Journal of Biosciences and Medicines
Vol.05 No.05(2017), Article ID:76480,12 pages
10.4236/jbm.2017.55004
Effects of Gaseous Drinks in Wistar Rats Esophagus
Thiago Dornelas de Oliveira1, Marcelli Eliotério Gaspar1, Larissa Menezes Viana Braga1, Joice Meire Rodrigues1,2, Rebeca Nogueira Falcão Santos3, Suelen Gaudino Moura3, Nayara Barbosa Bicalho3, Lamara Laguardia Valente Rocha4, Daniel Almeida da Costa5, Marcus Vinicius de Mello Pinto6
1Academic of the Graduate Course in Medicine of the University Center of Caratinga, Caratinga, Brazil
2Religious Sciences from the Pontifical Catholic University of São Paulo-PUC/SP, São Paulo, Brazil
3University Center of Caratinga, Caratinga, Brazil
4The Institute of Health Sciences at UNEC, Caratinga, Brazil
5The Medical School at the Center of Higher Education of Valenca, Valenca, Brazil
6The Cellurare Institute Center of Laser Treatment and Cell Therapy, Petrópolis, Brazil

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 3, 2017; Accepted: May 23, 2017; Published: May 26, 2017
ABSTRACT
The consumption of beverages and processed foods, mainly soft drinks, has been incorporated into the usual diet of children, adolescents and adults frequently in recent years. The present study aimed to study the effects of carbonated drinks on Wistar rats, based on the macroscopic and histological morphology of the esophagus. Fifteen Wistar rats were divided into three groups: control group; group fed with ration and cola-type refrigerant ad libitum and group fed with ration and gas industrialized water ad libitum, for a trial period of 90 days. For histopathology and morphology, the esophagus was removed, prepared in slides and stained with hematoxylin-eosin. Images of the sections were captured for analysis and classification of the inflammatory infiltrate. For morphometry, ten grid fields of 100 points were evaluated in the 40x eyepiece, totaling 3000 points per animal. The results demonstrate that the refrigerant treatment induced the following structural changes in the rats: significant reduction of weight in relation to the control group; inflammatory infiltrate predominantly diffuse mild to moderate and tissue edema. The rats treated with carbonated water had similar results to the control, besides signs of healing and tissue repair.
Keywords:
Soft Drinks Consumption, Inflammation, Carbonate Beverages

1. Introduction
The incorporation of industrialized beverage and foods into the eating habits of children, adolescents and adults has been a customary practice in the last years. Among the most consumed industrialized products, the refrigerant stands out [1] . According the Brazilian Association of Soft Drinks and Non Alcoholic Beverages (ABIR), the soft drink consists of an industrialized carbonated drink, non-alcoholic, with aromas and high refreshing power [2] .
Produced from sugar, the refrigerant is a drink without nutritional value, con- tributing thus with the excessive consumption of empty calories. Besides, his consumption can replace healthy foods with adequate nutritional values, which contribute to the human growth and development, such as natural juices, fruits and milk [3] [4] .
Despite its popularity, the increase in consumption of sugared beverages frequently is associated to the increase of energetic ingestion, weight gain, obesity and diabetes [5] . In a recent study, Eweis, et al., (2017) [6] evaluated the effect on rodents and humans of the carbon dioxide contained in carbonated beverages, concluding that carbon dioxide induces the release of ghrelin and increases the consumption of foods, thus increasing the risks of weight gain, obesity and hepatic steatosis.
The consumption of carbonated drinks has shown to be prejudicial to health in diverse researches [7] [8] [9] [10] . El-Terras, et al., (2016) [7] , for example, evaluated the effects of the consumption of three carbonated soft drinks on the brain of Wistar rats and on the genetic expression of monoamine oxidase and D2 dopamine receptors. The results demonstrated that carbonated beverages induced oxidative stress and change of the expression of certain genes associated with brain activity. Alkhedaide in his study also associated the consumption of soft drinks in rodents with the increase of oxidative stress biomarkers, and besides that with a disruption in the expression of certain genes associated with liver and kidney functions [8] [9] .
