International Journal of Clinical Medicine
Vol.5 No.3(2014), Article ID:42457,7 pages DOI:10.4236/ijcm.2014.53019
Improved Sustained Virological Response Following Treatment with Pegylated-Interferon Alpha-2b Compared with Alpha-2a, Both with Ribavirin, for Chronic Hepatitis C Infection with Genotypes 2 and 3
1School of Medicine and Pharmacology, Fremantle Hospital, University of Western Australia, Crawley, Australia; 2Hepatitis Services, Fremantle Hospital, Fremantle, Australia; 3Royal Perth Hospital, Perth, Australia.
Email: mollison@cyllene.uwa.edu.au
Copyright © 2014 Lindsay C. Mollison et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Lindsay C. Mollison et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received October 6th, 2013; revised November 3rd, 2013; accepted December 1st, 2013
KEYWORDS
Hepatitis C; Pegylated Interferon; Ribavirin; SVR; Multivariate Analysis; Genotype 2; Genotype 3
ABSTRACT
Purpose: The optimal formulation of pegylated interferon ( (PEG-IF() as a part of combination therapy with ribavirin (RBV) is uncertain for patients infected with hepatitis C Genotypes 2 and 3. Methods: A multivariate analysis of prospectively collected treatment data from two tertiary centres on 351 treatment naïve HCV Genotype 2 or 3 patients who received PEG-IF(-2a or b plus ribavirin. Results: Univariate analyses demonstrate that PEG-IF(-2b based on regimens achieved a higher sustained virological response (SVR) than PEG-IF(-2a (77.9% versus 62.0%, P = 0.0012). On multivariate analyses, PEG-IF(-2b appeared superior to PEG-IF(-2a with an odds ratio (OR) and 95% confidence interval (CI95) for SVR of 2.19 (CI95 1.35 - 3.52, P = 0.0005). Genotype was a significant predictor of outcome in the multivariate model with 80% of Genotype 2 but only 67.7% of Genotype 3 subjects achieving SVR (OR 2.66 [CI95 1.35 - 5.92]). Increasing age was negatively associated with SVR (OR 0.97 [CI95 0.94 - 0.99]). Some of the differences in SVR are explained by higher relapse rates with PEG-IF(-2a (P = 0.009). Conclusions: PEG-IF(-2b and RBV achieve higher SVR rates than PEG-IF(-2a and RBV in Genotypes 2 and 3 chronic HCV infections. There is less relapse with PEG-IF(-2b. Genotype 2 infections are considerably easier to cure. SVR is higher in younger patients. These findings should influence a choice of PEG-IF( in the era of direct acting anti-viral drugs in therapy of Genotypes 2 and 3.
1. Introduction
Chronic hepatitis C virus (HCV) infection is a significant public health issue. Worldwide, approximately 170 million people are infected [1]. The risk of developing chronic infection after acute exposure to HCV approaches 85% [2]. It is estimated that approximately 1% of the Australian community have chronic HCV infection [2]. Approximately 5% - 20% of chronically infected patients progress to cirrhosis in the long term with 3% - 5% of these patients developing hepatocellular carcinoma annually (HCC) [3].
Successful treatment of chronic HCV infection is defined by a negative PCR for HCV-RNA 6 months post therapy—a sustained virological response (SVR). Interferon (IF) alpha was the first established treatment [4], with further enhancement of outcomes seen after the addition of the antiviral nucleoside analogue, ribavirin (RBV) [5,6]. The advent of pegylated (PEG) forms of IF alpha led to further improvements in response rates [7,8]. The combination of PEG-IF with ribavirin has remained the standard of care throughout the 2000s [9-11].
With broadening of choices of therapy to include genotype specific direct acting antivirals (DAAs) [12,13] and the understanding that numerous host and viral factors affect outcomes, choices affecting therapy are likely to become highly individualised [14]. As such, any additional information that might affect the choice of IF backbone to that therapy is especially valuable. International guidelines [15] suggest that there is no practical difference between the two commercially available formulations: PEG-IFa-2a (Pegasys, Roche Pharmaceuticals, Geneva, Switzerland) and PEG-IFa-2b (Pegintron, Schering Plough Corporation, NJ, USA) both combined with RBV. A recent Cochrane review and meta-analysis [16] have reported improved SVR with PEG-IFa-2a, particularly for HCV Genotype 1. However, the evidence for the optimal choice of PEG-IFa in the treatment of other HCV genotypes is less clear.
The aim of this study was to compare treatment outcomes between PEG-IFa-2a and PEG-IFa-2b (plus RBV) in patients with Genotype 2 and 3 infections, in a “realworld” clinical environment, through a retrospective review of our experience in two large, tertiary-referral, hospital-based hepatitis treatment services in Australia.
2. Materials and Methods
Data on all patients treated for HCV between 2002 and 2008 were prospectively collected through the clinical hepatitis services of two tertiary metropolitan hospitals in Perth, Western Australia [Fremantle Hospital (FH) and Royal Perth Hospital (RPH)]. These two hospitals serve a population of approximately one million. Patient demographic factors, viral genotype, liver biopsy result, and treatment allocation were prospectively documented. HCV viral load at baseline was not routinely available at our centre during this time. The degree of histologic liver fibrosis was reported using the METAVIR score [17].
