Open Journal of Rheumatology and Autoimmune Diseases
Vol. 2 No. 4 (2012) , Article ID: 24902 , 4 pages DOI:10.4236/ojra.2012.24018
Early Arthritics
![]()
1Centro Nacional de Enfermedades Reumáticas (CNER), Hospital Universitario de Caracas, Caracas, Venezuela; 2Cátedra de Salud Pública, Universidad Central de Venezuela, Caracas, Venezuela.
Email: *luiskakuro@gmail.com
Received June 12th, 2012; revised July 20th, 2012; accepted August 3rd, 2012
Keywords: ACR criteria; Early Arthritis; Anti-CCP; Ctx-II; DAS28
ABSTRACT
To determine which patients with Early Inflammatory Polyarthritis (EIP) with less than a year of evolution of the disease without a definitive diagnosis, progressed to rheumatoid arthritis according to ACR1987 criteria, we developed a predictive model for classification of early rheumatoid arthritis, based on clinical characteristics by observation of theonset of the disease and to relate with certain laboratory variables. A total of 54 patients with arthritis of less than one year of evolution were evaluated. We conducted a physical examination and the following parameters were determined: DAS 28, HAQ, Rheumatoid Factor (RF), Citrullinated Peptide (anti-CCP) and C-Telopeptide (CTx-II); radiology of hands and feet was also carried out, and was assessed by the Sharp method modified by van der Heijde. The patient follow-up was performed every 3 months for 12 months, classifying them according to the development of self-limiting, persistent non-erosive, and persistent erosive arthritis, and according to definite diagnosis. We estimated the relative risk and 95% confidence intervals for the predictor variables considered. Overall, 80.4% of patients with EIP evolved to persistent arthritis. Most persistent arthritis was diagnosed as rheumatoid arthritis (67.4%). However 51.6% (16/31) were anti-CCP positive, 21/31 (67.7%) were RF, and 11/31 (35%) were CTx-II positive. The basal nodes and RF were able to predict the persistence of activity and symmetry; rheumatoid nodules predict the development of erosions. It is important to note that patients who had an high initial average by the Sharp method modified by van der Heijde tend to have a greater increase in erosion at 6 months compared to those who had an initial low average (Pearson correlation coefficient 0.40, p < 0.015). An initial erosive disease increases the risk of radiological progression.
1. Introduction
The early inflammatory polyarthritis concept (EIP) is not yet standardized, however consensus defined as the presence of arthritis of 3 or more joints in a period >6 weeks but less than 1 year and still not complete the criteria for a specified arthritide. Saraux and colleagues showed that the criteria of the American College of Rheumatology (ACR), developed in 1987, are not enough sensitive (66%) for the diagnosis of rheumatoid arthritis in the first two years of disease.
Researchers seek tools to predict precociously which patients with Early Inflammatory Polyarthritis (EIP) will develop rheumatoid arthritis; however, it has to be noted that initially the disease is not clinical, radiological and immunological characteristic [1].
Clinical, radiological and serological criteria for predicting the severity and persistence of the EIP have been explored. Several factors appear to influence the disease, but there is no consensus because the results are contradictory. [2]
It is noteworthy that in 70% of patients radiological damage develops in the first two years of the disease and the rate of progression is higher in the first year than in the second or third year. This suggests that the opportunity to change the course of the disease occurs early [3]. As noted, early intervention with drugs in order to interfere on the natural progression of the disease has been advocated [3-5].
The interest in developing new treatment strategies is a consequence of the negative impact of disease on functional capacity and moropne diagnostic test was limited, because these antibodies could only be detected by immunofluorescence. Subsequently a synthetic peptide containing citrulline, an amino acid in the filaggrin, which can be determined by analysis of enzyme-linked immunosorbent assay (ELISA), was developed [6]. A sensitivity of 76% and a specificity of 95% - 100% for the diagnosis of rheumatoid arthritis have been reported by this method [7].
Anticitrulline antibodies can be detected before the onset of the symptoms of rheumatoid arthritis, suggesting that these antibodies are involved in the pathogenesis of the disease [7].
Goldach-Mansky and colleagues showed that antibodies anticitrulline were the best marker to predict the diagnosis and erosions in the first year of the disease [8]. Visser et al., in their model for the diagnosis of early rheumatoid arthritis, included antibodies anticitrulline, which showed a strong association for persistence and were the best variable to predict erosions [9].
Other markers used to predict long-term progression of joint damage in early rheumatoid arthritis patients are the C-Telopeptide I and II (CTX-I and CTX-II) in urine, which are markers of bone destruction in bone and cartilage, respectively. Garnero and colleagues showed that high levels of CTx-I and CTX-II were independent predictors and increase the risk of radiological progression at 4 years of evolution in patients with EIP [10].