Others organic effects related to chronic ingestion of refrigerants are descry- bed on literature, such as the capacity to increase insulin sensitivity and promote changes in functional renal parameters, which was described by Celec, et al., (2010) [10] after three months treating Wistar rats with refrigerant. Histological alterations of rodents’ cerebellums were related by Eluwa, et al., (2013) [11] , who investigated the effect of aspartame contained in refrigerants. Lastly, alterations in lipid profiles were related by Botezelli, et al., (2010) [12] , who compared lipid content of Wistar rats treated with fructose-rich refrigerant with those who were treated with low-fructose refrigerant.
According Prado (2001) [13] , the consumption of certain foods, including carbonated beverages, promoted incompetence of the lower esophageal sphincter, delay in gastric emptying, abnormalities in the esophageal mucosa, among others, predisposing to gastroesophageal reflux.
Conforming to Kapicioglu, et al., (1999) [14] , who studded the relation between cola beverages consumption and esophageal mucosa damage, the cola has proliferative and regenerative effects on the esophageal mucosa, possibly caused by its irritating effect.
In fact, persistent oral regurgitation may lead to esophageal epithelial alterations or to the development of gastroesophageal reflux disease due tissue injury by the gastric acid [15] . Thus, there is no doubt that the chemical composition of an acidic beverage is important in the esophagitis physiopathology [14] . Besides, the carbon dioxide dissolved in drinks is able to influence the activity of taste receptors in the mouth, and results in neuromotor responses that may compromise swallowing [16] . Therefore, it is important to investigate the effects of carbonated drinks in the esophagus, in order to estimate the risk and ensure the safety to the consumer population.
Thus, the mainly purpose of this research was to study the effects of carbonated beverages in Wistar rats, during a period of ninety days of experimentation, based on the macroscopic and histological morphology of the esophagus. Besides that, it aimed to continue the study of Santos, et al., (2016) [17] , who investigated the effects of carbonated beverages in the digestive system organs of Wistar rats.
2. Material and Methods
2.1. Study Design and Histopathological Analysis
1) Rats and the experimental design
In the present study 15 Wistar rats were used, from the vivarium of the Federal University of Viçosa (UFV), with approximately 260 g each. The animals were divided into three groups, with five members per group, according to the model proposed by Eluwa, et al., (2013) [11] . In group 1, the animals were ration with feed and industrialized water without gas ad libitum. Group 2 was submitted to a with ration and hydration with cola-type refrigerant ad libitum and group 3 included rats submitted to the with feed with ration and water of the industrialized type with gas ad libitum and offered in the 24 hours of each treatment day in a period Experimental period of 90 days, according the model developed by El-Terras, et al., (2016) [7] . The weight of each rat was measured and registered on a daily basis. The drinks of all thirty rats were changed once daily in the amo- unt of 150 ml daily.
The carbonated beverage cola-type was chosen as an exogenous physiologic acid because millions of people consume it every day, besides being a strongly acidic (pH 2.5) (Kapicioglu, et al., 1999) [4] .
2) Sampling of esophagus
The 12-hour light/dark cycles were respected. For the histopathology and morphometry, the esophagus was removed, the fragments of which were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.2 - 7.4 at room temperature for 24 hours. After dehydration in increasing solutions of ethyl alcohol, the fragments were diafiltered in xylol and included in histological paraffin. Sections of 5 and 7 μm were obtained using a microtome (Ek Micro, Eikonal do Brasil). The material was then processed for hematoxylin-eosin staining and the Entelan-mounted blades. Four cuts were placed per slide, in a total of fifteen slides.
Two slides were randomly chosen per animal, and section images were captured directly from the light microscope (Ken-a-vision 2103) through a digital video camera (1.3 Mp-DinoEye model Dinolite brand with image analysis software). In this way, ten photos were obtained in the randomly chosen cuatros, totalizing ten photos/animal. These images were evaluated using Image Pro Plus 4.0 (Media Cybermetcs) image analysis software on a microcomputer.
3) Slide preparation for histopathological study
For histopathological analysis, five photos/animals obtained with 20x eyepieces were used, totaling twenty five photos per group. The presence of inflammatory infiltrate in the organ parenchyma was considered for this analysis, classified as intense, moderate or mild, in addition to the classification of diffuse or focal infiltrate.
4) Slide for morphometry study
For the morphometry, ten grid fields of 100 points in the 40x eyepiece were evaluated, totaling 3000 points per animal. The parameters related to possible structural alterations as inflammatory infiltrate and the evolution of healing and repair were considered.