The choice of PEG-IF formulation was at the treating physician’s discretion and was prescribed with RBV according to licensed indications. Haematological side effects were managed by dose reduction of RBV or PEGIF in a standard fashion. Sustained virological response was the primary outcome of interest and was defined by the presence of a negative HCV PCR result 6 months after cessation of treatment. Any patient who received at least one dose of PEG-IF therapy, but did not attain this outcome was considered to have not achieved SVR. An end of treatment (EOT) HCV PCR was considered a secondary outcome as was relapse during the 6 month period after cessation of treatment.
We used R for all basic and multivariate analyses [18]. Data are presented as median (interquartile range [IQR]) or proportions for continuous and categorical variables, respectively. Mann-Whitney U and Chi-squared tests were applied for univariate analyses for continuous and categorical variables, respectively. The METAVIR score was considered to be an ordinal variable. Multivariate analysis was performed using logistic regression. Variables other than age were included based on biological plausibility and P < 0.10 on univariate analysis. Backward stepwise logistic regression was applied and the most parsimonious model was chosen using Aikake’s Information Criterion (AIC). To account for the large amount of missing data for weight and fibrosis scores, we imputed missing data using the R package “AMELIA” [19]. Briefly, each variable of interest was defined as nominal, ordinal or continuous. Non-parametric, continuous variables were log-transformed and intuitive constraints placed on the possible output data. Following multivariate imputation, AMELIA provides visual and statistical diagnostics that ensure that the imputed data are representative of measured data and consistent with clinical expectations. The output from AMELIA consists of 5 complete datasets. Logistic regression modeling was then performed on each of the 5 completed imputed datasets and the final adjusted odds ratios determined by calculating the mean from each model [19].
