Open Journal of Rheumatology and Autoimmune Diseases
Vol.3 No.1(2013), Article ID:27841,8 pages DOI:10.4236/ojra.2013.31004
Dose and Administration Schedule Effect of Tiludronate on Joint Damage in the Model of Complete Freund Adjuvant Induced Monoarthritis in Rats
![]()
1Ceva Santé Animale, R&D, Libourne, France; 2Laboratoire de Pharmacologie Médicale, Facultés de Médecine et Pharmacie, Clermont-Ferrand, France; 3ANS Biotech, Facultés de Médecine et Pharmacie, Clermont-Ferrand, France.
Email: thierry.bertaim@ceva.com
Received November 6th, 2012; revised December 10th, 2012; accepted December 20th, 2012
Keywords: Tiludronate; Arthritis; Bone Remodelling; Hyperalgesia
ABSTRACT
The effects of dose and administration schedule of Tiludronate (TA) were assessed on joint lesions and hyperalgesia in a rat model of monoarthritis induced by injection of Complete Freund Adjuvant into the tibio tarsal joint of the hindpaw on day 0 (D0). Rats (n = 12/group) received subcutaneous injection of saline at D1, D4, D8 and D12; single dose (15 mg/kg; 60 mg/kg) at D1 or repeated doses (15 mg/kg) at D1, D4, D8 and D12 of TA; or daily injection of Meloxicam (1 mg/kg, from D1 to D12). Joint lesion severity, hindpaw volume and hyperalgesia were evaluated using radiography, plethysmometry and paw pressure, respectively. TA dose dependently reduced radiographic joint lesion (p < 0.001), including bone demineralisation and erosion, joint deformation and to a lesser extent, soft tissue and space articular narrowing. These results were supported by a significant limited increase of paw volume at the highest dose, independently of the administration schedule (60 mg/kg, 4 × 15 mg/kg) within D12-D28 (p < 0.01). In contrast, Meloxicam had no effect on radiographic joint lesion and significantly reduced paw volume only within D0-D12 (p < 0.01). Irrespective of dose and administration schedule, TA had a significant partial anti hyperalgesic effect (p < 0.05) within D0-D12 that was sustained until D28. In contrast, Meloxicam had a significant early anti hyperalgesic effect (p < 0.001) that was not sustained overtime. In conclusion, early TA administration showed a beneficial effect on joint lesion severity, with a partial anti hyperalgesic effect indicating that TA might be helpful for the management of arthritic pathologies with bone remodelling and osteolysis.
1. Introduction
Biphosphonates are one of the most effective classes of anti resorptive drugs. These substances are synthetic analogues of pyrophosphates and belong to 1) the simple biphosphonate group including clodronate, etidronate and tiludronate (TA) or 2) the amino biphosphonate group including alendronate, incandronate, zoledronate and ibandronate [1]. Their pharmacological effects result from their affinity for bone mineral and their inhibitory effects on osteoclasts, which in turn inhibit osteoclastic bone resorption [2,3]. Consequently, biphosphonates are leading drugs for the treatment of excessive bone resorption including Paget’s disease, tumour associated osteolysis and postmenopausal osteoporosis [4-6].
Biphosphonates were evaluated in rheumatoid and osteoarthritis [7]. These chronic inflammatory disorders affect several tissues of the joint inducing changes in the subchondral bone metabolism [8,9]. Subchondral bone is the site of numerous morphological transformations due to an altered equilibrium between osteoblast metabolism for bone formation through the synthesis of bone matrix and osteoclast activity accountable for degrading the periarticular bone. Therefore, excessive bone remodelling from increased bone resorption, especially at early stage, would justify the use of biphosphonates in such diseases. Therefore, some authors suggested that the schedule of drug administration and the stage of disease severity are two key points for optimal benefit from treatment [10].
In veterinary medicine, TA is used to treat horse osteoarthritic lesions and associated pain [11-13]. In horse bone spavin and navicular disease, TA inhibits bone resorption and increases bone mineral density [13,14]. However, its mechanism of action in the dysregulation of bone remodelling with an osteolytic component is not clearly understood.
Complete Freund’s Adjuvant (CFA) induced monoarthritis is a well established model for hyperalgesia and a model of choice to investigate bone remodelling [15,16]. Several biphosphonates have shown beneficial effect on hyperalgesia [17,18], two weeks after monoarthritis induction. Bone remodelling started early after CFA intra articular injection indicating that the early stage of the monoarthritic process is critical to assess any therapeutic agent which could act on bone remodelling/resorption.
A previous study showed that low (15 mg/kg) and high (60 mg/kg) doses of TA when given as a single subcutaneous injection after two weeks post induction in CFA induced monoarthritis in rats, reduced hyperalgesia (data not shown). As better therapeutic approaches are required to reduce bone damage, we evaluated whether TA administered early during monoarthritis might have an effect on joint structure and associated pain. Therefore, the efficacy of TA was assessed using two administration schedules, a single dose at induction, and repeated doses at regular intervals over the early phase of CFA induced monoarthritis in rats. Its effects on joint lesion and associated hyperalgesia were compared with the non steroid anti inflammatory drug, Meloxicam.
2. Materials and Methods
2.1. Animals
Sixty male Sprague Dawley rats (Charles River, Saint Germain sur L’Arbresle, France) weighing 180 to 200 g were housed in a temperature (22˚C ± 2˚C) and humidity (55% ± 10%) controlled environment with a 12 h light/ dark cycle. Rats were fed a standard diet (SAFE, France) and water ad libitum. After a seven days acclimatization period, rats were randomly assigned to different experimental groups (n = 5) consisting of 12 animals each. An examiner blinded to the treatment protocol conducted all experiments. The study was performed according to the guidelines of the Committee for Research and Ethical Issue of the I.A.S.P. (1983). The test facility accreditation number for the use of laboratory animals was (A63.113.15/DDSV04/154).