2. Methodology
To determine with EIP patients progressed to rheumatoid arthritis, we develop a longitudinal cohort study, with patients referred to triage of rheumatology service at the Hospital Universitariode Caracas (HUC). All patients attending the rheumatology clinic of HUC from May 2004 to November 2004 and who met the following inclusion criteria were included in the study:
1) Patients with an age equal to or greater than 18 years.
2) Patients with arthritis of three or more joints, as evidenced by the doctor with a duration equal to or greater than 6 weeks and less than 1 year.
3) Morning stiffness >30 minutes.
4) Bilateral compression pain in both feet(MTP: metatar-sophalangeal)
We excluded patients who showed:
1) Crystal disease.
2) Reactive arthritis.
3) Septic arthritis.
4) Arthritis due to sarcoidosis.
5) Lupus erythematosus or other collagen disease clearly defined.
6) Who had received disease-modifying drugs at the start of follow-up.
7) Presence of mono arthritis Protocol was evaluated by a technical committee and ethics committee of the HUC, all patients signed the consent form. Patients were evaluated at baseline, at 3 months and 6 months.
To calculate the sample size the analysis was based on survival using the Log Rank Test, and was intended to achieve a power of 80% for which the sample size should be 59 individuals; however, only a sample of 54 patients was achieved [11].
For the evaluation of pain to symmetrical compression of MTPs, interobserver variability was measured by Cohen’s Kappa coefficient which applies to dichotomous qualitative variables [12] and two researchers were trained in the determination of this sign for the existence of a maximum match, each observer was blind to the results of the other. The patient was placed in supine or sitting position with feet in neutral position and each foot was examined by palpation separately. The Kappa correlation was 0.88 (standard error 0.14) for bilateral compression of the MTPs, of 0.71 (standard error 0.16) for the variable “points out”, 0.71 for the variable “gestures” (standard error 0.16) and 1.0 for the variable “remove at pain” (standard error 0.0), which revealed a good correlation between the two observers.
At baseline and every 6 months PA and oblique radiographs of hands and feet were done and were analyzed by the Sharp method modified by van der Heijde [13].
3. System Variables
Main outcome measures are remission, persistence, and severity. This will define the clinical course: the nominal variable where classified as:
1) Natural remission: absence of arthritis in patients not taking disease-modifying drugs or steroids during the last 3 months of one-year follow-up period.
2) Persistence: the presence of arthritis in at least one joint and/or treatment with disease-modifying drugs or steroids in the last 3 months of one-year follow-up period.
3) Severity: erosive disease in at least one of the areas assessed by the Sharp method modified by van der Heijde (average greater than or equal to 1) after one year of follow-up.
This will generate three possible diagnoses:
1) Self-limiting arthritis: these correspond to patients who meet the criterion of natural remission.
2) Persistent non-erosive arthritis: these include patients meeting the criteria for persistence, but not for severity.
3) Persistent erosive arthritis: these include patients meeting the criteria for persistence and severity.
4. Statistical Analysis
As patients were enrolled in the study, data were stored in a database contained in the statistical program SPSS Version 10.00. At the end of sample collection a quantitative analysis of the information was performed, for which we applied descriptive statistical methods such as averages, percentages and standard deviations, prevalence rate, among others, as well as measures of association of risk with odds ratio and Fisher's exact test.
We estimated the Relative Risk (RR) and 95% confidence intervals for the variables considered as predictors. For comparison between subgroups the Log-rank test was use.
5. Results
A prospective study in rheumatology service at the University Hospital of Caracas during a period of twelve months, whose objective was to reveal predictors of persistence and severity in patients with EIP was conducted. During the sample collection period (August, September, October and November 2004), 54 patients with EIP attend the out-patient clinic, of whom 92% (50 patients) were female, mean age 42 ± 11.11 (1SD), 67% of patients had disease progression less than 6 months (Table 1).
Out of 54 patients included in the study, 8 were lost to follow-up (14.81%), 2 patients expressed their desire not to continue in the study and the other six patients were telephoned 3 times by cutoff date (at 6 months) and did not attend the clinic. One diabetic patient died from hypoglycemia while receiving a sulfonylurea (1.85%), but the 6 months evaluation was performed just before his death. The characteristics of the 54 patients at the beginning of the study revealed that 100% had arthritis of the hands and 94% had arthritis of three or more areas.