3. Results
During the study period, 730 patients with HCV received at least one dose of PEG-IF and RBV and were included in the analysis. Of these, 351 were infected with either HCV Genotypes 2 or 3. The baseline demographic and laboratory characteristics of the study population are presented in Table 1. The median (IQR) age at treatment was 42 (33 - 48) years. There was a predominance of male subjects (64.7%). A liver biopsy was performed on 54% of participants with the majority of these patients found to have early stage fibrosis. There was an even spread of prescription of the two interferon types across each of the study centres (48% and 54% for PEG-IFa-2a at FH and RPH, respectively [P = 0.30]). Details regarding weight were available for 48% of patients.
Comparisons according to PEG-IFa formulation by univariate analysis are shown in Table 2. The overall SVR rate was superior for PEG-IFa-2b compared to PEG-IFa-2a (77.9% versus 62.0%; odds ratio (OR) for SVR of 2.16 [CI95 1.35 - 3.46; P = 0.0012]) (Figure 1). The only other factor associated with SVR was lower age with the median age of those attaining an SVR being 41 years versus 44 years for non-SVR (OR 0.98 CI95 0.95 - 1.00, P = 0.04). Multivariate analysis confirmed a superior SVR with PEG-IFa-2b (OR 2.19; CI95 1.35 - 3.52). Patient age remained independently associated with SVR (OR 0.97; CI95 0.94 - 0.99). HCV Genotype 2 had a significantly higher SVR rate than Genotype 3 (OR 2.66; CI95 1.28 - 5.97).
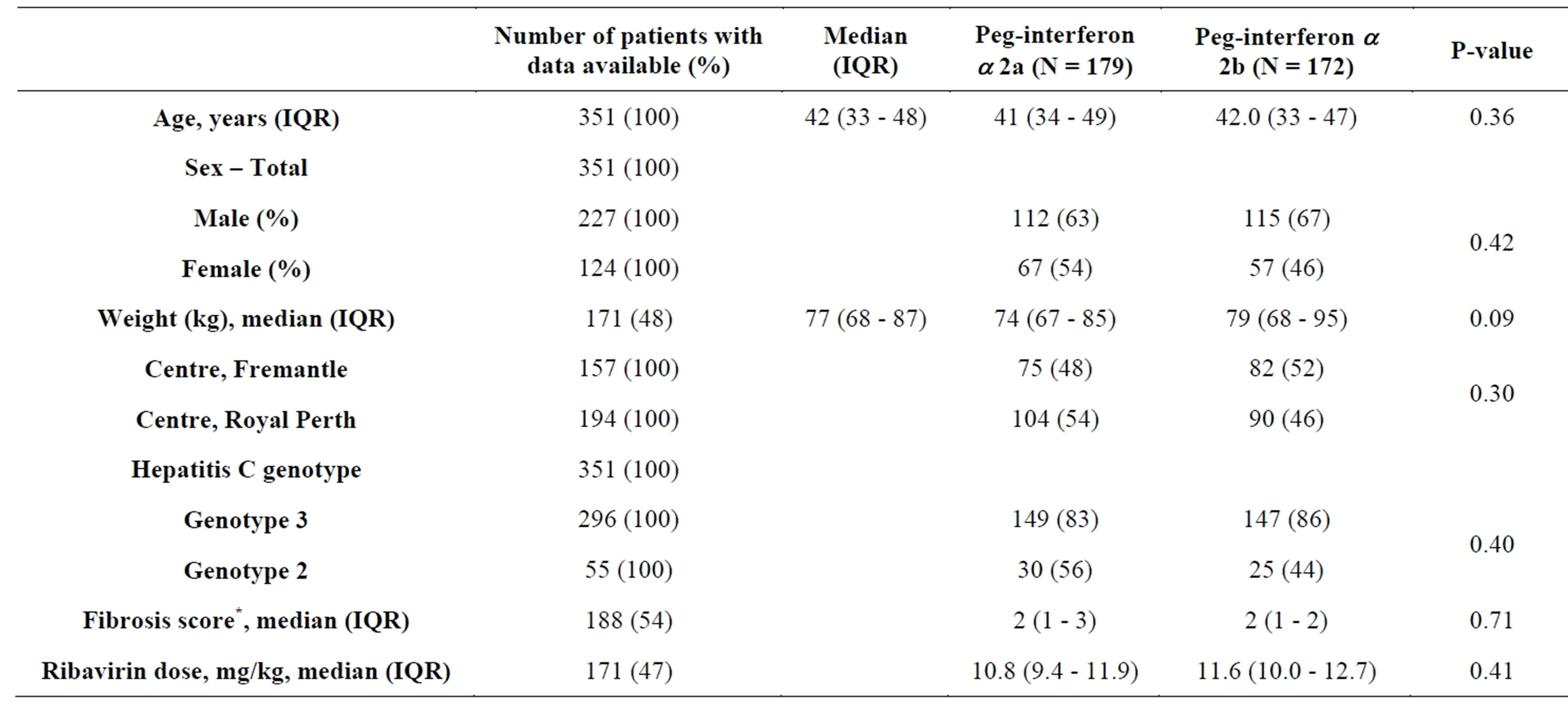
Table 1. Baseline demographics and univariate comparisons between patients receiving different formulations of pegylated interferon alpha. *METAVIR fibrosis score [17].
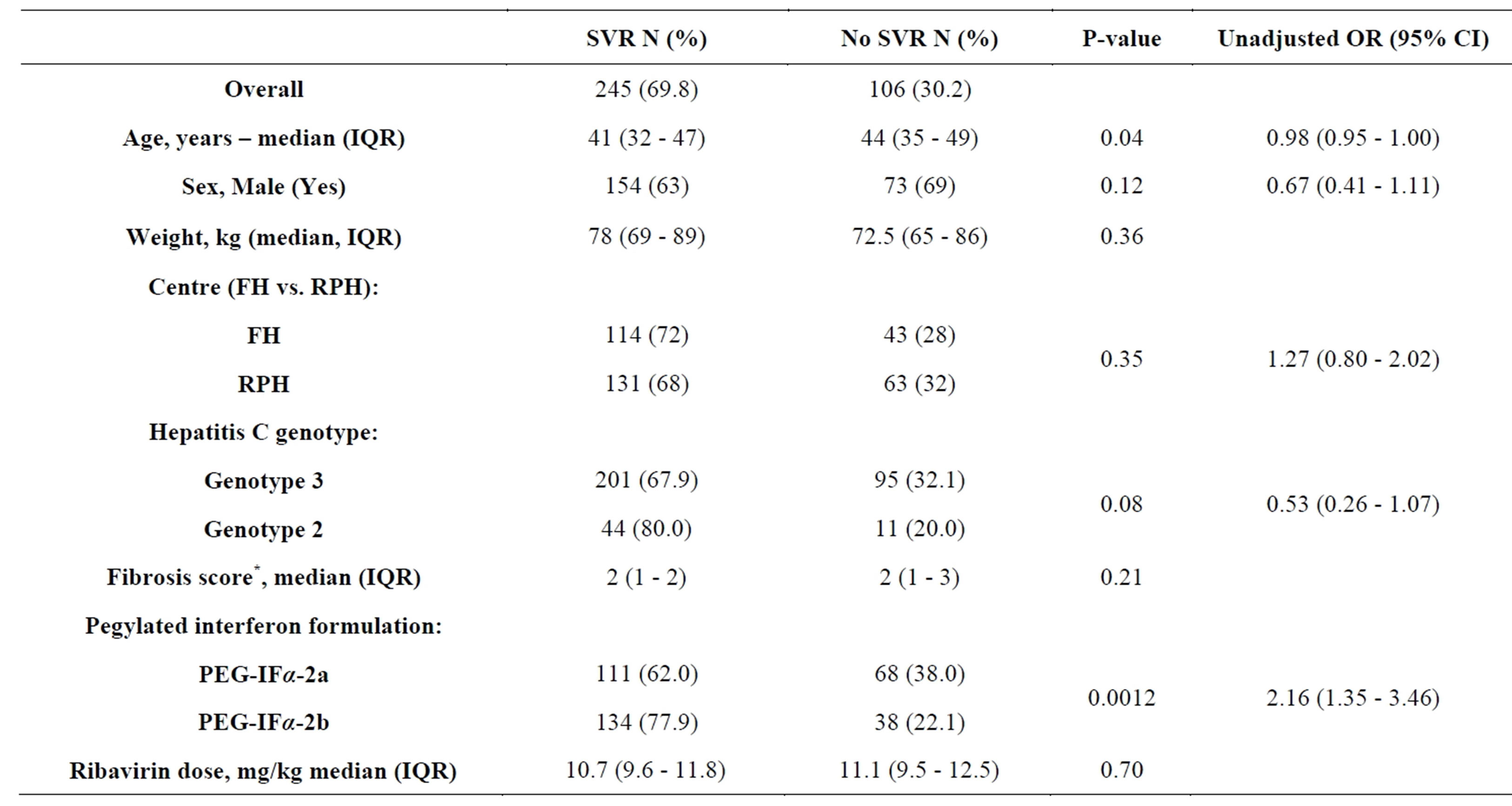
Table 2. Predictors of sustained virological response (SVR) in patients treated with pegylated-interferon and ribavirin for chronic hepatitis C infection with Genotypes 2 and 3. Data are shown as percentages (%) or medians with interquartile ranges (IQR). Odds ratios are given with 95% confidence intervals. *METAVIR fibrosis score [17].