2.2. Induction of Experimental Adjuvant Monoarthritis
Adjuvant arthritis was induced according to the adapted method of Butler et al. [19]. Briefly, 25 µl of a suspension of CFA (0640, DIFCO laboratories, USA) containing heat-inactivated Mycobacterium butyricum (5 mg/ml) in paraffin oil, Tween 80 and saline was injected on Day 0 (D0), into the rat tibio tarsal joint of the right hindpaw.
2.3. Drug and Treatment
After CFA administration (D0), monoarthritic rats received subcutaneous injection (5ml/kg Body Weight (BW)) of either vehicle (0.9% NaCl) at D1, D4, D8 and D12; daily injection of Meloxicam (1 mg/kg BW) from D1 to D12 (1 mg/kg × 12); or 15 mg/kg BW TA at D1 (15 mg/kg × 1), 60 mg/kg BW TA at D1 (60 mg/kg ×1) or 15 mg/kg BW TA at D1, D4, D8 and D12 (15 mg/kg × 4). Measurements were performed 30 min. after drug injection (if any). TA ((4-chlorophenyl)thiomethylene bisphosphonate; Batch number 127A) in disodium salt/ base powder was provided by Ceva Sante Animale (Libourne, France). A non steroid anti inflammatory drug, Meloxicam (Mobic, Batch number 625557) in solution (15 mg/1.5 ml) was purchased to Boehringer Ingerlheim (Paris, France).
2.4. Radiographic Evaluation of Joint Damage Severity
At D29, rats were sacrificed and radiographs of tibio tarsal joint of the right hindpaw were taken with an X-ray instrument (40 kV, 100 mA, 6/100 s) and Kodak MIN-R films. The severity of the lesions was evaluated using a five items scale, namely bone demineralisation (periarticular), bone erosion (small geodes, bone cysts, bone oedema), soft tissue (oedema), articular space narrowing and joint deformation (bone and cartilage) [20]. Each item was scored using a semi quantitative grade of severity from 0 to 4; 0: normal, 1: very mild, 2: mild, 3: moderate and 4: severe.
2.5. Paw Volume Measurement
The paw volume (ml) was evaluated before (D-1) and after (D1, D4, D8, D12, D14, D21, D28) CFA induced monoarthritis, using a plethysmometer (Ugo Basile, Comerio, Italy). At each time point (tn), the paw volume was expressed as the percentage of the pre-induction volume (D-1) according to the following formula: (Paw volume × 100)/pre-induction volume). Time effect Curve was described according to the treatments and the relevant periods of time, D0-D12 (period of treatment administration (if any) and monoarthritis induction), and D12-D28 (monoarthritic process implementation). The Area Under the time Effect Curve (AUEC) quantified the paw volume variation over D0-D12 (AUEC0-12) and D12-D28 (AUEC12-28) time period. AUEC was calculated by trapezoidal rule, each trapeze being calculated according to the following formula, [(En−1 − Ebl) + (En − Ebl)] × (tn−1 + tn)/2, where En was the paw volume expressed as percentage at tn, and tn was the time (Day). Ebl was equal to E0 and E12 when considering D0-D12 and D12- D28 time period, respectively. The AUEC of each animal was calculated by summing all trapezes of the period considered.
2.6. Mechanical Paw Pressure Hyperalgesia Measurement
Paw Pressure Hyperalgesia Threshold (PPHT) was evaluated before (D-1) and after (D1, D4, D8, D12, D14, D21, D28) CFA induced monoarthritis, using the modified Randall-Selitto test (Analgesimeter, Ugo Basile, Comerio, Italy) [21]. Pressure was applied as a linearly increasing mechanical force to the tibio tarsal joint of the right hindpaw and pain reaction threshold was recorded as the pressure (g) at which the rat elicited vocalization (cut-off at 750 g). At each time point (tn), PPHT (g) was expressed as the percentage relative to the pre-induction level (D-1) according to the following formula: (paw pressure × 100)/pre induction paw pressure). Time Effect curves were described according to the treatments, and the relevant periods of time, D0-D12 (period of treatment administration (if any) and monoarthritis induction, and D12-D28 (monoarthritic process implementation).
The AUEC quantified PPHT variation during D0-D12 (AUEC0-12) and D12-D28 (AUEC12-28) periods of time. AUEC was calculated by trapezoidal rule, each trapeze being calculated according to the following formula, [(En−1 − Ebl)+ (En − Ebl)] × (tn−1 + tn)/2, where En was the paw volume expressed as percentage at tn, and tn was the time (Day). Ebl was equal to E0 and E12 when considering D0-D12 and D12-D28 time period, respectively. The AUEC of each animal was calculated by summing all trapezes for the period considered.
2.7. Statistical Analysis
At each time point, results were expressed as mean ± S.E.M., and time effect curves were described according to treatment, from D0 to D28. Statistical analysis were performed in comparison to vehicle, for quantitative variables e.g. radiological total lesion scores (0 - 20), AUEC for PPHT and paw volume, using one-way analysis of variance (ANOVA), followed by a Tukey Honestly Significant Difference test when the F-value was significant. For radiological lesion subscores (0 - 4) non parametric tests (Wilcoxon and Kruskal-Wallis tests) were used (Statgraphics® Centurion XV, USA SigmaStat). Significance was set at *: p < 0.05; **: p < 0.01; ***: p < 0.001.
3. Results
3.1. Effect of TA on Joint Damage
CFA injection into the rat tibio tarsal joint of the right hindpaw induced a monoarthritic process characterized by severe radiological joint damage (Figure 1). Examination of the radiographs in TA treated rats revealed a clear decrease in arthritic lesions that was not observed in vehicle and Meloxicam treated rats.
