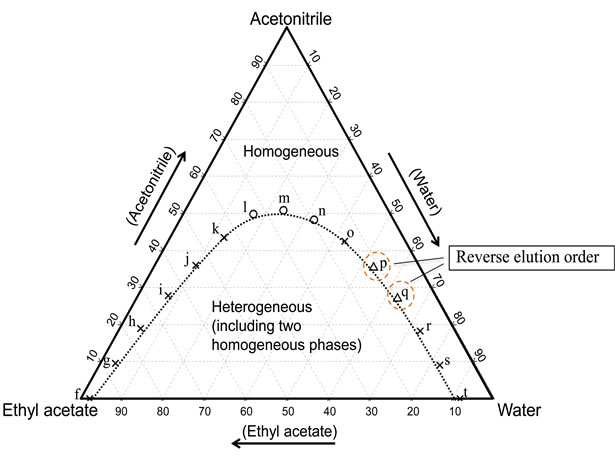
Figure 6. Phase diagram for water-acetonitrile-ethyl acetate mixture (20˚C) and the component ratios of the solvents for the chromatograms obtained by the TRDC system with tube temperature 0˚C. The component ratios of the carrier solvents f)-t) are plotted in the diagram with the symbols (○, Δ, and ×). 1-Naphthol and 2,6-naphthalenedisulfonic acid were eluted in this order with carrier solutions l)-n), while they were eluted in the reverse order with the carrier solutions p) and q).
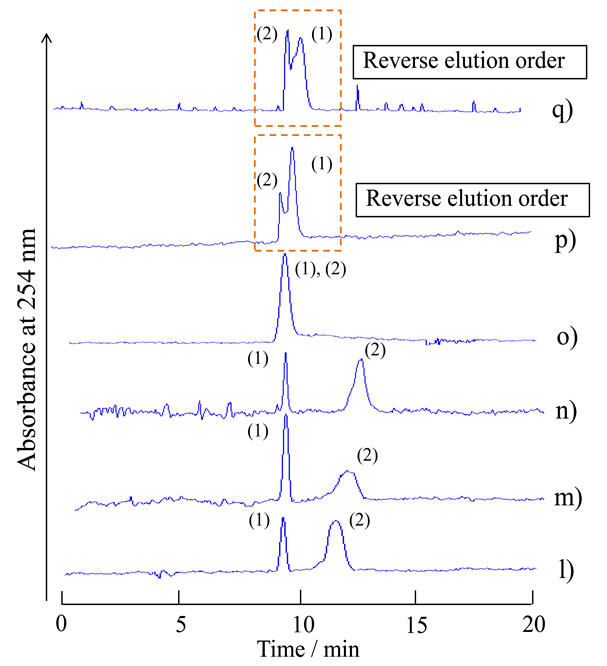
Figure 7. Chromatograms of a mixture of 1-naphthol and 2,6-naphthalenedisulfonic acid by the TRDC system using various component ratios of water-acetonitrile-ethyl acetate mixture as carrier solutions that were positioned near the boundary curve in the phase diagram. Tube temperature 0˚C. (1) 1-Naphthol and (2) 2,6-naphthalenedisulfonic acid were eluted in this order with carrier solutions l)-n), while they were eluted in the reverse order with carrier solutions p) and q). The component ratios of carrier solutions l)-p) used for the chromatograms are plotted in the phase diagram of Figure 6.
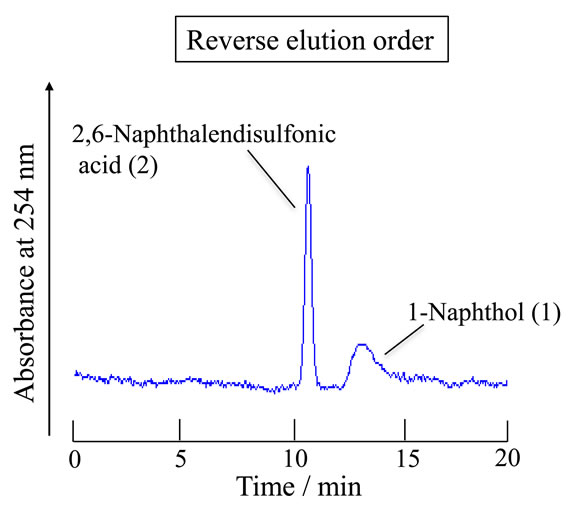
Figure 8. Chromatograms of a mixture of (1) 1-naphthol and (2) 2,6-naphthalenedisulfonic acid by the TRDC system. Capillary effective length, 200 cm (total length 220 cm); carrier, water-acetonitrile-ethyl acetate of 15:3:2 volume ratio; tube temperature, 0˚C; and flow rate, 0.8 μL∙min–1.
our previous work [7]. However, there were also differences in separation behavior between them. In the fusedsilica capillary tube, the resolutions obtained with the organic solvent-rich carrier solution were better than those of the water-rich carrier solution. On the other hand, in the PTFE capillary tube, the resolutions obtained with the water-rich carrier solution were better than those of the organic solvent-rich carrier solutions as reported previously [7]. The interaction between the outer phase of water-rich and fused-silica inner wall as well as the outer phase of organic solvent-rich and PTFE (polymer material) inner wall may give prior conditions for making the outer phase (pseudo-stationary phase) in the capillary tube, while the interaction between the outer phase of water-rich and polymer material inner wall as well as the outer phase of organic solvent-rich and fused-silica inner wall may result in conditions inconvenient for forming the outer phase in the capillary tube.