Logistic regression on the 5 imputed datasets from AMELIA did not identify any additional independent variables associated with SVR (data not shown).
In our study, patients with HCV Genotype 2 were significantly older (median 48 years versus 40, P < 0.001) and fewer were male (53% versus 67%, P = 0.04) than patients with Genotype 3. Genotype-specific logistic regression did not identify any variable to be independently associated with SVR for Genotype 2, whilst only PEGIFα-2b (OR 2.64; CI95 1.59 - 4.44) and lower age (OR 3.34; CI95 1.42 - 10.1, P = 0.01) were independently associated with SVR for Genotype 3.
In analyses for end of treatment HCV RNA PCR negativity (EOT response) PEG-IFα-2b formulation (OR
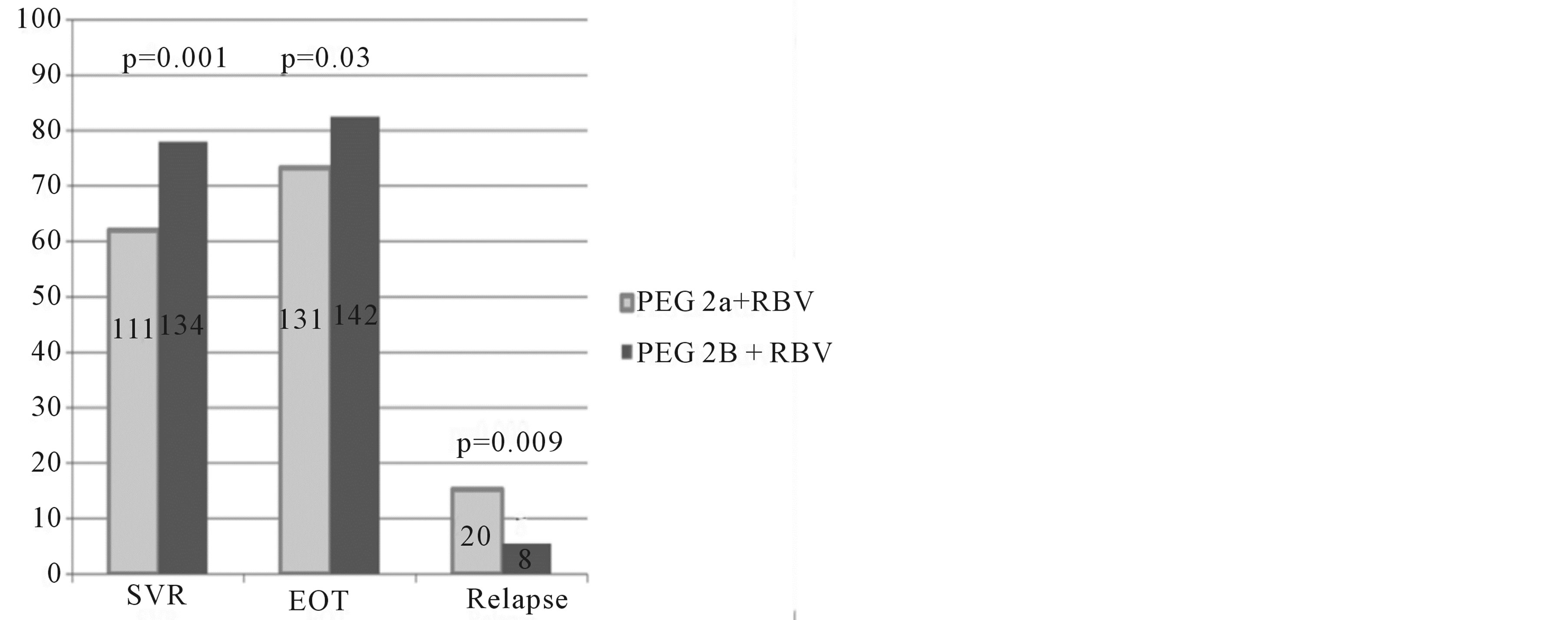
Figure 1. Intention to treat analysis for virologic response rates by pegylated interferon alpha type for the study population. Percentage (y axis) and numbers (text in columns) of sustained virological response (SVR), end of treatment response with viral undetectability (EOT), and relapse after EOT, with Chi-square P values comparing pegylated (PEG) interferon a 2a and 2b plus ribavirin (RBV) in Genotype 2 and 3 infected patients.
1.79; CI95 1.07 - 3.03, P = 0.03) and Genotype 2 (OR 3.34; CI95 1.42 - 10.1, P = 0.01) were independently associated with EOT response. Subsequent relapse was higher with PEG-IFα-2a (P = 0.009) (see Figure 1).