Total joint lesion scores were significantly and dose dependently reduced after a single injection of TA at 15 mg/kg × 1 (p < 0.05) and 60 mg/kg × 1 (p < 0.001), and a repeated injection at 15 mg/kg × 4 (p < 0.001) in comparison to vehicle (Figure 2). The highest dose of TA (15 mg/kg × 4 and 60 mg/kg × 1) had a significantly lower total joint lesion score than Meloxicam (p < 0.05). Meloxicam had no significant effect on joint lesions when compared to vehicle.
The decrease of joint lesion was confirmed for all subscores: TA induced a significant dose dependent decrease in bone demineralisation (p < 0.001), bone erosion (p < 0.001), joint deformation (p < 0.01), and to a lesser extent soft tissue (p < 0.05) and space articular narrowing (p < 0.01) (Figure 3).
3.2. Effects of TA on Hindpaw Volume
Paw volume variation was characterized by three different phases during the experimental period (Figure 4).
At D1, after CFA injection into the tibio tarsal joint hindpaw, paw volume increased by nearly 50% of the pre-induction volume, in all treated groups.
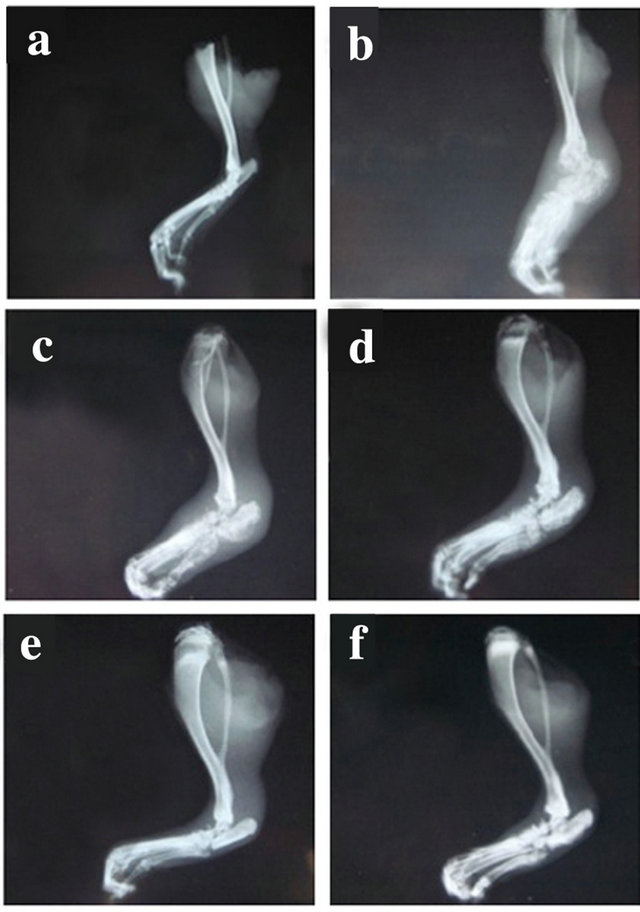
Figure 1. Representative radiographs of joint lesion severity. Xray radiographs of hindpaws (n = 12/group) were collected at D29 from (a) Naïve rats and monoarthritic rats treated with (b) Vehicle (0.9% NaCl); (c) Meloxicam 1 mg/ kg × 12; (d) TA 15 mg/kg × 1; (e) TA 60 mg/kg × 1 and (f) TA 15 mg/kg × 4.
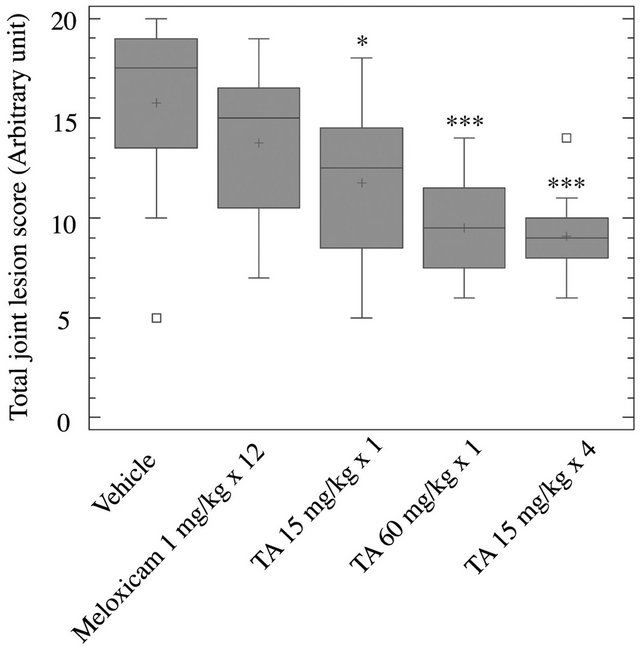
Figure 2. Radiographic analysis of joint lesions. At D29, hindpaws were collected from rats (n = 12/group) treated with vehicle (0.9% NaCl), Meloxicam (1 mg/kg × 12) and TA (15 mg/kg × 1; 60 mg/kg × 1; 15 mg/kg × 4). Total joint lesion scores (Box and Whisker plot) are expressed as mean ± S.E.M in arbitrary unit. Significance was set at: *p < 0.05, **p < 0.01, ***p < 0.001.