4. Conclusion
Chromatograms of model analytes were examined by the TRDC system using a fused-silica capillary tube from the viewpoint of the phase diagram of ternary mixed solvents. The separation performances of the analytes were similar to those obtained with a PTFE capillary tube described in our previous work [7]. With the organic solvent-rich carrier solution, model analytes, hydrophobic 1-naphthol and hydrophilic 2,6-naphthalenedisuflonic acid, were separated in this order, while they were eluted in the inverse order with the water-rich carrier solution, 2,6-naphthalenedisuflonic acid was detected first, followed by 1- naphthol detection. However, in the fused-silica capillary tube resolutions obtained with the organic solvent-rich carrier solution were better than those of the water-rich carrier solution. The interaction between the outer phase of water-rich and fused-silica surface may provide better conditions than that between the outer phase of organic solvent-rich and fused-silica surface for forming the outer phase in the fused-silica capillary tube.
5. Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also supported by “Advanced Study for Integrated Particle Science, and Technology,” Strategic Development of Research Infrastructure for Private Universities, the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
REFERENCES
- S. Terabe, “Micellar Electrokinetic Chromatography,” Analytical Chemistry, Vol. 76, No. 13, 2004, pp. 240A-246A. doi:10.1021/ac0415859
- C. A. Lucy, A. M. MacDonald and M. D. Gulcev, “NonCovalent Capillary Coatings for Protein Separations in Capillary Electrophoresis,” Journal of Chromatography A, Vol. 1184, No. 1-2, 2008, pp. 81-105. doi:10.1016/j.chroma.2007.10.114
- K. Otsuka, “Chiral Separations Using Avidin as a Chiral Selector and Highly Sensitive Detection Using Thermal Lens Microscopy in Capillary Electrophoresis,” Chromatography, Vol. 28, No. 1, 2007, pp. 1-7.
- H. Small, F. L. Saunders and J. Solc, “Hydrodynamic Chromatography. A New Approach to Particle Size Analysis,” Advances in Colloid and Interface Science, Vol. 6, No. 4, 1976, pp. 237-266. doi:10.1016/0001-8686(76)85004-X
- R. Umehara, M. Harada and T. Okada, “Wide-Bore Hydrodynamic Chromatography Sub-Second Range,” Journal of Separation Science, Vol. 32, No. 3, 2009, pp. 472- 478. doi:10.1002/jssc.200800504
- N. Jinno, M. Murakami, K. Mizohata, M. Hashimoto and K. Tsukagoshi, “Fluorescence Observation Supporting Capillary Chromatography Based on Tube Radial Distribution of Carrier Solvents under Laminar Flow Conditions,” Analyst, Vol. 136, No. 5, 2011, pp. 927-932. doi:10.1039/c0an00820f
- N. Jinno, M. Hashimoto and K. Tsukagoshi, “Experimental Consideration of Capillary Chromatography Based on Tube Radial Distribution of Ternary Mixture Carrier Solvents under Laminar Flow Conditions,” Analytical Sciences, Vol. 27, No. 3, 2011, pp. 259-264. doi:10.2116/analsci.27.259
- M. Murakami, N. Jinno, M. Hashimoto and K. Tsukagoshi, “Tube Radial Distribution Phenomenon of Ternary Mixed Solvents in a Microspace under Laminar Flow Conditions,” Analytical Sciences, Vol. 27, No. 8, 2011, pp. 793-798. doi:10.2116/analsci.27.793
- N. Jinno, M. Murakami, M. Hashimoto and K. Tsukagoshi, “Analytical Conditions and Separation performance of Capillary Chromatography Based on the Tube Radical Distribution of Aqueous-Organic Mixture Carrier Solvents under Laminar-Flow Conditions,” Analytical Sciences, Vol. 26, No. 7, 2010, pp. 737-742. doi:10.2116/analsci.26.737
- S. Ishimoto, Y. Kudo, N. Jinno, M. Hashimoto and K. Tsukagoshi, “Introduction of Fluorescence and Chemiluminescence Detection to Capillary Chromatography Based on Tube Radial Distribution of Water-Hydrophilic-Hydrophobic Organic Mixture Carrier Solvents,” Analytical Methods, Vol. 2, No. 9, 2010, pp. 1377-1381. doi:10.1039/c0ay00378f
- S. Fujinaga, N. Jinno, M. Hashimoto and K. Tsukagoshi, “Use of Tube Radial Distribution of Ternary Mixed Carrier Solvents for Introduction of Absorption Reagent for Metal Ion Separation and Online Detection into Capillary,” Journal of Separation Science, Vol. 34, No. 20, 2011, pp. 2833-2839. doi:10.1002/jssc.201100404
NOTES
*Corresponding author.