4. Discussion
Our study demonstrates that PEG-IFa-2b provides higher SVR cure rates in chronic HCV infection Genotypes 2 and 3 than PEG-IFa-2a, when both are combined with RBV. Additionally, we observe a higher SVR for HCV Genotype 2 than for Genotype 3.
Patients and clinicians now face ever more individualised and complex therapeutic choices flowing from recent developments such as the discovery of the importance of the IL28B polymorphism on response rates in Genotype 1 infection [20-22] and the arrival in the last 12 months of DAAs [13]. Incorporating our findings into this mix may allow therapy in the future to be even more effective and individualised [14].
Throughout the 2000s, therapy for chronic HCV infection has been based on the use of PEG-IFa-2a or 2b, in combination with RBV [12]. Yet the choice of PEG-IFa backbone has remained controversial despite the large IDEAL study [23]. In this study, 3070 treatment naïve HCV Genotype 1 patients, were enrolled in a 3-armed, open label, randomised, parallel group, multicentre study that compared two different PEG-IFa-2b dosing regimens with PEG-IFa-2a, all given for 48 weeks. There was no statistically significant difference in SVR between the groups.
Two meta-analyses, published simultaneously, have reported superiority of PEG-IFa-2a when compared with PEG-IFa-2b for HCV Genotype 1 infection [16,24]. Because of inconsistencies in study selection, inclusion criteria and randomisation stratification, critics have urged caution in applying these results to HCV treatment in all clinical situations [25].
Such a situation is treatment of HCV Genotypes 2 and 3. In the Cochrane analysis, only 5 studies were selected for inclusion in the Genotype 2/3 sub-group analyses [16]. These studies included those only published in abstract form, [26] studies enrolling HIV co-infected patients [27] and studies of previous non-responders, [28] often with small patient numbers [27,28]. Furthermore, because the reported SVR was particularly high in at least two studies, it is likely that they lacked statistical power to detect differences of limited magnitude [26,29,30].
At least one other study seems not to have been included at all [31]. In this study, no statistically significant difference in SVR was observed between different formulations, but the authors noted lower relapse rates with PEG-IFa-2b and RBV suggesting that cost effectiveness issues might favour that choice, irrespective of SVR, a proposition also favoured by other commentators [32].
Given the limitations of other studies and their associated meta-analyses, the present study, involving over 350 patients in a “real-world” clinical environment extends the literature on this topic. As in the Genotype 1 IDEAL [23], study we found a similar EOT response with PEGIFa-2a and 2b, but higher relapse rates within the PEGIFa-2a group. In HCV Genotype 1 infection, relapse has been postulated to be related to levels of RBV or IF drug exposure during treatment [33,34]. In Australia, weight based on RBV is not licensed for HCV Genotype 2 and 3 infections treated with PEG-IFa-2a. Despite this in our population, there were no significant differences in weight or RBV dosage between the two groups, so RBV dose is unlikely to explain the observed differences in SVR. Therefore, the formulation of PEG-IFa may be relevant. Commentaries on different pharmacokinetics, binding characteristics, and interferon exposures between the two brands broadly suggest that PEG-IFa-2b exhibits greater virological activity [35-37].
The other important finding in the present study is that SVR for HCV Genotype 2 is better than that for Genotype 3. This phenomenon has been recognised elsewhere and questions the validity of continuing to group these HCV genotypes together [30].
Similar to findings in previous studies, we also observed improved SVR in patients treated at younger ages [38]. The discovery of the role of IL28B polymorphisms in response in Genotype 1 infection has occurred since our study was completed [20-22] and IL28B data were not available for our analysis. Subsequently, other authors using both brands of either PEGor non PEG-IFa together with RBV have found similar IL28B polymorphisms may be important in SVR in infections with other HCV genotypes [38]. However, there is no reason to suspect that there would be any differences in IL28B polymorphisms between our groups treated with either PEG-IF formulation.
This study is limited in that it did not include information about adherence to therapy, a factor important to the outcome [39]. Meta-analyses suggest that PEG-IFa-2a is associated with fewer adverse events leading to treatment discontinuation [16]. Although speculative, this could underestimate the magnitude of the beneficial SVR response observed in our patients with HCV Genotypes 2 and 3 treated with PEG-IFa-2b.
With the addition of direct acting antivirals (DAAs) to the therapy of HCV [12,13], the importance of a difference between the two forms of PEG-IFa might appear irrelevant. But with the high cost of DAAs, increasingly personalised choices based on DAA, viral and host characteristics, and excellent results with the present standard of care in patients with HCV Genotypes 2 and 3, the choice of the optimal PEG-IFa will remain important for those who do not need or who cannot afford new DAAs.
Acknowledgements
We wish to thank all the patients who were treated in our units. We acknowledge the effort of the various members of our teams involved in documenting patient data and collecting it for these analyses.
REFERENCES
- D. Lavanchy, “The Global Burden of Hepatitis C. Liver International,” Official Journal of the International Association for the Study of the Liver, Vol. 29, Suppl. 1, 2009, pp. 74-81.
- Digestive Health Foundation, “Facts about Hepatitis C,” The Gastroenterological Society of Australia, 2007.