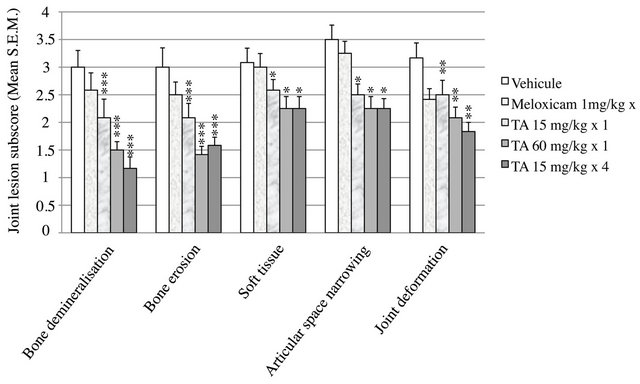
Figure 3. Radiographic analysis of joint lesions subscores. At D29, hindpaws were collected from rats (n = 12/group) treated with vehicle (0.9% NaCl), Meloxicam (1 mg/kg × 12) and TA (15 mg/kg × 1; 60 mg/kg × 1; 15 mg/kg × 4). Joint lesion subscores, i.e. bone demineralisation, bone erosion, soft tissue, articular space narrowing and joint deformation were scored using a semi quantitative grade of severity from 0 to 4 where 0: normal, 1: very mild, 2: mild, 3: moderate and 4: severe. Subscores are expressed as mean ± S.E.M in arbitrary unit. Significance was set at: *p < 0.05, **p < 0.01, ***p < 0.001.
Over the D1-D12 period, paw volume stayed rather unchanged in vehicle and TA treated rats (Figure 4). It was significantly reduced after Meloxicam treatment, with a maximal 25% decrease of pre-induction volume at D8 as assessed by AUEC0-12 (p < 0.01) (Figure 5).
Over the D12-D28 period, paw volume in vehicle treated rats increased by 137% of pre induction volume
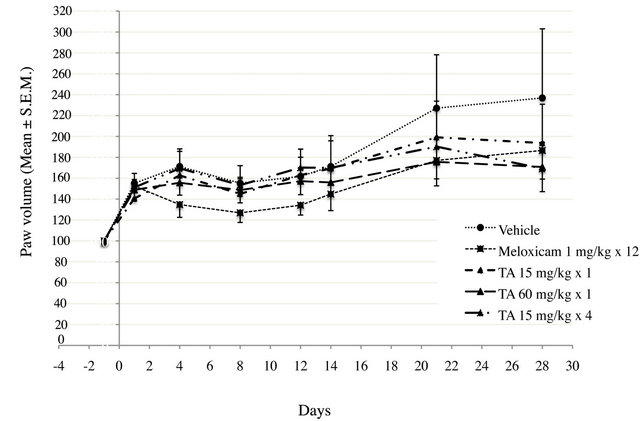
Figure 4. Paw volume (ml) in monoarthritic rats (n = 12/ group) treated with vehicle (0.9% NaCl), Meloxicam (1 mg/kg × 12) and TA (15 mg/kg × 1; 60 mg/kg × 1; 15 mg/kg × 4) was measured by plethysmometry at D1, D4, D8, D12, D14, D21 and D28. Paw volume is expressed as percentage relative of pre-induction volume. Results are expressed as mean ± S.E.M.
at D28 (Figure 4). The paw volume of Meloxicam treated rats increased by 80% of pre induction volume at D28 and the Meloxicam curve had a slope similar to that of the vehicle. This increase was significant (p < 0.01) as assessed by AUEC12-28 (Figure 5). Interestingly, the increase in paw volume was significantly lower in rats treated with TA 60 mg/kg × 1 (p < 0.05) and TA 15 mg/kg × 4 (p < 0.01).
3.3. Paw Pressure Hyperalgesia Threshold (PPHT)
Over the experimental period, PPHT variation was characterized by two phases, which differ according to treatment (Figure 6).
In vehicle treated rats, PPHT progressively decreased from D0 to D12, to reach a lower level (−56% of preinduction level). Then, PPHT stayed rather constant up to D28, meaning that a steady monoarthritic hyperalgesia settled.
In TA treated rats, independently of dose and administration schedule, PPHTs decreased progressively from D1 to D12 (Figure 6). PPHTs were significantly higher than vehicle, meaning that monoarthritic hyperalgesia was lower (p < 0.01) (Figure 7). Then, from D12 to D28, PPHTs were maintained up to the end of the experimental period. In comparison to Meloxicam, TA, irrespective of the dose, had only a partial anti hyperalgesic effect (p < 0.005).
In Meloxicam treated rats, PPHT variation over the experimental period differs in comparison to vehicle and TA groups (Figures 6 and 7). From D1 to D4, PPHT slightly increased indicating an anti nociceptive rather than an anti hyperalgesic effect (Figure 6). Then, PPHT
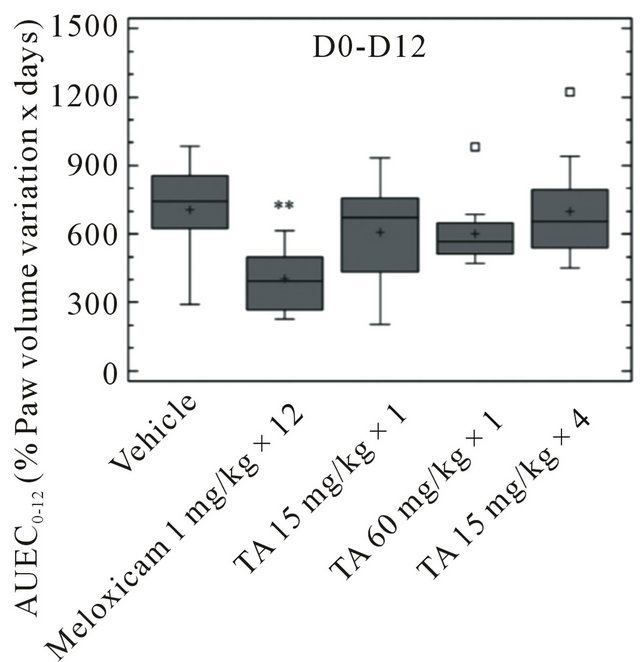
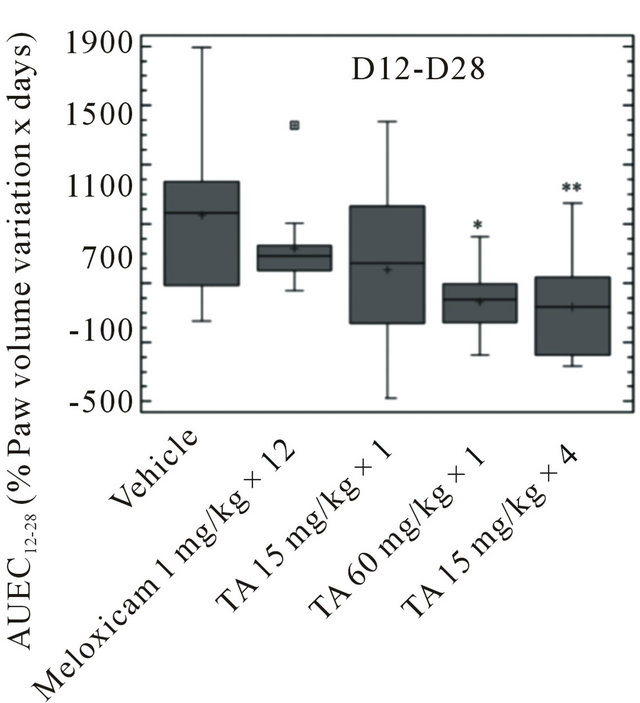
Figure 5. Paw volume (ml) in monoarthritic rats (n = 12/group) treated with vehicle (0.9% NaCl), Meloxicam (1 mg/kg × 12) and TA (15 mg/kg × 1; 60 mg/kg × 1; 15 mg/kg × 4) was measured by plethysmometry at D1, D4, D8, D12, D14, D21 and D28. Paw volume variations are expressed as AUEC0-12 and AUEC12-28 (Box and Whisker plot). Significance was set at: *p < 0.05, **p < 0.01, ***p < 0.001.
