International Journal of Clinical Medicine
Vol.4 No.7A1(2013), Article ID:34566,4 pages DOI:10.4236/ijcm.2013.47A1004
Oncogenic Osteomalacia Associated with Phosphaturic Mesenchymal Tumor of the Knee: Case Presentation and Review of the Literature*
![]()
1Department of Surgery, Orthopaedics and Traumatology, University of Verona, Verona, Italy; 2Department of Radiotherapy, University Hospital, Verona, Italy.
Email: #tommasomaluta@yahoo.it
Copyright © 2013 Eugenio Vecchini et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 23rd, 2013; revised May 30th, 2013; accepted June 10th, 2013
Keywords: Four Paraneoplastic Syndrome; Oncogenic Osteomalacia; Phosphaturia; Phosphaturic Mesenchymal Tumors; Knee Localization; Hypophosphatemia; Fibroblast Growth Factor 23 (FGF23); Sero-Negative Spondilo-Arthritis; Complete Recovery; Kidney Tubular Reabsorption
ABSTRACT
Oncogenic osteomalacia (OOM) is an uncommon metabolic and bone disease caused by fibroblast growth factor 23 (FGF23), a phosphaturic factor produced by phosphaturic mesenchymal tumors (mixed connective tissue variant, PMTMCTV) characterized by phosphate leakage from kidneys and subsequent hypophosphatemia. In this paper, we present the case of a patient, 42-year-old woman affected by left side limp and pain involving lumbar spine, pelvis and hip joints, referred to the Rheumatology Department of our Hospital for the treatment of a suspected sero-negative spondilo-arthritis. During hospitalization patient began an immuno-suppressive therapy with TNF-alpha inhibitors associated with Pamidornate, Indometacin, Esomeprazole and vitamin D3. Nevertheless pain did not decrease and a new examination found a worst hypophosphatemia (1 mg/dl) with normal Ca and PTH’s plasma values. During the same check-up a painful bulge on the anterior part of the right knee was observed and the Magnetic Resonance Imaging scan revealed an ovular solid lesion in the soft tissue closed to the upper part of the patella. Histological analysis identified the lesion as a PMTMCTV. After surgical removal patient got complete recovery. We will discuss about diagnostic evaluation, differential diagnosis and treatment.
1. Introduction
Tumor-induced osteomalacia, also known as oncogenic osteomalacia (OOM), is an acquired disorder characterized by marked mineral derange and adjustment of skeletal metabolism [1,2]. Its clinical expressions are hypophosphatemia (because of renal phosphate wasting), osteomalacia, bone distress, proximal muscle weakness, pathological fractures and by this way functional disability. OMM is commonly associated with small and slowgrowing mesenchymal tumors, usually hardly detected [3]. These masses excrete humoral factors such as fibroblast growth factor 23 (FGF23), which inhibits phosphate’s renal tubular reabsorpition and impairs 1,25(OH)2D3’s synthesis. Therefore there is a reduction of circulating- 25(OH)2-vitamin D despite hypophosphatemia [4]. Both biomechanical sings and bone disease can be completely solved by the complete removal of the tumor.