- World Health Organization (WHO), “Hepatitis C fact sheet,” World Health Organization, Geneva, 2011.
- J. H. Hoofnagle, K. D. Mullen, D. B. Jones, V. Rustgi, A. Di Bisceglie, M. Peters, J. G. Waggoner, Y. Park and E. A. Jones, “Treatment of Chronic Non-A, Non-B Hepatitis with Recombinant Human Alpha Interferon. A Preliminary Report,” The New England Journal of Medicine, Vol. 315, No. 25, 1986, pp. 1575-1578. http://dx.doi.org/10.1056/NEJM198612183152503
- J. G. McHutchison, S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M.-H. Ling, S. Cort and J. K. Albrecht, for the Hepatitis Interventional Therapy Group, “Interferon Alfa-2b alone or in Combination with Ribavirin as Initial Treatment for Chronic Hepatitis C. Hepatitis Interventional Therapy Group,” The New England Journal of Medicine, Vol. 339, No. 21, 1998, pp. 1485-1492. http://dx.doi.org/10.1056/NEJM199811193392101
- T. Poynard, P. Marcellin, S. S. Lee, C. Niederau, G. S., Minuk, G. Ideo, et al., “Randomised Trial of Interferon Alpha2b plus Ribavirin for 48 Weeks or for 24 Weeks versus Interferon Alpha2b plus Placebo for 48 Weeks for Treatment of Chronic Infection with Hepatitis C Virus,” International Hepatitis Interventional Therapy Group (IHIT),” Lancet, Vol. 352, No. 9138, 1998, pp. 1426-1432. http://dx.doi.org/10.1016/S0140-6736(98)07124-4
- K. L. Lindsay, C. Trepo, T. Heintges, M. L. Shiffman, S. C. Gordon, J. C. Hoefs, E. R. Schiff, Z. D. Goodman, M. Laughlin, R. Yao and J. K. Albrecht, “A Randomized, Double-Blind Trial Comparing Pegylated Interferon Alfa- 2b to Interferon alfa-2b as Initial Treatment for Chronic Hepatitis C,” Hepatology, Vol. 34, No. 2, 2001, pp. 395- 403. http://dx.doi.org/10.1053/jhep.2001.26371
- M. Simin, J. Brok, D. Stimac, C. Gluud and L. L. Gluud, “Cochrane Systematic Review: Pegylated Interferon plus Ribavirin vs. Interferon plus Ribavirin for Chronic Hepatitis C,” Alimentary Pharmacology & Therapeutics, Vol. 25, No. 10, 2007, pp. 1153-1162. http://dx.doi.org/10.1111/j.1365-2036.2007.03294.x
- M. P. Manns, J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, et al., “Peginterferon Alfa-2b plus Ribavirin Compared with interferon alfa-2b plus Ribavirin for Initial Treatment of Chronic Hepatitis C: A Randomised Trial,” Lancet, Vol. 358, 9286, 2001, pp. 958-965. http://dx.doi.org/10.1016/S0140-6736(01)06102-5
- M. W. Fried, M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Häussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman and J. Yu, “Peginterferon Alfa-2a plus Ribavirin for Chronic Hepatitis C Virus Infection,” The New England Journal of Medicine, Vol. 347, No. 13, 2002, pp. 975-982. http://dx.doi.org/10.1056/NEJMoa020047
- S. Zeuzem, T. Berg, B. Moeller, H. Hinrichsen, S. Mauss, H. Wedemeyer, C. Sarrazin, D. Hueppe, E. Zehnter and M. P. Manns, “Expert Opinion on the Treatment of Patients with Chronic Hepatitis C,” Journal of Viral Hepatitis, Vol. 16, No. 2, 2009, pp. 75-90. http://dx.doi.org/10.1111/j.1365-2893.2008.01012.x
- J. H. Hoofnagle, “A Step forward in Therapy for Hepatitis C,” The New England Journal of Medicine, Vol. 360, No. 18, 2009, pp. 1899-1901. http://dx.doi.org/10.1056/NEJMe0901869
- D. M. Jensen, “A New Era of Hepatitis C Therapy Begins,” The New England Journal of Medicine, Vol. 364, No. 13, 2011, pp. 1272-1274. http://dx.doi.org/10.1056/NEJMe1100829
- H. Ochi, C. N. Hayes, H. Abe, Y. Hayashida and T. Uchiyama, N. Kamatani, Y. Nakamura and K. Chayama, “Toward the Establishment of a Prediction System for the Personalized Treatment of Chronic Hepatitis C,” The Journal of Infectious Diseases, Vol. 205, No. 2, 2012, pp. 204-210. http://dx.doi.org/10.1093/infdis/jir726
- European Association for the Study of the Liver, “EASL Clinical Practice Guidelines: Management of Hepatitis C Virus Infection,” Journal of Hepatology, Vol. 55, No. 2, 2011, pp. 245-264. http://dx.doi.org/10.1016/j.jhep.2011.02.023
- T. Awad, K. Thorlund, G. Hauser, D. Stimac, M. Mabrouk and C. Gluud, “Peginterferon Alpha-2a Is Associated with Higher Sustained Virological Response than Peginterferon Alfa-2b in Chronic Hepatitis C: Systematic Review of Randomized Trials,” Hepatology, Vol. 51, No. 4, 2010, pp. 1176-1184. http://dx.doi.org/10.1002/hep.23504
- P. Bedossa and T. Poynard, “An Algorithm for the Grading of Activity in Chronic Hepatitis C. The METAVIR Cooperative Study Group,” Hepatology, Vol. 24, No. 2, 1996, pp. 289-293. http://dx.doi.org/10.1002/hep.510240201
- R Development Core Team, “R: A Language and Environment for Statistical Computing,” R Foundation for Statistical Computing, Vienna, 2012.