Bilateral painful compression was present in 63% of patients at the start and was only 24% for the cutoff date. 72% had morning stiffness, only 3 patients (5%) had rheumatoid nodules. The initial DAS 28 was 4.92 (SD 1.03) and decreased to 3.66 at 6 months. The HAQ and ESR decreased from 1.22 (SD 0.78) to 0.66 (SD 0.78) and 37.48 (SD 32.98) to 29.61 (SD 22.88) at 6 months follow up respectively.
With respect to laboratory tests, RF was the most frequently positive parameter with 55.5%, followed by antiCCP with 48% and CTx-II with 33.7%.
Of the 46 patients who completed the study, 19.6% had self-limiting arthritis, and 80.4% had persistent arthritis. Of the patients with persistent arthritis 32.6% had nonerosive disease and 47.8% had erosive disease (Figure 1).
Regarding the use of DMARDs, the most used drug was methotrexate. At the start of follow-up methotrexate was prescribed to 42.6% of patients and 6 months were receiving up to 56.5%; the second most used type of drugs at 6 months were antimalarial followed by leflunomide and sulfasalazine. Only one patient received therapy antiTNF-a (Table 1)
Most of the population showed a persistent arthritis (80.4%). Of the 37 patients with persistent arthritis 31 (67.4%) had rheumatoid arthritis and 4 (8.7%) had seronegativespondyloarthropathy, being these two pathologies the most common diagnoses for this group. Of the 9 patients with self-limiting EIP, 6 (13%) had a transient undifferentiated arthritis, the other three had nodular osteoarthritis, fibromyalgia and mixed connective tissue disease (Table 2).
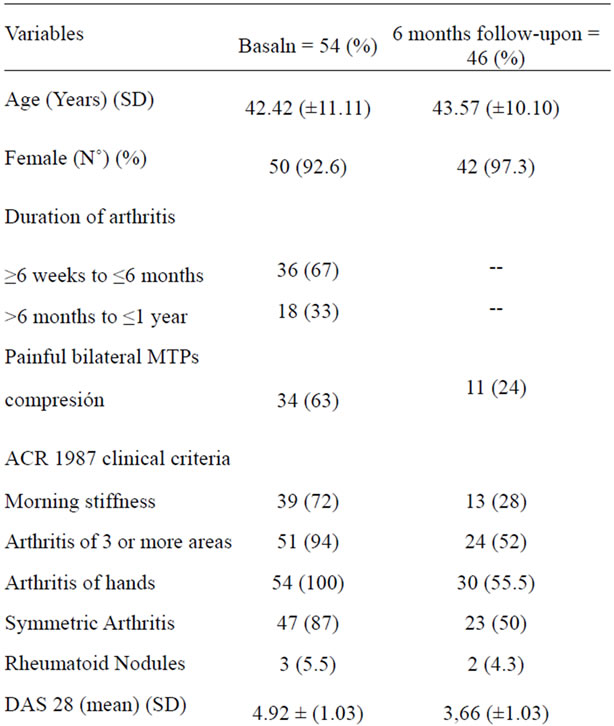
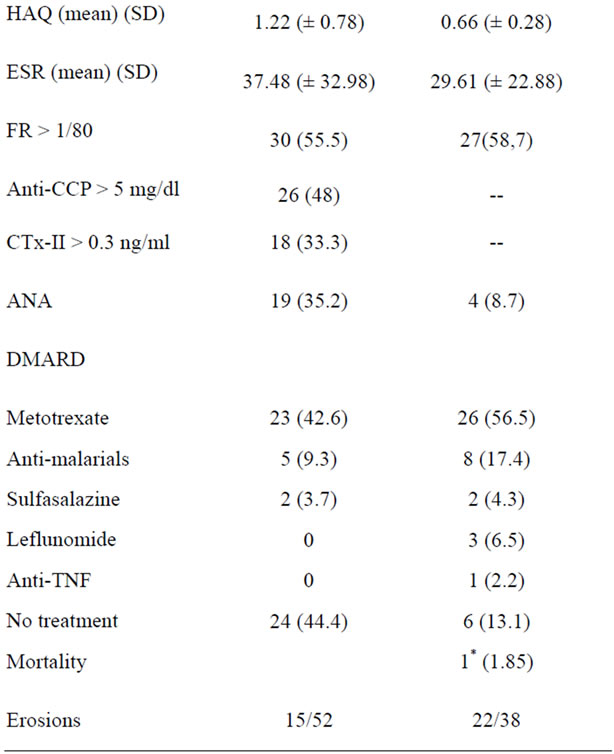
Table 1. Characteristics of the population at baseline and at 6 months follow-up.