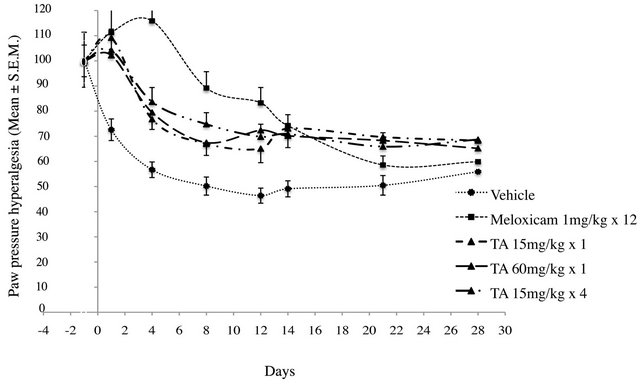
Figure 6. Paw Pressure Hyperalgesia Threshold (PPHT, g) in monoarthritic rats (n = 12/group) treated with vehicle (0.9% NaCl), Meloxicam (1 mg/kg × 12), and TA (15 mg/kg × 1; 60 mg/kg × 1; 15 mg/kg × 4) was measured at D-1, D1, D4, D8, D12, D14, D21 and D28. PPHT is expressed as the percentage relative to pre-induction level. Results are expressed as mean ± S.E.M.
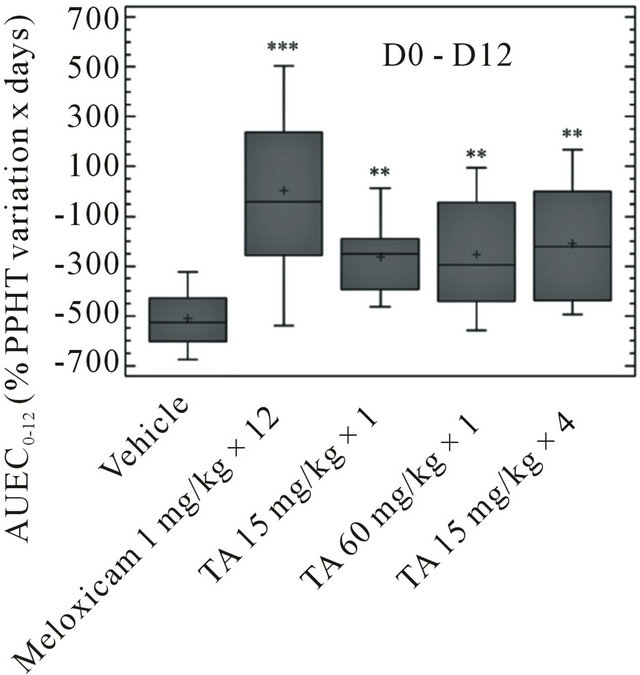

Figure 7. Paw Pressure Hyperalgesia Threshold (PPHT, g) in monoarthritic rats (n = 12/group) treated with vehicle (0.9% NaCl), Meloxicam (1 mg/kg × 12), and TA (15 mg/kg × 1; 60 mg/kg × 1; 15 mg/kg × 4) was measured at D-1, D1, D4, D8, D12, D14, D21 and D28. PPHT variations are expressed as AUEC0-12 and AUEC12-28 (Box and Whisker plot). Significance was set at: *p < 0.05, **p < 0.01, ***p < 0.001.
decreased progressively from D4 until the end of the experimental period. This decrease was more marked after D12 (Meloxicam treatment discontinuation) and reached a level close to vehicle at D28 (Figure 6).
The AUEC0-12 of Meloxicam was significantly higher than vehicle and TA (p < 0.001), showing a clear anti hyperalgesic effect during this period (Figure 7). The AUEC12-28 was significantly lower than vehicle (p < 0.001), indicating that hyperalgesia worsened in Meloxicam treated rats after D12.