- J. Honaker, G. King and M. Blackwell, “Amelia II: A Program for Missing Data,” Journal of Statistical Software, Vol. 45, No. 7, 2011, pp. 1-47.
- D. Ge, J. Fellay, A. J. Thompson, J. S. Simon, K. V. Shianna, T. J. Urban, E. L. Heinzen, P. Qiu, A. H. Bertelsen, A. J. Muir, M. Sulkowski, J. G. McHutchison and D. B. Goldstein1, “Genetic Variation in IL28B Predicts Hepatitis C Treatment-Induced Viral Clearance,” Nature, Vol. 461, No. 7262, 2009, pp. 399-401. http://dx.doi.org/10.1038/nature08309
- V. Suppiah, M. Moldovan, G. Ahlenstiel, T. Berg, M. Weltman, M. L. Abate, M. Bassendine, U. Spengler, G. J Dore, E. Powell, S. Riordan, D. Sheridan, A. Smedile, V. Fragomeli, T. Müller, M. Bahlo, G. J. Stewart, D. R. Booth and J. George for the Hepatitis C Study, “IL28B Is Associated with Response to Chronic Hepatitis C Interferon-Alpha and Ribavirin Therapy,” Nature Genetics, Vol. 41, No. 10, 2009, pp. 1100-1104. http://dx.doi.org/10.1038/ng.447
- Y. Tanaka, N. Nishida, M. Sugiyama, M. Kurosaki, K. Matsuura, N. Sakamoto, et al., “Genome-Wide Association of IL28B with Response to Pegylated InterferonAlpha and Ribavirin Therapy for Chronic Hepatitis C,” Nature Genetics, Vol. 41, No. 10, 2009, pp. 1105-1109. http://dx.doi.org/10.1038/ng.449
- J. G. McHutchison, E. J. Lawitz, M. L. Shiffman, AJ Muir, GW Galler, J McCone, L. M. Nyberg, W. M. Lee, R. H. Ghalib, E. R. Schiff, J. S. Galati, B. R. Bacon, M. N. Davis, P. Mukhopadhyay, K. Koury, S. Noviello, L. D. Pedicone, C. A. Brass, J. K. Albrecht and M. S. Sulkowski for the IDEAL Study Team, “Peginterferon alfa-2b or alfa-2a with Ribavirin for Treatment of Hepatitis C infection,” The New England Journal of Medicine, Vol. 361, No. 6, 2009, pp. 580-593. http://dx.doi.org/10.1056/NEJMoa0808010
- S. M. Alavian, B. Behnava and S. V. Tabatabaei, “The Comparative Efficacy and Safety of Peginterferon Alpha-2a vs. 2b for the Treatment of Chronic HCV infection: A Meta-Analysis,” Hepatitis Monthly, Vol. 10, No. 2, 2010, pp. 121-131.
- D. Kershenobich, L. Munoz, R. Male, J. Gaytan and F. Sanchez, “Proceed with Caution: Peginterferon Alpha-2a versus peginterferon alfa-2b in Chronic Hepatitis C. A Systematic REVIEW of Randomized trials,” Hepatology, Vol. 52, No. 6, 2010, pp. 2240-2241. http://dx.doi.org/10.1002/hep.24025
- A. Kolakowska, H. Berok, M. Wasilewski and A. Horbon, “Relevance between Fibrosis and Response to Treatment with Peginterferon Alfa2a vs Alfa2b with Ribavirin in Chronic Hepatitis C Genotype 3 Patients. Randomized open label study,” Hepatology [ABSTRACT], Vol. 48, 2008, p. 1278.