2.2. Systematization and Data Analysis
For the statistical analysis, the software Sigmastat Statistical Analysis System, version 1.0 (Jandel Scientific) was used. Analysis of variance was applied; the t- student test was used for comparison between two groups and multiple comparison by the Student Newmann Keuls method, with significance at p < 0.05. Measurements were presented as mean ± standard deviation.
2.3 Ethical Considerations
This work was forwarded and approved by the Ethics Committee on the Use of Animals of the University Center of Caratinga-MG (CEUA-UNEC).
3. Results
In order to evaluate the possible harmful effect of the refrigerant and the carbonated drink in the esophagus of the animals, parameters such as animal weight, morphometry and histopathology of the esophagus were analyzed.
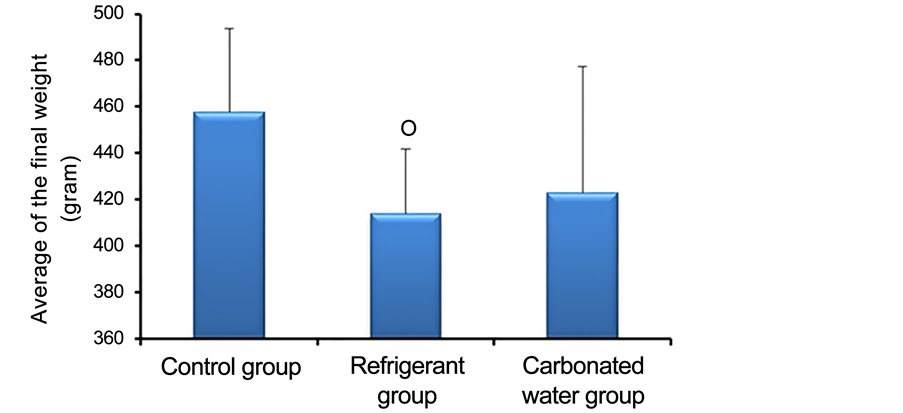
Regarding the weight at the end of the experiment, significant differences were observed between the experimental groups as recorded in Figure 1. The analysis of Figure 1 shows that the animals that consumed refrigerant (413.7 ± 28.1) presented significant weight Lower than controls (457.6 ± 36.3). Those who ingested carbonated water (423.1 ± 54.2) did not present significant differences in weight at the end of the experiment when compared to controls (457.6 ± 36.3) and to the group that treated with refrigerant (413.7 ± 28.1).
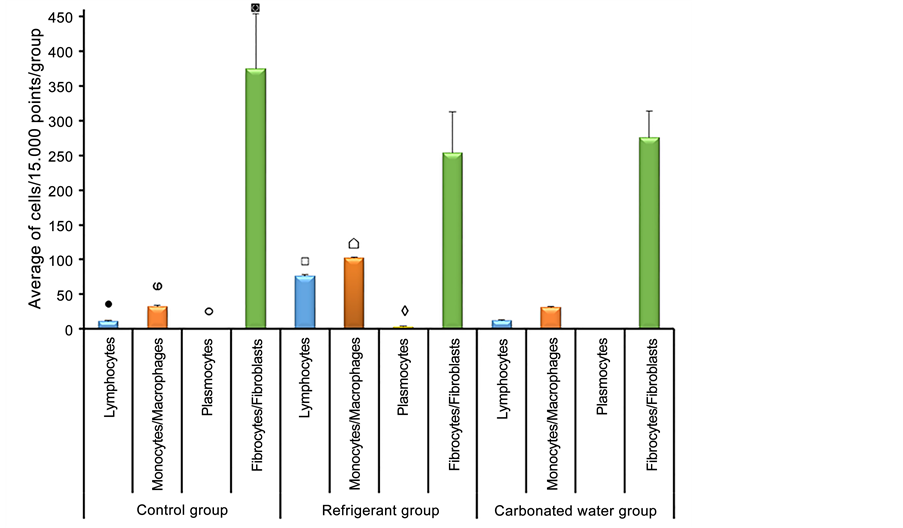
● Significant difference between lymphocytes of Control group x Refrigerant group (Analysis of significant variant (Q = 64.4 and p < 0.01));
Figure 1. Average of the final weight of the control animals (n = 5) and of the groups treated with refrigerant (n = 5) and carbonated water (n = 5) during ninety days.○ P = 0.0105 for Control x Refrigerant.