2. Case Report
In November 2009, a 42-year-old woman affected by left side limp and pain involving lumbar spine, pelvis and hip joints, referred to the Rheumatology Department of our Hospital for the treatment of a suspected sero-negative spondilo-arthritis. Patient complained of a migrating pain localised at both shoulders and small joints of hands and feet arisen seven months earlier; a bone scan revealed a huge tracer accumulation on left jaw’s side, on both shoulders, on several costal arches and vertebral bodies, on the left elbow, on small joints of both hands, on the sacrum and on the left femoral head. At the moment of the admission to Our Department the joints interested with pain were: right shoulder (during passive motion), left hip, both sides of the sacroiliac joint, thoracic and lumbar joints during flexion, extension and lateral movements, many distal-interphalangeal feet joints and metatarsal joints bilaterally. Laboratory testing with complete blood count, CRP, ESR, fibrinogen and protein profile, ferritin, electrolytes, liver function, creatinine, CPK, LDH, TSH and T4 were normal. Rhematoid factor and anti-CCP antibodies were not detected; ANA, anti-ENA, anti-cardiolipin, LAC, anti-beta2glicoprotein 1 were all negative. Complement consumption was negative and crioglobulines were absent. Plasma vitamin D was 30.2 ng/ml, CTX and PTH were both normal. Folic acid was 5 ng/ml, vitamin B12 415 pg/ml, Homocysteine 9.8 micromol/L. CEA, CA19-9, CA125, CA15-3, NSE and AFP were absent, as Anti-Entamoeba hystilitica and anti-Taenia solium antibodies. Markers for HLA B27, quantiferon test were negative. Urinalysis revealed few bacteria. The final diagnosis was sero-negative-spondiloarthritis. Therefore patient began an immuno-suppressive therapy