The highest percentage of patients with rheumatoid arthritis had positive rheumatoid factor (70%), followed by anti-CCP with 61.5% and CTx-II with 61%. There were 6 patients without rheumatoid arthritis who had rheuma-
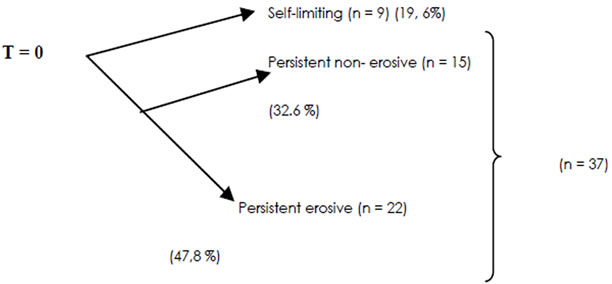
Figure 1. Diagram of the evolution of patients with EIP at cutoff date, T = 12 month n = 46.
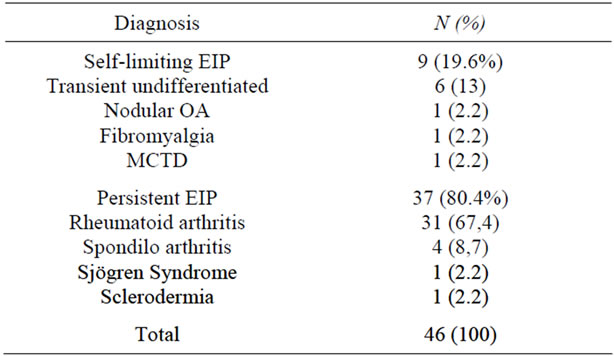
Table 2. Frequency and percentage distribution according to final diagnosis at 12 months follow-up.
toid factor and anti-CCP positive and only 4 had an increased CTx-II. Of the patients with CTx-II positive, three had a seronegativespondyloarthropathy. Patients who had any positive test were not considered, but lost to follow up because we were not able to establish the definitive diagnosis (Table 3).
The basal rheumatoid nodules and positive rheumatoid factor were enough to predict which patients would present persistence of disease activity. A duration of symptoms longer than 6 months, basal symmetry, anti-CCP and CTX-II positive, increased the relative risk for persistent activity for patients who did not have these risk factors, although this difference was not statistically significant (Table 4).
Symmetry, rheumatoid nodules and basal erosions allow predicting which patients will develop erosive disease at 6 months. The evolution of more than six months, the RF, and CTX-II increased the relative risk of developing erosions at 6 months compared with patients who did not meet these conditions; however this difference was not statistically significant. The positivity for the three serological markers (RF + AntiCCP + CTx-II) or at least the presence of two thirds of them increased the risk of persistent disease in addition to predict the development of erosions (Table 4).
6. Discussion
The diagnosis of early rheumatoid arthritis often pre-
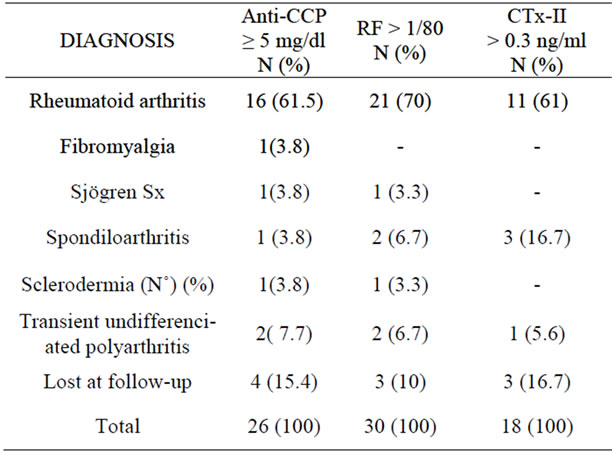
Table 3. Frequency and percentage distribution according to Anti-CCP values, RF, CTx-II positive and type of diagnosis at 12 months follow-up.