ⱷSignificant difference between monocytes/macrophages of Control group x Refrigerant group (Analysis of significant variance (Q = 70.2 and p < 0.01));
○ Significant difference between plasmocytes of Control group x Refrigerant group (Analysis of significant variance (Q = 2.8 and p < 0.01));
◙Significant difference between fibrocytes/fibroblasts of Control group x Refrigerant group (Analysis of significant variance (Q = 120.8 and p < 0.05));
□ Significant difference between lymphocytes of Refrigerant group x Carbonated water group (Analysis of significant variance (Q = 63.4 and p < 0.01));
⌂Significant difference between monocytes/macrophages of Refrigerant group x Carbonated water group (Analysis of significant variance (Q = 70.8 and p < 0.01));
◊ Significant difference between plasmocytes of Refrigerant group x Carbonated water group (Analysis of significant variance (Q = 3.0 and p < 0.01)).
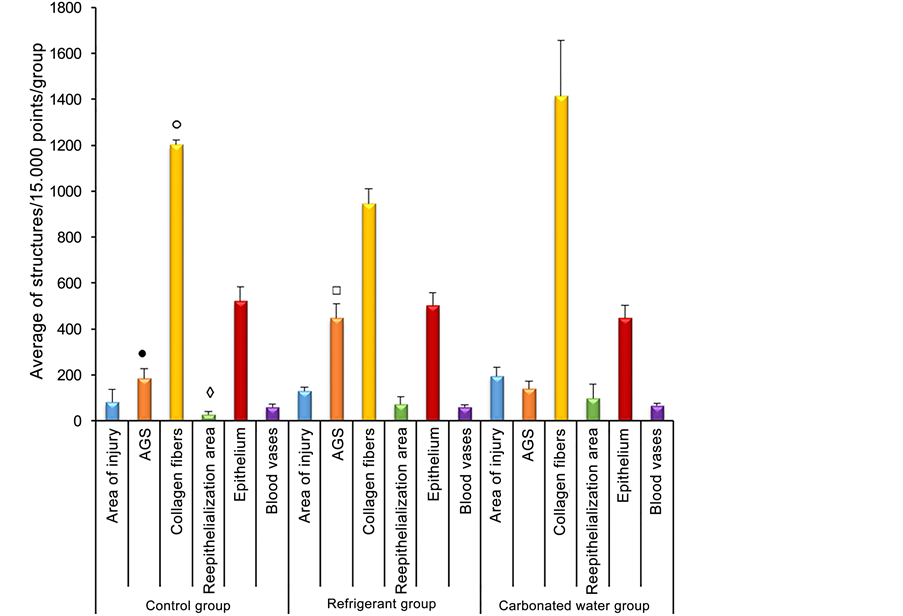
● Significant difference between AGS of Control group x Refrigerant group (Analysis of significant variance (Q = 262.4 and p < 0.01));
○ Significant difference between collagen fibers of Control group x Refrigerant group (Analysis of significant variance (Q = 120.8 and p < 0.05));
◊ Significant difference between reepithelialization area of Control group x Carbonated water group (Analysis of significant variance (Q = 71.0 and p < 0.05));
□ Significant difference between AGS of Refrigerant group x Carbonated water group (Analysis of significant variance (Q = 306.6 and p < 0.01)).
Regarding morphometry, the data obtained are available in Figure 2 and Figure 3. Figure 2 shows that there was a significant difference when comparing the control group (lymphocytes (11 ± 1.30), monocytes/macrophages (32 ± 1.87), Plasma cells (0 ± 0.45), fibrocytes/fibroblasts (375 ± 79.18)) with the refrigerant group (lymphocytes (76 ± 2.30), monocytes/macrophages (102 ± 1.64),
Figure 2. Average of inflammatory infiltrate cells (15.000 points/group) in control ani- mals (n = 5) and groups treated with refrigerant (n = 5) and carbonated water (n = 5) during ninety days. ANOVA, Tukey’s test.
Figure 3. Average of marking structures of healing and repair evolution (15.000 points/ group) in control animals (n = 5) and the groups treated with refrigerant (n = 5) and carbonated water (n = 5) during ninety days. Amorphous Ground Substance (AGS). ANOVA, Tukey’s test.
plasma cells 3 ± 1.58), fibrocytes/fibroblasts (254 ± 58.57)), i.e., an increase in inflammatory cells and the reduction of fibrocytes and fibroblasts were observed in the refrigerant treated group. It was also possible to identify a significant difference when comparing the lymphocytes, monocytes/macrophages and plasma cells of the group that consumed refrigerant to the group treated with carbonated water (lymphocytes (12 ± 1.30), monocytes/macrophages (31 ± 1.14) and plasma cells (0 ± 0)). The comparison of the other data of this figure did not present significant results.