with TNF-alpha inhibitors associated with Pamidornate, Indometacin, Esomeprazole and vitamin D3. Nevertheless in the following months pain did not decrease and for this reason in February 2010 the TNFalpha inhibitor therapy was stopped. New examination found a worst hypophosphatemia (1 mg/dl) with normal Ca and PTH’s plasma values. During the same check-up a painful bulge on the anterior part of the right knee was observed and a MRI examination revealed a solid with ovular shape, 40 mm major axis and 25 mm thickness, regular profile, homogeneous matrix, hyperintense T2 signal and hypointense T1 signal in the viewed sequences. No signs of malignancy (Figure 1). This lesion was surgically completely removed in September 2010; during surgery it macroscopically appeared greyish and well capsulated (Figures 2 and 3) and its histopathological diagnosis was of a mesenchymal tumor (mixed connective-tissue) (Figure 4). Looking at the MRI scan report (small size, regular profile with no signs of malignancy) we decided to excise completely the lesion before biopsy, which could seem as invasive as what we have done. Two months after surgery the patient was completely asymptomatic and her phosphate was normal (0.82 mmol/L). The patient’s follow-up was every six months and by the time of writing she is completely asymptomatic for pain and her clinical tests are normal.
3. Discussion
OOM is a paraneoplastic disorder generally due to benign mesenchymal tumors secreting hormonal factors as FGF23, which inhibit renal tubular reabsorption of
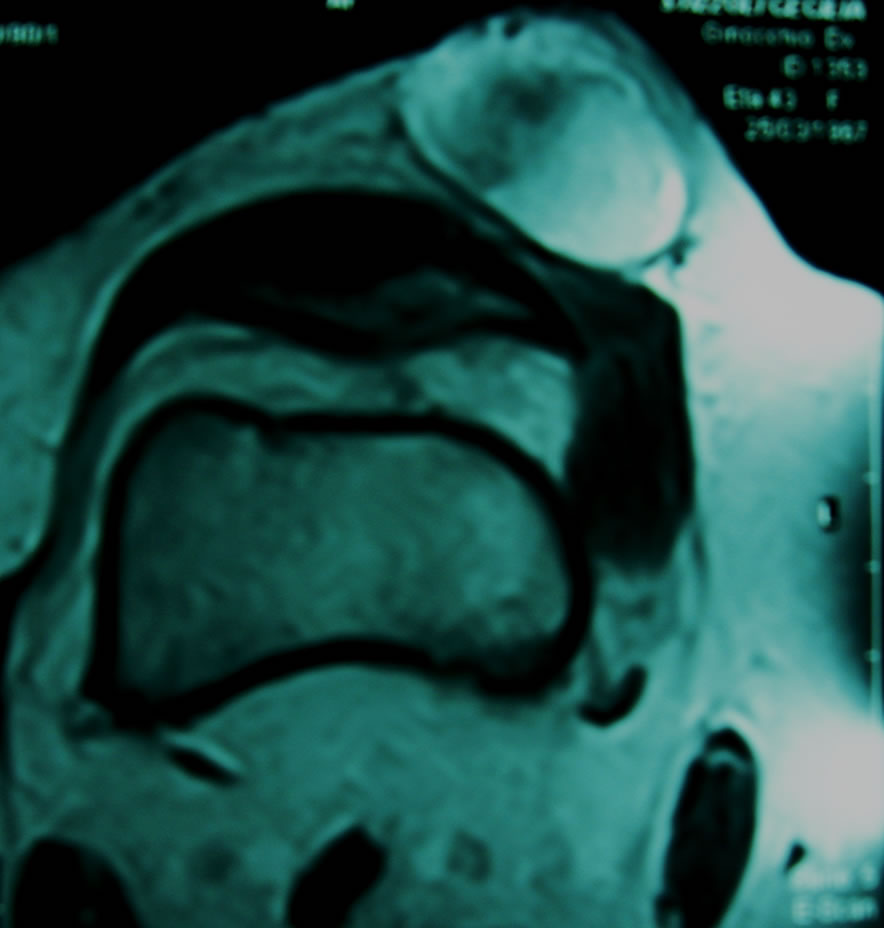
Figure 1. MRI image of the lesion.
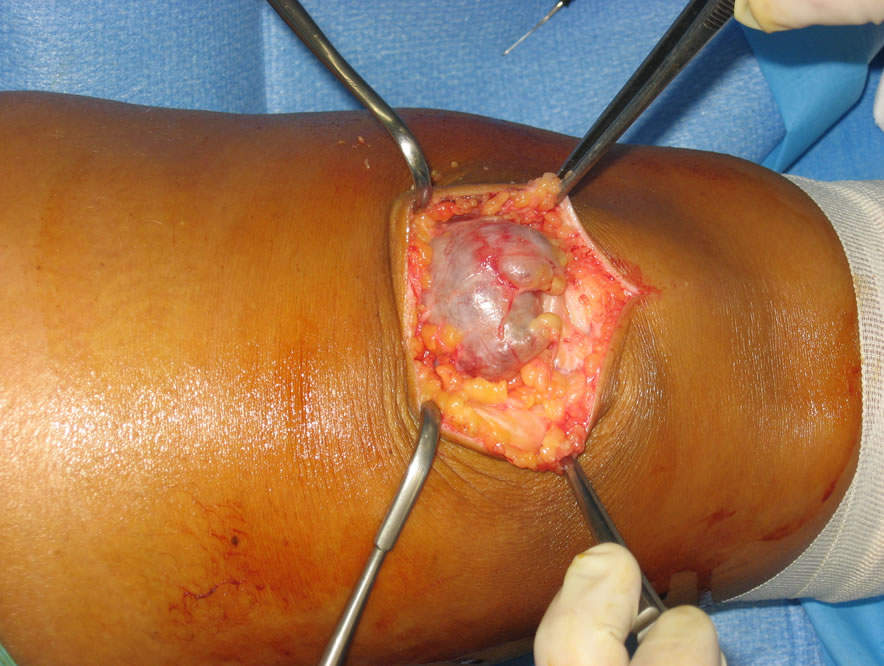
Figure 2. Surgical excision: appearance of a greyish wellcapsulated lesion in the anterior portion of the right knee.