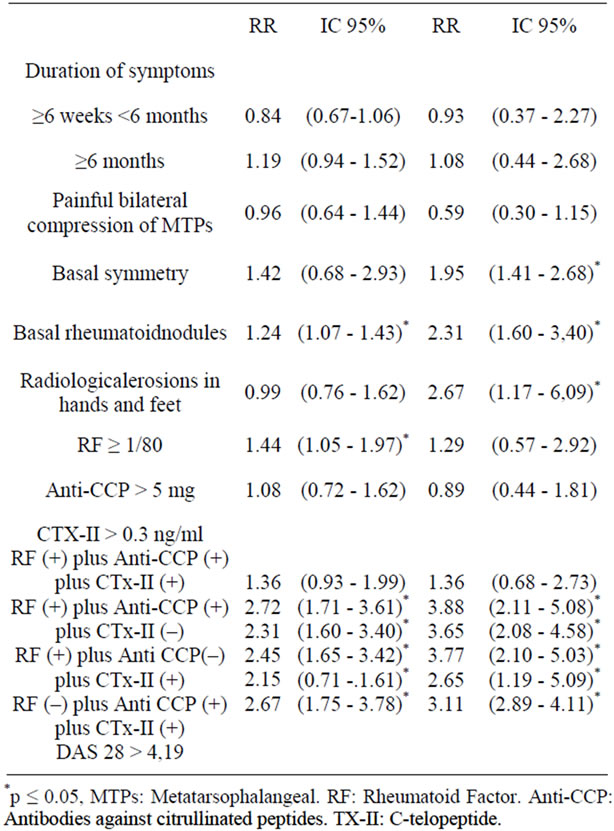
Table 4. Persistence of the activity and severity by radiological damage at 12 months follow-up.
sents difficulties. The biggest problem is that the disease reveals typical clinical features and radiological damage over time and not in the initial stages. Therefore, it is necessary to sharpen on the clinical, serological detection of different antibodies and early radiographic changes [2].
The evaluation of factors or predictive tests for a defined condition requires that general population have a reasonable proportion of patients with the disease. The prevalence of rheumatoid arthritis is low, therefore studies should be carried out in groups at high risk of developing the disease, as the sample evaluated in this trial.
Most of the patients in our sample had arthritis for more than 6 weeks and less than 6 months, which reveals that a high percentage of patients with early polyarthritis were included in the study.
With respect to ACR 1987 clinical criteria, it is noteworthy that at baseline 100% of patients had arthritis in their hands, 94% arthritis of three or more joints and 87% symmetrical arthritis, which made it a sample with high potential to develop rheumatoid arthritis, but not so in all cases. These findings suggest that the initial population showed more affection of the hands than the feet.
To our knowledge, this is the first study that simultaneously evaluates an antibody as the rheumatoid factor and the anti-CCP and a marker of cartilage degradation as the CTx-II. At the start of the investigation the paraclinical examination most often reported was RF with 55.5%, followed by anti-CCP with 48% and CTx-II with 33.3%. In other populations with undifferentiated arthritis the detection of antibodies was much lower, with 21% for both rheumatoid factor and anti-CCP [14]. In the study of Charnie and colleagues, only 14.08% (89 patients out of 632 patients) had a high value of urinary CTX-II [15].
80.4% of the population showed a persistent EIP, and from these 67.4% were rheumatoid arthritis, followed by seronegativespondyloarthropathy with 8.7%. In a population of 524 patients, Visser and colleagues showed that only 40.3% had persistent arthritis after a 2-year follow up period. However, of the 211 patients with persistent arthritis 66.4% developed rheumatoid arthritis and 3.3% had a seronegativespondyloarthropathy [9]. Values in patients with persistent arthritis who developed rheumatoid arthritis were similar.
The study shows that 13% of patients with undifferentiated arthritis acted in the evolution of the disease as a self-limited arthritis. Of 313 patients with self-limiting arthritis evaluated by Visser and colleagues, 33% had undifferentiated arthritis [9]. These differences could be explained by the dissimilar sizes of the samples, different follow-up time or the provenance of the studied populations.
It was interesting to note the comparison between the antibodies studied and the cartilage degradation marker investigated. Among patients who developed rheumatoid arthritis at 6 months it was determined that the RF was positive in 70%, anti-CCP in 61.5% and CTx-II in 61% of the patients. The study by Gossec et al. of patients with early rheumatoid arthritis found a 68% of RF-IgM and 58.9% of anti-CCP. [16] These similarities show that in properly selected patients with a clinical signs and symptoms of EIP, serological markers are presented in a high percentage of patients and can be very useful for establishing a definitive diagnosis.
It has been widely published that the anti-CCP surpasses in specificity to rheumatoid factor [17]. In this study there were 6 patients without rheumatoid arthritis who were RF and anti-CCP positive. By contrast, the study highlighted that the CTx-II was present in only four patients without rheumatoid arthritis, of whom 3 had a seronegativespondyloarthropathy and 1 had a transient undifferentiated polyarthritis. Patients with seronegativespondyloarthropathy recorded the highest titers of CTx-II; this may be the subject of further study.