Figure 3 shows that there was a significant difference when buying the control group (amorphous fundamental substance (SFA) (185 ± 42.81) and collagen fibers (1203 ± 18.42)) with the refrigerant treated group (448 ± 61.24), collagen fibers (947 ± 62.65), that is, an increase in SFA and reduction of collagen was observed, suggesting tissue edema. It was also possible to identify a significant difference when comparing the re-epithelialization area of the control group (28 ± 11.15) with the group receiving aerated water (99 ± 61.43), suggesting a tissue repair mechanism in the group treated with water Gaseified In addition, the comparison between the SFA of the group that consumed refrigerant with the group treated with carbonated water (141 ± 31.58) presented a significant difference, reinforcing the idea of edema in the esophagus of the group that consumed refrigerant.
The results of the histopathological analysis are recorded in Figure 4 and allow observing the main changes installed by the ingestion of refrigerant and carbonated water in the groups used in this experiment.
In the esophagus of the animals that ingested the refrigerant (Figure 4), it was possible to identify a diffuse inflammatory infiltrate in 87% of the cuts and focal inflammation in 13% of the analyzed cuts. Chronic inflammation installed could be classified as mild or moderate in 80% of the cuts and in 20% that inflammation was intense. In the underlying muscle tissue, a mild inflammatory infiltrate was also observed in 7.5% of the samples. In all fifteen analyzed fields in this group they showed inflammation.
In the group of animals fed with carbonated water (Figure 4), diffuse mild inflammation was also observed in 13% of the sample. In 87% of the analyzed fields, inflammation was not observed. In both groups treated with refrigerant or carbonated water it was possible to observe areas of injury.
During the analysis of the control group (Figure 4), the esophageal tissue with normal characteristics was observed, evidencing the non-keratinized stratified squamous epithelium, with its own thin lamina and muscular layer of the mucosa without inflammatory infiltrate. Blood vessels could be visualized by irrigating the underlying connective tissue.
4. Discussion
Soda is often indicated as an important risk factor for weight gain because of its low satiety power and high sugar concentration [18] [19] . An American study in Texas evaluated 15,283 adolescents (7573 boys and 7748 girls), showing that the daily consumption of only one can of soda can lead to a weight gain of 15 kg in a year [20] .
In this study, the relationship between soda intake and weight gain was not established. The weight loss observed in the group that consumed soda may be related to the harmful effect of the beverage on its intestinal physiology. Since the animals that consumed soda, presented from the third week, a picture of diarrhea, with liquid and foul-smelling stools that persisted throughout the expe-
Figure 4. Sections of esophagus stained by HE. (A) Control animal. Presence of blood vessel (V), connective tissue where fibrocytes/fibroblasts (arrows), integral epithelium (1) and smooth muscle tissue (2), 20x. (B) Animal subjected to treatment with refrigerant. It is observed the presence of intense and diffuse inflammatory infiltrate (arrows), 20x. (C) Animal subjected to treatment with carbonated water. Absence of inflammatory infiltrate and presence of thin and scaly epithelium (1), 20x. (D) Animal subjected to treatment with refrigerant. It is observed presence of intense focal inflammation (arrows) in the connective and muscular tissue. 20x. (E) Animal subjected to treatment with refrigerant. Presence of epithelial degeneration/necrosis (1) and edema in connective and muscular tissue (arrows), 20x. (F) Animal subjected to treatment with refrigerant. Presence of intense and diffuse inflammation (arrows), 40x.
rimental period. This finding is consistent with Mackenzie et al., (1992) [21] , who, when treating rats with Caramel IV, a component of soft drinks, noticed this same intestinal effect.
The weight comparison between the control group and the group that consumed aerated water showed no significant difference. However, other studies report that animals submitted to treatment with carbonated water had a greater weight gain than the control group [22] .