4. Discussion
Subcutaneous administration of TA induced a significant and dose dependent decrease of CFA induced monoarthritic joint lesions in rats including bone demineralisation and erosion, joint deformation, and to a lesser extent soft tissue and articular space narrowing. Irrespective of the administration schedule (60 mg/kg × 1, 15 mg/kg × 4), the highest dose of TA showed a marked protective effect on joint lesions as assessed by radiography. This beneficial effect was supported by a significant limited increase in hindpaw volume over the D12-D24 experimental period. Irrespective of the dose and administration schedule, TA had a significant early and partial anti hyperalgesic effect even after only one injection at D0 that was maintained up to D28. In contrast, Meloxicam had no effect on radiographic joint lesion. It had a significant anti hyperalgesic effect assessed by PPHT variations, which was supported by an early reduction of hindpaw volume during the pathological process over the D1-D12 experimental period. However, these effects progressively decreased and disappeared after treatment discontinuation at D12.
CFA injection into the rat tibio tarsal hindpaw is known to induce changes in bone morphology and joint structure associated with hyperalgesia [15-17]. These processes start early within two weeks post-injection and lead to a steady painful chronic monoarthritis [19,22].
Biphosphonates are known to reduce bone disorders with complicated pain [18,23,24]. More recently, increase in the subchondral bone remodelling rate and bone marrow attrition has been shown to appear early and to be predictive of the pathological mechanisms responsible for structural joint impairments [9,25]. Based on these observations and to further investigate the effect of TA, the present study assessed the potential effect of TA as a bone anti resorptive drug on joint structure lesions. For that purpose, different administration schedules within the first two weeks of the monoarthritic process, combined with different doses, single low and high (15 and 60 mg/kg) versus repeated low doses (15 × 4 mg/kg) were studied. Although TA regimens were higher than those used by other groups (5 mg/kg/day), all doses were well tolerated [2,26]. Furthermore, the study assessed the anti hyperalgesic effect of TA in comparison to Meloxicam, which is known to significantly improve pain in pathological processes such as rheumatoid arthritis and osteoarthritis [27].
Our results are in agreement with previous studies showing that TA dose dependently improved monoarthritic joint damage [2,13,15,17,25,28,29]. Using a single Xray evaluation four weeks after CFA induced monoarthritis, we showed the articular protective effect of TA even after administration of a single low dose. However, further investigations using imaging technology (X-ray, Magnetic Resonance Imaging) would be required over the monoarthritic process (different time points, longer period of analysis) to refine the present observations. The highest dose of TA (60 mg/kg) offers a better articular protective effect than the low dose (15 mg/kg) at the end of the experimental period. However, no difference was evidenced between the single (60 mg/kg) and the repeated low dose (4 × 15 mg/kg). This observation shows that the articular protective effect of TA is independent of the administration schedule and indicates the flexibility of TA administration. In clinical practice, biphosphonates are administered either by daily oral route or single intravenous injection in patients suffering from osteoporosis. In view of the present results, TA administration offers a flexible therapeutic scheme. This property might improve its convenience in daily practice and confer a better safety margin if any.
As previously reported with other biphosphonates, TA has an anti hyperalgesic effect [17]. The present study showed that this effect is independent of the administration schedule (single versus repeated), and the dose (high versus low). However, this effect is only partial and is sustained up to the end of the experimental period, in contrast to Meloxicam. This could be due to the high and persistent affinity of biphosphonates on bone.
Thus, the articular protective and the anti hyperalgesic effects of TA might be the result of its well known inhibitory activity on osteoclasts [30]. TA, and more widely biphosphonates, have a preventive effect on excessive proton secretion in bone, known to create a microenvironment acidosis [17,31,32]. In turn, this acidic microenvironment induces 1) bone mineral matrix degradation and subsequent structural joint damage, and 2) subchondral bone acid sensory neuron sensitization contributing to joint hyperalgesia [17,18]. The use of biphosphonates as a monotherapy and/or at an inadequate period of time, i.e. when bone remodelling and osteolysis are not present, might explain their limited clinical effects on joint damage and pain. Thus, in clinical practice, it is of prime importance to target the right patient at the right moment in arthritis therapy managed by biphosphonates [10,33].
TA, as an anti osteoclastic drug, and Meloxicam, as a cyclooxygenase inhibitor, might complement each other over the monoarthritic process. In view of the present results and as suggested by other groups, it would be of interest to evaluate the beneficial effect of TA in combination with a non steroid anti inflammatory drug on joint lesion severity in arthritic conditions [34,35]. This combination could offer interesting therapeutic strategies in the treatment of arthritic conditions.
In conclusion, our findings showed that TA has 1) a protective effect on joint lesions and 2) a partial anti hyperalgesic effect in experimental monoarthritis. This biphosphonate offers a flexible early treatment option with respect to the dose and administration schedule in the management of joint lesion severity and hyperalgesia. Thus, early TA administration might be helpful for the management of arthritic pathologies with bone remodelling and osteolysis.
5. Acknowledgements
The authors would like to thank Dr. Gilles Chaudieu for X-ray radiographs, Dr Philippe Bertin for radiographical analysis, and Dr. Florence Pousset for writing this manuscript.
REFERENCES
- M. J. Rogers, “New Insights into the Molecular Mechanisms of Action of Biphosphonates,” Current Pharmaceutical Design, Vol. 9, No. 32, 2003, pp. 2643-2658. doi:10.2174/1381612033453640
- J. P. Bonjour, P. Ammann, A. Barbier, J. Caverzasio and R. Rizzoli, “Tiludronate: Bone Pharmacology and Safety,” Bone, Vol. 17, No. 5, 1995, pp. 473S-477S. doi:10.1016/8756-3282(95)00344-9
- G. Shu, K. Yamamoto and M. Nagashima, “Differences in Osteoclast Formation between Proximal and Distal Tibial Osteoporosis in Rats Adjuvant Arthritis: Inhibitory Effects of Biphosphonates on Osteoclasts,” Molecular Rheumatology, Vol. 16, No. 6, 2006, pp. 343-349. doi:10.1007/s10165-006-0515-1
- C. Roux and M. Dougados, “Treatment of Patients with Paget’s Disease of Bone,” Drugs, Vol. 58, No. 5, 1999, pp. 823-830. doi:10.2165/00003495-199958050-00005
- P. D. Delmas, “Treatment of Postmenopausal Osteoporosis,” Lancet, Vol. 359, No. 9322, pp. 2018-2026. doi:10.1016/S0140-6736(02)08827-X
- R. G. Russell, Z. Xia, J. E. Dunford, U. Oppermann, A. Kwaasi, P. A. Hulley, K. L. Kavanagh, J. T. Triffitt, M. W. Lundy, R. J. Phipps, B. L. Barnett, F. P. Coxon, M. J. Rogers, N. B. Watts and F. H. Ebetino, “Bisphosphonates: an Update on Mechanisms of Action and How These Relate to Clinical Efficacy,” Annual New York Academic Sciences, Vol. 1117, No. 1, 2002, 2007, pp. 209-257.