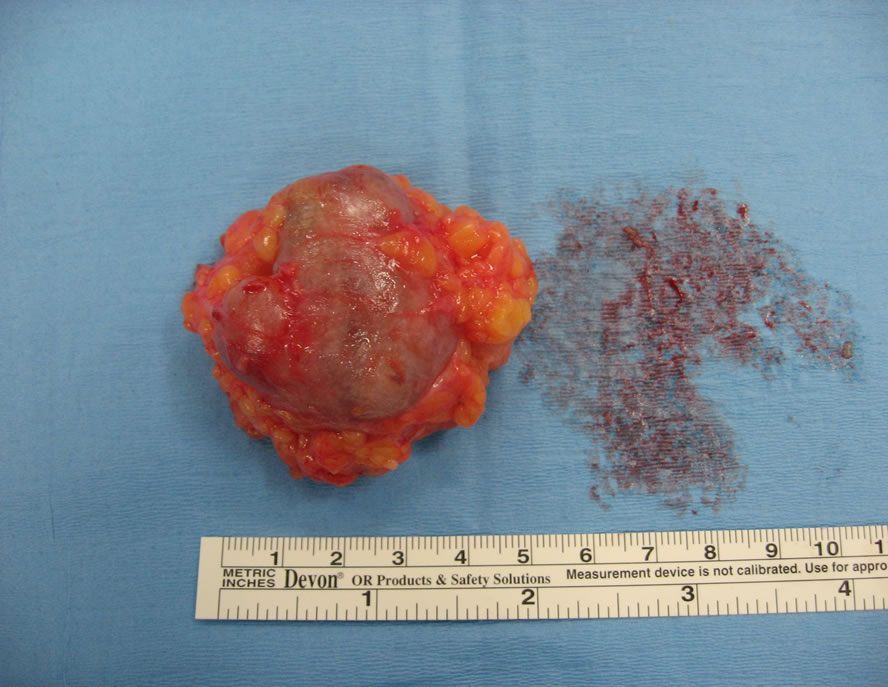
Figure 3. Enucleation of the lesion, which shows a prominent microvasculature.
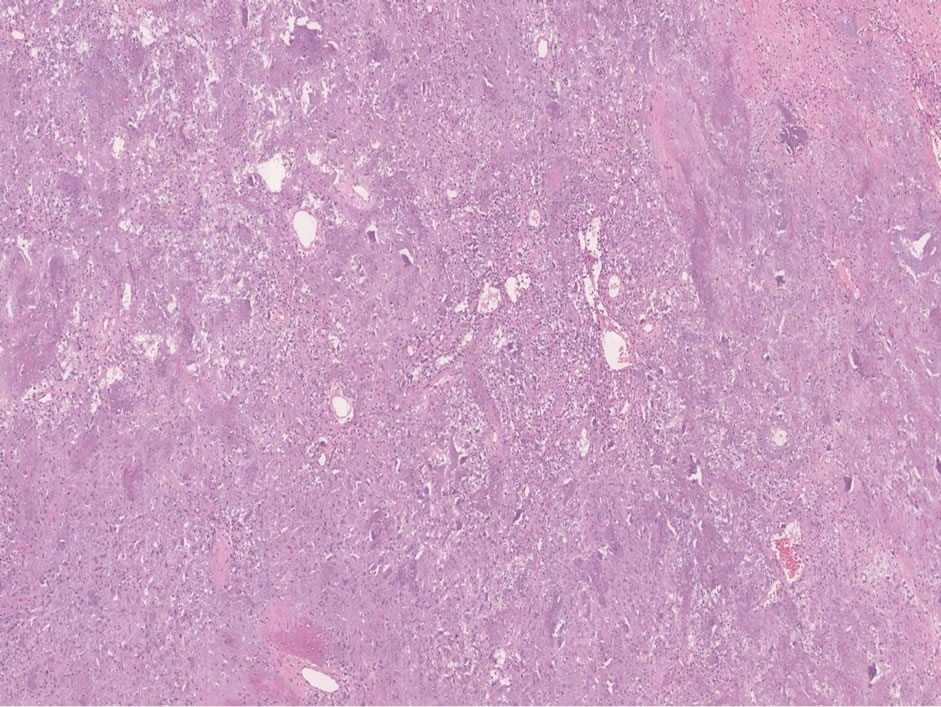
Figure 4. The tumor is composed of small spindle cells embedded in a “grungy” myxoid matrix, often containing areas of hemorrhage, fat and foci of cartilage.