These results could promote the identification of markers of cartilage degradation in EIP, as there are studies that concluded that high levels of CTx-I and urinary CTX-II predict an increased risk of radiological progression at one year and 4 years in patients with early rheumatoid arthritis [10,15]. In our study the determination of CTx-II also showed an increased risk in the persistence and the development of the disease, although this difference was not statistically significant. In our study the determination of this marker was in blood, which could facilitate their use in clinical practice.
The presence of bilateral compression pain of the MTPs was not statistically significant, and did not show an increased risk for persistence of disease activity, or the radiological damage; on the contrary, in the study of Visser et al. bilateral metatarsophalangeal compression it did predict both the persistence as erosions in a span of two years [9].
In our study, examining the X-ray of hands and feet, we found that patients had a greater and significant radiological damage in hands than in feet. This is opposed to observe in European populations, where early rheumatoid arthritis erosions are more frequent in the small joints of the feet [18-20].
While the greatest initial radiological damage was at hand, the progression was greater in feet at 6 months. Van der Heijde et al. observed similar behavior in patients with early rheumatoid arthritis [21]. Our population seems to have differences with respect to patients of European countries in relation to the initial condition of the joints of hands and feet [18-20]. Baseline data indicate that 100% of patients at beginning of the study had signs of inflammation in the joints of the hands in contrast with 63% who reported pain on bilateral compression of the MTPs. Verifying the initial condition of the joints of the hands seems to be more important than feet examination in our region.
While not a goal in our study we emphasize that basal symmetry increased relative risk for persistent activity; but did not achieved statistically significance. On the other hand, the basal symmetry did allow the prediction of erosion on radiographs at 12 months follow up.
Van Gaal and colleagues in a multivariate analysis that included the ACR 1987 criteria to predict evolution of undifferentiated arthritis to rheumatoid arthritis in one year, the symmetry presented an OR (odds ratio) of 2.6 (1.1 to 60); p < 0.028. Therefore, the symmetry may be a clinical variable to consider as predictor of EIP [14].
Although basal rheumatoid nodules were able to predict both persistence of the activity and development of erosions at 6 months, their presence is rare in early arthritis. In fact, only 5.5% of the patients in the study had rheumatoid nodules in the first assessment and this percentage did not increase during the first 6 months after follow-up.
Radiological erosions at the start of the disease failed to predict persistence of activity, but logically it increased the relative risk for the severity. Likely, the low number of patients in whom the radiological study was done and the short follow-up period of time did not provide opportunity to establish the statistical significance of this variable. However, it is important to note that patients who had a high initial average by the Sharp method modified by van der Heijde tend to have a greater increase in erosion at 6 - 12 months compared to those who had an initial low average (Pearson correlation coefficient 0.40, p < 0.015). This is consistent with other studies where it has been established that the presence of erosions in hands and feet are able to predict which patients will develop rheumatoid arthritis [6,22].
With regard to laboratory test results, the RF allowed to predict which patients were to persist with symptoms of disease activity. There was an increased relative risk to determine which patients will develop erosion, although this value was not statistically significant. A large group of studies have found that the RF is a predictor of persistence, development of erosion and useful in predicting which patients will progress to rheumatoid arthritis [6- 14].
Serological markers separately were inefficient in predicting the persistence or radiological erosions at 6 months, not even the new marker anti-CCP, in contrast to the studies of Visser et al., where the anti-CCP increased the OR to 4.58 for persistence and was the best predictor of erosion at two years. (9) Other studies demonstrate that the anti-CCP does predict which patients will develop rheumatoid arthritis. [21,23]
The CTx-II increased the RR of persistence of disease activity and development of erosions at 6 months, although this difference was not statistically significant. The urinary CTX-II has been shown to predict increased risk of radiological progression in 89 patients with rheumatoid arthritis less with than 3 years of evolution [15]. Type II collagen represents the major cartilage collagen fiber, constituting 30% - 90%. The degradation of collagen type II is considered an excellent marker of cartilage degradation and is considered an early sign of disease in rheumatoid arthritis. [21]
In the light of the results obtained, the CTx-II seems an interesting marker to be considered in patients with EIP. This confirms the result that shows that up to 61% of the 13 patients with CTx-II positive developed rheumatoid arthritis at 12 months follow-up.Due to the extensive choice in the exclusion criteria for this cohort allowed us to differentiate the patient with EIP who would develop chronic, which is why we developed a simple but practical screening test to discriminate patients who consult primary care physicians, family doctors or non-specialist practitioners (Figure 2).
InEIP about 40% of patients going to self-limiting arthritis, in this study we added 2 clinical variables (morning stiffness and bilateral compression) with it, get a larger number of patients who developed arthritis persistent.