- C. O. Bingham, J. C. Buckland-Wright, P. Garnero, S. B. Cohen, S. M. Dougado and S. Adarni, “Risedronate Decreases Biochemical Markers of Cartilage Degradation but Does Not Decrease Symptoms or Slow Radiographic Progression in Patients with Medial Compartment Osteoarthritis of the Knee E Results of the Two-Year Multinational Knee Osteoarthritis Structural Arthritis Study,” Arthritis Rheumatology, Vol. 54, No. 11, 2006, pp. 3494- 3507. doi:10.1002/art.22160
- J. Martel-Pelletier, D. Lajeunesse and J. P. Pelletier, “Etiopathogenesis of Osteoarthritis,” In: W. J. Koopman and L. W. Moreland, Eds., A Textbook of Rheumatology, Lippincott, Williams & Wilkins Publishers, Baltimore, 2005, pp. 2199-2226.
- S. Kwan Tat, D. Lajeunesse, J. P. Pelletier and J. MartelPelletier, “Targeting Subchondral Bone for Treating Osteoarthritis: What is the Evidence?” Best Practice Research Clinical Rheumatology, Vol. 24, No. 1, 2010, pp. 51-70. doi:10.1016/j.berh.2009.08.004
- B.W. Strassle, L. Mark, L. Leventhal, M. J. Piesla, X. Jian Li, J. D. Kennedy and S. S. Glasson, “Inhibition of Osteoclasts Prevents Cartilage Loss and Pain in a Rat Model of Degenerative Joint Disease,” Osteoarthitis and Cartilage, Vol. 18, No. 10, 2010, pp. 1319-1328. doi:10.1016/j.joca.2010.06.007
- C. Delguste, H. Amory, M. Doucet, C. Piccot-Crozollet, D. Thibaud, P. Garnero, J. Detilleux and O. M. Lepage, “Pharmacological Effects of Tiludronate in Horses after Long-Term Immobilization,” Bone, Vol. 41, No. 3, 2007, pp. 414-421. doi:10.1016/j.bone.2007.05.005
- V. Coudry, D. Thibaud, B. Riccio, F. Audigi, D. Didierlaurent and J. M. Denoix, “Efficacy of Tiludronate in the Treatment of Horses with Signs of Pain Associated with Osteoarthritic Lesions of the Thoracolumbar Vertebral Column,” American Journal of Veterinary Research, Vol. 68, No. 3, 2007, pp. 329-337. doi:10.2460/ajvr.68.3.329
- M. R. Gough, D. Thibaud and R. K. Smith, “Tiludronate Infusion in the Treatment of Bone Spavin: A Double Blind Placebo-Controlled Trial,” Equine Veterinary Journal, Vol. 42, No. 5, 2010, pp. 381-387. doi:10.1111/j.2042-3306.2010.00120.x
- J. M. Denoix, D. Thibaud and B. Riccio, “Tiludronate as a New Therapeutic Agent in the Treatment of Navicular Disease: A Double-Blind Placebo-Controlled Clinical Trial,” Equine Veterinary Journal, Vol. 35, No. 4, 2003, pp. 407-413. doi:10.2746/042516403776014226
- H. Harada, T. Nakayama, T. Nanaka and T. Katsumata, “Effects of Bisphosphonates on Joint Damage and Bone Loss in Rat Adjuvant-Induced Arthritis,” Inflammation Research, Vol. 53, No. 2, 2004, pp. 45-52. doi:10.1007/s00011-003-1214-4
- H. Vermeirsch, R. Biermans, P. Salmon and T. Meert, “Evaluation of Pain Behavior and Bone Destruction in Two Arthritic Models in Guinea Pig and Rat,” Pharmacology Biochemistry and Behavior, Vol. 87, No. 3, 2007, pp. 349-359. doi:10.1016/j.pbb.2007.05.010
- M. Nagae, T. Hiraga, H. Wakabayashi, L. Wang, K. Iwata and T. Yoneda, “Osteoclasts Play a Part in Pain Due to the Inflammation Adjacent to Bone,” Bone, Vol. 39, No. 5, 2006, pp. 1107-1115. doi:10.1016/j.bone.2006.04.033
- M. Nagae, T. Hirage and T. Yoneda, “Acidic Microenvironment Created by Osteoclasts Causes Bone Pain Associated with Tumor Colonization,” Journal of Bone and Mineral Metabolism, Vol. 25, No. 2, 2007, pp. 99-104. doi:10.1007/s00774-006-0734-8
- S. H. Butler, F. Godefroy, J. M. Besson and J. Weil-Fugazza, “A Limited Arthritic Model for Chronic Pain,” Pain, Vol. 48, No. 1, 1992, pp. 73-81. doi:10.1016/0304-3959(92)90133-V
- O. Barou, M. H. Lafage-Proust, C. Martel, T. Thomas, F. Tirode, N. Laroche, A. Barbier, C. Alexandre and L. Vico, “Biphosphonate Effects in Rat Unloaded Hindlimb Bone Loss Model: Three-Dimensional Microcomputed Tomographic, Histomorphometric, And Densitometric Analyses,” Journal of Pharmacology and Experimental Therapy, Vol. 291, No. 1, 1999, pp. 321-328.