Another parameter investigated in this study is the characterization of the inflammatory infiltrate installed in the esophageal tissue. After the ninety-day experimental period, the animals in the refrigerant-treated group showed incr- eased mononuclear inflammatory cells (lymphocytes, plasma cells, monocytes and macrophages) characterizing a chronic inflammatory infiltrate. This finding is consistent with the study by Hamaguchi et al., (2003) [23] that studied the expression of cytokines and molecules in rats with chronic esophagitis, observing an inflammatory esophageal infiltrate consisting mainly of macrophage, twenty- one days after induction of reflux Chronic acid. It is important to emphasize that the esophageal mucosa responds to aggression in different ways, and the macroscopic appearance of esophagitis varies with the duration and severity of the disease [24] .
Chronic inflammation is triggered by the presence of the damaging agent and the concomitant occurrence of destruction and tissue repair. The characteristic infiltrate of this process consists of mononuclear cells (monocytes, macrophages and lymphocytes), signs of angiogenesis and fibrosis. Several stimuli may induce chronification of the inflammatory process, such as intracellular bacteria, chemicals and even physical agents [25] .
In addition, it was identified the increase of SFA in the esophagus of animals that consumed soft drinks, suggesting edema. The fundamental substance is an amorphous material that binds tissues and fluids allowing the diffusion of gases and metabolic compounds. The increase of the tissue fluids within the amorphous matrix of the fundamental substance occurs in areas of injury and inflammation [26] .
Despite the known growth factors that stimulate fibroblast proliferation, matrix secretion, collagen and leukocyte recruitment, this study evidenced the relative reduction of fibroblasts and collagen fibers in the groups treated with refrigerant [27] . This can be justified by the expressive increase of SFA in relative values, thus not excluding an absolute increase of fibroblasts and collagen in this group.
As for the effect of aerated water in relation to the morphometric analysis, results similar to the control could be observed, no inflammatory infiltrate or har- mful effects were observed. However, recent studies have shown that carbonated and citrus-flavored water have dental erosive potential that can be verified with a scanning electron microscope [28] .
It was also possible to notice the increase in the area of re-epithelialization in the animals that consumed gaseified water in relation to the control, raising the hypothesis of possible previous tissue injury. According to Enwemeka (2004) [29] , tissue healing is suggested by the reduction of the inflammatory process and edema, increase of phagocytosis and collagen synthesis and reepithelialization. Reepithelialization of the esophageal mucosa takes on average 30 days, but if there is destruction of the submucosa, 120 or more days may be necessary for its recovery [30] [31] .
As for the histopathological analysis performed in the present study, the esophagus of the animals that ingested refrigerant had a diffuse inflammatory infiltrate in 87% of the cuts and focal in 13% of the cuts. Chronic inflammation was classified as mild or moderate in 80% of the cuts and as intense in 20%. These findings are consistent with Santos et al., (2016) [17] who studied the effect of the coolant on the liver of rats, identifying in the parenchyma of this gland the presence of diffuse inflammatory infiltrate characterized as mild in 64% of the fields, moderate in 32% And intense in 8% of the samples.
5. Conclusion
The results demonstrate that treatment with refrigerant induced the following structural changes in the rats: significant reduction of weight in relation to the control group; inflammatory infiltrate predominantly diffuse mild to moderate and tissue edema. However, the rats treated with carbonated water had similar results to the control, besides signs of the mechanism of healing and repair. The lack of articles that evaluate the effects of refrigerant and carbonated water mainly considering the morphological alterations in the esophagus made it difficult to discuss the results. In view of the findings reported here, it is suggested that new studies be undertaken to understand the systemic effects of carbonated drinks in the human body.
Acknowledgements
To all who directly or indirectly contribute to this work.
Cite this paper
de Oliveira, T.D., Gaspar, M.E., Braga, L.M.V., Rodrigues, J.M., Santos, R.N.F., Moura, S.G., Bicalho, N.B., Rocha, L.L.V., da Costa, D.A. and de Mello Pinto, M.V. (2017) Effects of Gaseous Drinks in Wistar Rats Esophagus. Journal of Biosciences and Medicines, 5, 32-43. https://doi.org/10.4236/jbm.2017.55004
References
- 1. Bleil, S.I. (1998) O padrão alimentar ocidental: Consideracoes sobre a mudanca de hábitos no Brasil. Cad Debate, 6, 1-25.
- 2. Willett, W.C. (2001) Eat, Drink, and Be Healthy: The Harvard Medical School Guide to Healthy Eating. Simon and Schuster, New York.