7. Conclusions
The highest percentage of patients with EIP developed a persistent arthritis, where rheumatoid arthritis was the most common pathology. The RF, anti-CCP and CTX-II were present at baseline in most patients with EIP who developed rheumatoid arthritis at 6 months.
In the EIP population studied, the initial clinical and radiological condition of the hand was more important than those of the feet.
Most patients with EIP were treated early with a DMARD, improving at 6 months in clinical and laboratory parameters, but progression of erosive disease continued. Basal nodules and RF were the variables that could predict early persistence in patients with EIP.
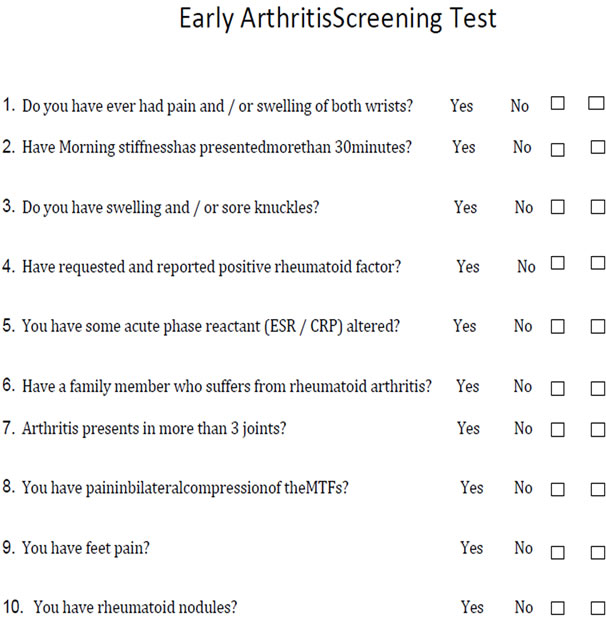
Figure 2. Early arthritis screening test.
Symmetry and rheumatoid nodules at baseline were able to predict which patients would develop an erosive disease at 6 months. The patients with basal erosive disease are more likely to suffer an increase in average number of erosions measured by the Sharp method modified by van der Heijde, compared with patients without the disease.
REFERENCES
- A. Saraux, J. Berthelot, G. Chalès, C. Lehenaff, J. Thorel, S. Hoang, et al., “Ability of the American College of Rheumatology 1987 Criteria to predict Rheumatoid Arthritis Patients with Early Arthritis and Classification,” Arthritis Rheuma, Vol. 44, No. 11, 2001, pp. 2485-2491. doi:10.1002/1529-0131(200111)44:11<2485::AID-ART428>3.0.CO;2-S
- P. Emery and D. Symmons, “What Is Early Rheumatoid Arthritis, Definition and Diagnosis,” Bailliére’s Clinical Rheumatology, Vol. 11, No. 1, 1997, pp. 13-26. doi:10.1016/S0950-3579(97)80030-1
- M. Weinblatt, “Rheumatoid Arthritis: Treat Now, Not Later,” Annals of Internal Medicine, Vol. 124, No. 8, 1996, pp. 773-774.
- T. Möttönen, P. Hannonén, M. Leirisalo-Repo, M. Nissila, H. Kautiainen, M. Korpela, et al., “Comparison of Combination Therapy with Single—Drug Therapy in Early Rheumatoid Arthritis: A Randomised Trial,” Lancet, Vol. 353, No. 9164, 1999, pp. 1568-1573. doi:10.1016/S0140-6736(98)08513-4
- M. Boers, A. Verhoeven, H. Markusse, M. Vandelaae, R. Westhovens, J. van Dendere, et al., ”Randomised Comparison of Combined Step-Down Prednisolone, Methotrexate and Sulphasalazine with Sulphasalazine Alone in Early Rheumatoid Arthritis,” Lancet, Vol. 350, No. 7094, 199, pp. 309-318. doi:10.1016/S0140-6736(97)01300-7
- D. L. Scott, “The Diagnosis and Prognosis of Earlyarthritis: Rationalefor New Prognosticcriteria,” Arthritis & Rheumatism, Vol. 46, No. 2, 2002, pp. 286-290. doi:10.1002/art.10134
- C. Reparon-Schuijt, W. J. E.Van Eschw, C. Kooten, G. Schellekens, B. Jong, et al., “Secretion of Anti-Citrulline— Containing Peptide ANTIBODY by B Lymphocytes in Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 44, No. 1, 2001, pp. 41-47. doi:10.1002/1529-0131(200101)44:1<41::AID-ANR6>3.0.CO;2-0
- H. Doyle and M. Mamula, “Post Translational Protein Modifications: New Flavours in the Menu of Autoantigens,” Current Opinion in Rheumatology, Vol. 14, No. 3, 2002, pp. 244-249. doi:10.1097/00002281-200205000-00009
- H. Visser, S. le Cessie, F. Breedueld, J. Hazes, et al., “How to Diagnose Rheumatoid Arthritis Early. A Prediction Model for Persistent (Erosive) Arthritis,” Arthritis & Rheumatism, Vol. 46, No. 2, 2002, pp. 357-365. doi:10.1002/art.10117