- L. O. Randall and J. J. Selitto, “A Method for Measurement of Analgesic Activity on Inflamed Tissue,” Archives Intrenationales de Pharmacodynamie et de Thérapie Vol. 111, No. 4, 1957, pp. 409-419.
- A. Bendele, J. Mc Comb, T. Gould, T. Mc Abee, G. Sennello and E. Chlipalla, “Animal Model of Arthritis: Relevance to Human Disease,” Toxicology Pathology, Vol. 27, No. 1, 1999, pp. 134-142. doi:10.1177/019262339902700125
- C. Stein, M. J. Millan and A. Nerz, “Unilateral Inflammation of the Hindpaw in Rats as a Model of Prolonged Noxious Stimulation: Alterations in Behavior and Nociceptive Thresholds,” Pharmacology Biochemistry and Behavior, Vol. 31, No. 2, 1988, pp. 445-451. doi:10.1016/0091-3057(88)90372-3
- H. Murakami, T. Nakamura, H. Tsurukami, M. Abe, A. Barbier and K. Suzuki, “Effects of Tiludronate on Bone Mass, Structure and Turnover at the Epiphyseal, the Primary and the Secondary Spongiosae in the Proximal Tibia of Growing Rats after Sciatic Neurectomy,” Journal of Bone and Mineral Research, Vol. 9, No. 9, 1994, pp. 1355-1364. doi:10.1002/jbmr.5650090906
- Y. H. Koh, S. H. Hong, H. S. Kang, C. Y. Chung, K. H. Koo, H. W. Chung, J. H. Cha and K. R. Son, “The Effects of Bone Turnover Rate on Subchondral Trabecular Bone Structure and Cartilage Damage in the Osteoarthritic Rat Model,” Rheumatology and Internal Medicine, Vol. 30, No. 9, 2010, pp. 1165-1171.
- J. P. Barbier, P. Bonjour, M. C. Geusens and F. L. De Vernejoul, “Tiludronate: A Biphosphonate with a Positive Effect on Bone Quality in Experimental Models,” Journal of Bone and Mineral Research, Vol. 6, Suppl. 1, 1991, p. S531.
- K. D. Peterson and T. J. Keefe, “Effects of Meloxicam on Severity of Lameness and Other Clinical Signs of Osteoarthritis in Dogs,” Journal of American Veterinary Medicine Association, Vol. 225, No. 7, 2004, pp. 1056- 1060. doi:10.2460/javma.2004.225.1056
- P. Amman, R. Rizzoli, J. Caverzasio, T. Shigematsu, D. Slosman and J. P. Bonjour, “Effects of the Bisphosphonate Tiludronate on Bone Resorption, Calcium Balance, and Bone Mineral Density,” Journal of Bone and Mineral Research,, Vol. 8, No. 12, 1993, pp. 1491-1498.
- T. Hayami, M. Pickarski, G. A. Wesolowski, J. McLane, A. Bone, J. Destefano, G. A. Rodan and T. Le Duong, “The Role of Subchondral Bone Remodelling in Osteoarthritis: Reduction of Cartilage Degeneration and Prevention of Osteophyte Formation by Alendronate in the Rat Anterior Cruciate Ligament Transection Model,” Arthritis Rheumatology, Vol. 50, No. 4, 2004, pp. 1193- 1206. doi:10.1002/art.20124
- P. David, H. Nguyen, A. Barbier and R. Baron, “The Bisphosphonate Tiludronate Is a Potent Inhibitor of the Osteoclast Vacuolar H+-ATPase,” Journal of Bone and Mineral Research, Vol. 11, No. 10, 1996, pp. 1498-507. doi:10.1002/jbmr.5650111017
- H. Murakami, N. Takahashi, S. Tanaka, I. Nakamura, N. Udagawa, S. Nakajo, K. Nakaya, M. Abe, Y. Yuda, F. Konno, A. Barbier and T. Suda, “Tiludronate Inhibits Protein Tyrosine Phosphatase Activity in Osteoclasts,” Bone, Vol. 20, No. 5, 1997, pp. 399-404. doi:10.1016/S8756-3282(97)00025-2
- K. Suzuki, S. Takeyama, Y. Sakai, S. Yamada and H. Shinoda, “Current Topics in Parmacological Research on Bone Metabolism: Inhibitory Effects of Biphosphonates on the Differentiation and Activity of Osteoclasts,” Journal Pharmacology Sciences, Vol. 100, No. 3, 2006, pp. 189-194. doi:10.1254/jphs.FMJ05004X2
- D. A. Walsh and V. Chapman, “Bisphosphonates for Osteoarthritis,” Arthritis Research Therapy, Vol. 13, No. 5, 2011, pp. 128-134. doi:10.1186/ar3448
- B. Le Goff, E. Soltner, C. Charrier, Y. Maugars, F. Rédini, D. Heymann and J. M. Berthelot, “A Combination of Methotrexate and Zoledronic Acid Prevents Bone Erosions and Systemic Bone Mass Loss in Collagen Induced Arthritis,” Arthritis Research Therapy, Vol. 11, No. 6, 2009, pp. R185-R191. doi:10.1186/ar2877
- M. D. Jones, C. Tran, G. Li, W. P. Maksymowych, R. Zernicke and M. Doschak, “In Vivo Microfocal Computed Tomography and Micro-Magnetic Resonance Imaging Evaluation of Antiresorptive and Anti-Inflammatory Drugs As Preventive Treatments of Osteoarthritis in the Rat,” Arthritis Rheumatology, Vol. 62, No. 9, 2010, pp. 2726-2735. doi:10.1002/art.27595