- 3. Grimm, G.C., Harnack, L. and Story, M. (2004) Factors Associated with Soft Drink Consumption in School-Aged Children. Journal of the American Dietetic Association, 104, 1244-1249.
https://doi.org/10.1016/j.jada.2004.05.206 - 4. Vartanian, L.R., Schwartz, M.B. and Brownell, K.D. (2007) Effects of Soft Drink Consumption on Nutrition and Health: A Systematic Review and Meta-Analysis. American Journal of Public Health, 97, 667-675.
https://doi.org/10.2105/AJPH.2005.083782 - 5. Malik, V.S., Schulze, M.B. and Hu, F.B. (2006) Intake of Sugar-Sweetened Beverages and Weight Gain: A Systematic Review. The American Journal of Clinical Nutrition, 84, 274-288.
- 6. Eweis, D.S., Abel, F. and Stiban, J. (2017) Carbon Dioxide in Carbonated Beverages Induces Ghrelin Release and Increased Food Consumption in Male Rats: Implications on the Onset of Obesity. Obesity Research & Clinical Practice, 645.
https://doi.org/10.1016/j.orcp.2017.02.001 - 7. El-Terras, A., Soliman, M.M., Alkhedaide, A., Attia, H.F., Alharthy, A. and Banaja, A.E. (2016) Carbonated Soft Drinks Induce Oxidative Stress and Alter the Expression of Certain Genes in the Brains of Wistar Rats. Molecular Medicine Reports, 13, 3147-3154.
- 8. Alkhedaide, A., Soliman, M.M., Salah-Eldin, A.E., Ismail, T.A., Alshehiri, Z.S. and Attia, H.F. (2016) Chronic Effects of Soft Drink Consumption on the Health State of Wistar Rats: A Biochemical, Genetic and Histopathological Study. Molecular Medicine Reports, 13, 5109-5117.
https://doi.org/10.3892/mmr.2016.5199 - 9. Alkhedaide, A., Soliman, M.M. and Ibrahim, Z.S. (2016) Carbonated Soft Drinks Alter Hepaticcytochrome P450 Isoform Expression in Wistar Rats. Biomedical Reports, 5, 607-612.
- 10. Celec, P., Palffy, R., Gardlík, R., Behuliak, M., Hodosy, J., Jáni, P., Bozek, P. and Sebeková, K. (2010) Renal and Metabolic Effects of Three Months of Decarbonated Cola Beverages in Rats. Experimental Biology and Medicine (Maywood), 235, 1321-1327.
https://doi.org/10.1258/ebm.2010.010051 - 11. Eluwa, M.A., Inyangmme, I.I., Akpantah, A.O., Ekanem, T.B., Ekong, M.B., Asuquo, O.R. and Nwakanma, A.A. (2013) A Comparative Study of the Effect of Diet and Soda Carbonated Drinks on the Histology of the Cerebellum of Adult Female Albino Wistar Rats. African Health Sciences, 13, 541-549.
https://doi.org/10.4314/ahs.v13i3.1 - 12. Botezelli, J.D., Dalia, R.A., Reis, I.M., Barbieri, R.A., Rezende, T.M., Pelarigo, J.G., Codorno, J., Goncalves, R. and Mello, M.A. (2010) Chronic Consumption of Fructose Rich Soft Drinks Alters Tissue Lipids of Rats. Diabetology & Metabolic Syndrome, 2, 43.
- 13. Prado, F.C., Ramos, J. and Valle, J.R. (2001) Atualizacao terapêutica. 20a Edition, Artes Médicas, Sao Paulo.
- 14. Kapicioglu, S., Bakil, A., Reis, A. and Tekelioglu, Y. (1999) Cola Drinks Consumption and Oesophagitis. Diseases of the Esophagus, 12, 306-308.
https://doi.org/10.1046/j.1442-2050.1999.00022.x - 15. Goldberg, H.I., Dodds, W.J., Gee, S., Montgomery, C. and Zboralske, F.F. (1969) Role of Acid and Pepsin in Acute Experimental Oesophagitis. Gastroenterology, 56, 223.