phosphate and impair the synthesis of 1,25(OH)2D3. The optimal treatment is the complete surgical removal of the tumor, which results in a quick and complete recovery from symptoms, in a normalization of biochemical alterations and in a re-mineralization of the bone. These tumors may have different appearance which may be due to their misdiagnosis with various other mesenchymal tumors. Though they are generally composed by small, bland cells that produce a distinctive smudgy matrix and a well-developed capillary network; its matrix calcifies in an unusual flocculent or “grungy” pattern which often generates an osteoclast-rich reaction. In a subset of cases is also shown a fibro-histiocytic reaction and a woven bone production. These type of tumors are difficult to be detected because often of small dimensions and localized everywhere in the body [5]. The isolated localization in the knee has already been reported as well as at the first metacarpal bone or at the cervical spine; they can be, in some cases multicentric [6-9].
The existing difficulty for what concerns these tumors is the diagnosis, because even MRI scan has its limits.
Five subtypes of somatostatin receptors (SSTRs) have been identified in many normal tissues as in in-vitro mesenchymal tumors; these findings suggest a potential radiolabelled role of somatostatin’s analogues in mesenchymal tumors’ imaging. In some cases the protracted unsuccess in detecting the tumor is overcame by the use of IN111-octreotide scintigraphy; recently PET-CT has shown good results in detecting these tumors [10].
Pre-operative localisation is essential and could be reached by many imaging modalities such as CT scans or MRI, or somatostatin receptor scintigraphy or PET-TC [11-13].
From a biochemical point of view OOMs are characterized by hypophosphatemia, elevation of alkaline phosphatase, low circulating 1,25(OH)2D3 and increased concentration of FGF23; all these values are similar to the ones we found in the hereditary forms of metabolic bone diseases as X-linked hypophosphatemic rickets (XLHR) and autosomal dominant hyphosphatemic rickets (ADHR) but while in these conditions the biochemical abnormalities are permanents, in OOM they normalize after PMTMCT’s removal [14]. However OOM, ADHR and XLHR are very similar and it means that they may involve the same phosphate-regulating pathway.
In this paper we describe the case of a patient affected with OOM, originating from a right knee tumor in which we obtained a complete clinical recovery after tumor removal; this result confirms the ones described in literature.
REFERENCES
- M. J. Econs and M. K. Drezner, “Tumor-Induced Osteomalacia—Unveiling a New Hormone,” The New England Journal of Medicine, Vol. 330, No. 23, 1994, pp. 1679- 1681. doi:10.1056/NEJM199406093302310
- A. L. Folpe, J. C. Fanburg-Smith, S. D. Billings, et al., “Most Osteomalacia-Associated Mesenchymal Tumors Are a Single Histopathologic Entity: An Analysis of 32 Cases and a Comprehensive Review of the Literature,” The American Journal of Surgical Pathology, Vol. 28, No. 1, 2004, pp. 1-30. doi:10.1097/00000478-200401000-00001
- R. Kumar, “Tumor-Induced Osteomalacia and the Regulation of Phosphate Homeostasis,” Bone, Vol. 27, No. 3, 2000, pp. 333-338. doi:10.1016/S8756-3282(00)00334-3
- M. K. Drezner, “Tumor-Induced Osteomalacia,” Reviews in Endocrine and Metabolic Disorders, Vol. 2, No. 2, 2001, pp. 175-186. doi:10.1023/A:1010006811394
- S. M. Jan de Beur, E. A. Streeten, A. C. Civelek, E. F. McCarthy, L. Uribe, S. J. Marx, O. Onobrakpeya, L. G. Raisz, N. B. Watts, M. Sharon and M. A. Levine, “Localisation of Mesenchymal Tumors by Somatostatin Receptor Imaging,” Lancet, Vol. 359, No. 9308, 2002, pp. 761- 763. doi:10.1016/S0140-6736(02)07846-7
- A. Szumera-Ciećkiewicz, K. Ptaszyński, A. Pawełas and P. Rutkowski, “Oncogenic Osteomalacia Associated with Phosphaturic Mesenchymal Tumour, Mixed Connective Tissue Type of the Knee,” Polish Journal of Pathology, Vol. 60, No. 4, 2009, pp. 193-198.