- M. Laguno, C. Cifuentes, J. Murillas, S. Veloso, M. Larrousse, A. Payeras, L. Bonet, F. Vidal4, A. Milinkovic1, A. Bassa, C. Villalonga, I. Pérez, C. Tural, M. MartínezRebollar, M. Calvo, J. L. Blanco, E. Martínez, J. M. Sánchez-Tapias, J. M. Gatell and J. Mallolas, “Randomized Trial Comparing Pegylated Interferon Alpha-2b versus Pegylated Interferon Alpha-2a, Both plus Ribavirin, to Treat Chronic Hepatitis C in Human Immunodeficiency virus Patients,” Hepatology, Vol. 49, No. 1, 2009, pp. 22-31. http://dx.doi.org/10.1002/hep.22598
- G. Scotto, V. Fazio, C. Fornabaio, A. Tartaglia, R. Di Tullio, A. Saracino and G. Angarano, “Peg-Interferon Alpha-2a versus Peg-Interferon Alpha-2b in Nonresponders with HCV Active Chronic Hepatitis: A Pilot Study,” Journal Interferon and Cytokine Research, Vol. 28, No. 10, 2008, pp. 623-629. http://dx.doi.org/10.1089/jir.2007.0116
- M. G. Rumi, A. Aghemo, G. M. Prati, R. D’Ambrosio, MF Donato, R Soffredini, E. Del Ninno, A. Russo and M. Colombo, “Randomized Study of Peginterferon-Alpha2a plus Ribavirin vs Peginterferon-alpha2b plus Ribavirin in Chronic Hepatitis C,” Gastroenterology, Vol. 138, No. 1, 2010, pp. 108-115. http://dx.doi.org/10.1053/j.gastro.2009.08.071
- A. Ascione, M. De Luca, M. T. Tartaglione, F. Lampasi, G. G. Di Costanzo, A. G. Lanza, F. P. Picciotto, G. Marino-Masrsilia, L. Fontanella and G. Leandro, “Peginterferon Alfa-2a plus Ribavirin Is More Effective than Peginterferon Alfa-2b plus Ribavirin for Treating Chronic Hepatitis C virus Infection,” Gastroenterology, Vol. 138, No. 1, 2010, pp. 116-122. http://dx.doi.org/10.1053/j.gastro.2009.10.005
- A. Escudero, F. Rodriguez, M. A. Serra, J. A. Del Olmo, F. Montes and J. M. Rodrigo, “Pegylated α-Interferon-2a Plus Ribavirin Compared with Pegylated α-Interferon-2b Plus Ribavirin for Initial Treatment of Chronic Hepatitis C Virus: Prospective, Non-Randomized Study,” Journal of Gastroenterology and Hepatology, Vol. 23, No. 6, 2008, pp. 861-866. http://dx.doi.org/10.1111/j.1440-1746.2008.05397.x
- D. C. Malone, T. T. Tran and F. F. Poordad, “Cost-Efficacy Analysis of Peginterferon Alfa-2b Plus Ribavirin Compared with Peginterferon Alfa-2a Plus Ribavirin for the Treatment of Chronic Hepatitis C,” Journal of Managed Care Pharmacy, Vol. 11, No. 8, 2005, pp. 687-694.
- K. R. Reddy, M. L. Shiffman, T. R. Morgan, S. Zeuzem, S. Hadziyannis, F. M. Hamzeh, T. L. Wright and M. Fried, “Impact of Ribavirin Dose Reductions in Hepatitis C Virus Genotype 1 Patients Completing Peginterferon Alfa-2a/Ribavirin Treatment,” Clinical Gastroenterology and Hepatology, Vol. 5, No. 1, 2007, pp. 124-129. http://dx.doi.org/10.1016/j.cgh.2006.10.008
- S. Zopf, C. Herold, E. G. Hahn and M. Ganslmayer, “Peginterferon Alfa-2a Relapse Rates Depend on WeightBased Ribavirin Dosage in HCV-Infected Patients with Genotype 1: Results of a Retrospective Evaluation,” Scandinavian Journal of Gastroenterology, Vol. 44, No. 4, 2009, pp. 486-490. http://dx.doi.org/10.1080/00365520802647400
- C. Dhalluin, A. Ross, W. Huber, P. Gerber, D. Brugger, B. Gsell and H. Senn, “Structural, Kinetic, and Thermodynamic Analysis of the Binding of the 40 kDa PEG-Interferon-α2a and Its Individual Positional Isomers to the Extracellular Domain of the Receptor IFNAR2,” Bioconjugate Chemistry, Vol. 16, No. 3, 2005, pp. 518-527. http://dx.doi.org/10.1021/bc049780h
- M. Silva, J. Poo, F. Wagner, M. Jackson, D. Cutler, M. Grace, R. Bordens, C. Cullen, J. Harvey and M. Laughlin, “A Randomised Trial to Compare the Pharmacokinetic, Pharmacodynamic, and Antiviral Effects of Peginterferon Alfa-2b and Peginterferon Alfa-2a in Patients with Chronic Hepatitis C (COMPARE),” Journal of Hepatology, Vol. 45, No. 2, 2006, pp. 204-213. http://dx.doi.org/10.1016/j.jhep.2006.03.008
- M. O. Silva, “Compare Trial: Updated Data,” Journal of Hepatology, Vol. 49, No. 2, 2008, pp. 288-289. http://dx.doi.org/10.1016/j.jhep.2008.05.009
- C. Sarrazin, S. Susser, A. Doehring, C. M. Lange, T. Muller, C. Schlecker, E. Herrmann, J. Lötsch and T. Berg, “Importance of IL28B Gene Polymorphisms in Hepatitis C Virus Genotype 2 and 3 Infected Patients,” Journal of Hepatology, Vol. 54, No. 3, 2011, pp. 415-421. http://dx.doi.org/10.1016/j.jhep.2010.07.041
- J. G. McHutchison, M. Manns, K. Patel, T. Poynard, K. L. Lindsay, C. Trepo, J. Dienstag, W. M. Lee, C. Mak, J. J. Garaud and J. K. Albrecht, “Adherence to Combination Therapy Enhances Sustained Response in Genotype-1- Infected Patients with Chronic Hepatitis C,” Gastroenterology, Vol. 123, No. 4, 2002, pp. 1061-1069. http://dx.doi.org/10.1053/gast.2002.35950