- 16. Moritaka, H., Kitade, M., Sawamura, S., Takihara, T., Awano, I., Ono, T., Tamine, K. and Hori, K. (2014) Effect of Carbon Dioxide in Carbonated Drinks on Linguapalatal Swallowing Pressure. Chemical Senses, 39, 133-142.
https://doi.org/10.1093/chemse/bjt062 - 17. Santos, R.N.F., Moura, S.G., Bicalho, N.B., Pereira, M.A., Ferreira, H.D.C., Sampaio, M.B., Costa, R.S., Neto, W.L. and Rocha, L.L.V. (2016) Efeitos de bebidas gaseificadas em diferentes órgaos do sistema digestório. Brazilian Journal of Surgery and Clinical Research, 13, 6-13.
- 18. Berkey, C.S. (2004) Sugar-Added Beverages and Adolescent Weight Change. Obesity Research, 12, 778-788.
https://doi.org/10.1038/oby.2004.94 - 19. Gomez-Martinez, S., Martín, A., Romeo, J., Castillo, M., Mesena, M., Baraza, J.C., Jiménez-Pavón, D., Redondo, C., Zamora, S. and Marcos, A. (2009) Is Soft Drink Consumption Associated with Body Composition? A Cross-Sectional Study in Spanish Adolescents. Nutricion Hospitalaria, 24, 97-102.
- 20. Ranjit, N., Evans, M.H., Byrd-Williams, C., Evans, A.E. and Hoelscher, D.M. (2011) Dietary and Activity Correlates of Sugar-Sweetened Beverage Consumption among Adolescents. Pediatrics, 126, 754-761. https://doi.org/10.1542/peds.2010-1229
- 21. Mackenzie, K.M., Boysen, B.G., Field, W.E., Petsel, S.R.W., Chappel, C.L., Emerson, J.L., et al. (1992) Toxicity Studies of Caramel Colour III and 2-Acetyl-4(5)-Tetra-Hydroxybutyl Imidazole in F344 Rats. Food and Chemical Toxicology, 30, 417-425.
https://doi.org/10.1016/0278-6915(92)90069-W - 22. Santiago, J.R.F. (2006) Efeitos de ingestao de água gaseificada sobre o peso, morfologia gástrica e parametros laboratorias da funcao metabólica de ratos. [tese] Botucatu: Universidade Estadual Paulista, Faculdade de Medicina de Botucatu.
- 23. Hamaguchi, M., Fujiwara, Y., Takashima, T., Hayakawa, T., Sasaki, E., Shiba, M., Watanabe, T., Tominaga, K., Oshitani, N., Matsumoto, T., Higuchi, K. and Arakawa, T. (2003) Increased Expression of Cytokines and Adhesion Molecules in Rat Chronic Esophagitis. Digestion, 68, 189-197. https://doi.org/10.1159/000075698
- 24. Vieira, M.C., Pisani, J.C. and Mulinari, R.A. (2004) Patologia do esofago distal deve complementar a endoscopia digestiva alta. The Journal of Pediatrics, 80, 197-202.
- 25. Medzhitov, R. (2008) Origin and Physiological Roles of Inflammation. Nature, 454, 428-435.
https://doi.org/10.1038/nature07201 - 26. Nanci, A. (2013) Ten Cate Histologia Oral. 8a Edition, Elsevier, Rio de Janeiro.
- 27. Aarabi, S., Longaker, M.T. and Gurtner, G.C. (2007) Hypertrophic Scar Formation Following Burns and Trauma: New Approaches to Treatment. PLoS Medicine, 4, 234.
https://doi.org/10.1371/journal.pmed.0040234 - 28. Brown, C.J., Smith, G., Shaw, L, Parry, J. and Smith, A.J. (2007) The Erosive Potential of Flavoured Sparkling Water Drinks. International Journal of Paediatric Dentistry, 17, 86-91.
https://doi.org/10.1111/j.1365-263X.2006.00784.x - 29. Enwemeka, C.S., Parker, J.C., Dowdy, D.S., Harkness, E.E., Sanford, L.E. and Woodruff, L.D. (2004) The Efficacy of Low-Power Lasers in Tissue Repair and Pain Control: A Meta-Analysis Study. Photomedicine and Laser Surgery, 22, 323-329.
https://doi.org/10.1089/pho.2004.22.323 - 30. Kikendal, C.J.W. (1991) Caustic Ingestion Injuries. Gastroenterology Clinics of North America, 20, 847-857.
- 31. Berthet, B., Castellani, P., Brioche, M.I., Assdourian, R. and Gauthier, A. (1996) Early Operation for Severe Corrosive Injury of the Upper Gastro Intestinal Tract. European Journal of Surgery, 162, 951-955.