- F. K. Hu, F. Yuan, C. Y. Jiang, D. W. Lv, B. B. Mao, Q. Zhang, Z. Q. Yuan and Y. Wang, “Tumor-Induced Osteomalacia with Elevated Fibroblast Growth Factor 23: A Case of Phosphaturic Mesenchymal Tumor Mixed with Connective Tissue Variants and Review of the Literature,” Chinese Journal of Cancer, Vol. 30, No. 11, 2011, pp. 794-804. doi:10.5732/cjc.011.10013
- M. Akhter, P. A. Sugrue, R. Bains and Y. A. Khavkin, “Oncogenic Osteomalacia of the Cervical Spine: A Rare Case of Curative Resection and Reconstruction,” Journal of Neurosurgery: Spine, Vol. 14, No. 4, 2011, pp. 453- 456. doi:10.3171/2010.11.SPINE09750
- N. R. Peterson, D. J. Summerlin and S. R. Cordes, “Multiple Phosphaturic Mesenchymal Tumors Associated with Oncogenic Osteomalacia: Case Report and Review of the Literature,” Ear, Nose & Throat Journal, Vol. 89, No. 6, 2010, pp. E11-E15.
- P. Suryawanshi, M. Agarwal, R. Dhake, S. Desai, B. Rekhi, K. B. Reddy and N. A. Jambhekar, “Phosphaturic Mesenchymal Tumor with Chondromyxoid Fibroma-Like Feature: An Unusual Morphological Appearance,” Skeletal Radiology, Vol. 40, No. 11, 2011, pp. 1481-1485. doi:10.1007/s00256-011-1159-6
- S. Fukumoto, Y. Takeuchi, A. Nagano and T. Fujita, “Diagnostic Utility of Magnetic Resonance Imaging Skeletal Survey in a Patient with Oncogenic Osteomalacia,” Bone, Vol. 25, No. 3, 1999, pp. 375-377. doi:10.1016/S8756-3282(99)00170-2
- J. L. Dupond, H. Mahammedi, D. Prie, F. Collin, H. Gil, O. Blagosklonov, B. Ricbourg, N. Meaux-Ruault and B. Kantelip, “Oncogenic Osteomalacia: Diagnostic Importance of Fibroblast Growth Factor 23 and F-18 Fluorodeoxyglucose PET/ct Scan for the Diagnosis and Follow-Up in One Case,” Bone, Vol. 36, No. 3, 2005, pp. 375-378. doi:10.1016/j.bone.2005.01.001
- E. Hesse, E. Moessinger, H. Rosenthal, F. Laenger, G. Brabant, T. Petrich, K. F. Gratz and L. Bastian, “Oncogenic Osteomalacia: Exact Tumor Localization by CoRegistration of Positron Emission and Computed Tomography,” Journal of Bone and Mineral Research, Vol. 22, No. 1, 2007, pp. 158-162. doi:10.1359/jbmr.060909
- K. B. Jonsson, R. Zahradnik, T. Larsson, K. E. White, T. Sugimoto, Y. Imanishi, T. Yamamoto, G. Hampson, H. Koshiyama, O. Ljunggren, K. Oba, I. M. Yang, A. Miyauchi, M. J. Econs, J. Lavigne and H. Juppner, “Fibroblast Growth Factor 23 in Oncogenic Osteomalacia and X-Linked Hypophosphatemia,” New England Journal of Medicine, Vol. 348, No. 17, 2003, pp. 1656-1663. doi:10.1056/NEJMoa020881
NOTES
*Conflict of interest: none. Before being included into the study patient has been completely informed and gave her consensus.
#Corresponding author.