- P. Garnero, R. Landewé, M. Boers, A. Verhoeven, S. van Linden, S. Christgau, et al., “Association of Baseline of Markers of Bone and Cartilage Degradation with LongTerm Progression of Joint Damage in Patients with Early Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 46, No. 11, 2002, pp. 2847-2856. doi:10.1002/art.10616
- L. Freedman, “Tables of the Number of Patients Required in Clinical Trials Using the Logrank Test,” Statistic in Medicine, Vol. 1, No. 2, 1982, pp. 121-129. doi:10.1002/sim.4780010204
- J. Cohen, “A Coefficient of Agreement for Nominal Class,” Educational and Psychological Measurement, Vol. 20, 1960, pp. 37-46. doi:10.1177/001316446002000104
- D. Van der Heijde, T. Dankert, F. Nieman, R. Rau and M. Boers, “Reliability and Sensitivity to Change a Simplification of Sharp/Van Der Heijde Radiological Assessment in Rheumatoid Arthritis,” Rheumatology, Vol. 38, No. 10, 1999, pp. 941-947. doi:10.1093/rheumatology/38.10.941
- F. A. van Gaalen, S. P. Linn-Rasker, W. T. van Verooij, B. A. de Jong, F. C. Breedveld, C. L. Verweij, et al., “Autoantibodies to Cyclic Citrullinated Peptides Predict Progression to Rheumatoid Arthritis in patients with Undifferentiated Arthritis. A prospective Cohort Study,” Arthritis & Rheumatism, Vol. 50, No. 3, 2004, pp. 709-715. doi:10.1002/art.20044
- Charni, F. Juillet and P. Garnero, “Urinary Type II Collagen Helical Peptide (HELIX-II) as a New Biochemical Marker of Cartilage Degradation in Patients with Ostearthritis and Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 52, No. 4, 2005, 1081-1090.
- L. Goosec, M. Dougados, P. Goupille, A. Cantagrel, J. Sibilia, O. Meyer, et al., “Prognostics Factor for Remission in Early Rheumatoid Arthritis: A Multiparameter Prospective Study,” Annals of the Rheumatic Diseases, Vol. 63, No. 6, 2004, pp. 675-680. doi:10.1136/ard.2003.010611
- G. Schellekens, H. Visser, B. A. W. de Jong, F. H. J. van den Hoogen, J. M. W. Hazes, F. C. Breedveld, et al., “The Diagnostic Properties of Rheumatoid Arthritis Antibodies Recognizing a Cyclic Citrullinated Peptide,” Arthritis & Rheumatism, Vol. 43, No. 1, 2000, pp. 155-163. doi:10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3
- P. Brennan, B. Harrison, E. Barrett, K.Chakravarty, D. Scott and A. Silma, “A Simple Algorithm to Predict the Early Rheumatoid Arthritis: Prospective Cohrte Study,” British Medical Journal, Vol. 313, 1996, pp. 471-476. doi:10.1136/bmj.313.7055.471
- D. van der Heijde, M. A. van Leeuwen, P. L. van Riel, A. M. Koster, M. A. van Hof, M. H. van Rijswijk, et al., “Biannual Radiographic Assessment of Patients with Early Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 35, No. 1, 1992, pp. 26-34. doi:10.1002/art.1780350105
- D. Scott and A. Silma, “A Simple Algorithmto Predict the Development of Radiological Erosions in Patients with Early Rheumatoid Arthritis: Prospective Cohort Study,” British Medical Journal, Vol. 313, No. 471, 1996, pp. 471-476.
- A. van der Heide, C. Remme, D. Hofman, J. Jacobs and J. Bijlsma, “Prediction of Progression of Radiologic Damage in Newly Diagnosed Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 38, No. 10, 1992, pp. 26-34.
- L. M. A. Jansen, I. E. van der Horst-Brwinsma, D. van Schaardenburg, P. D. Bezemer and B. A. C. Dijkmans, “Predictors of Radiographic Joint Damage in Patients with Early Rheumatoid Arthritis,” Annals of the Rheumatic Diseases, Vol. 60, No. 10, 2001, pp. 924-927. doi:10.1136/ard.60.10.924
- J. Fries, P. W. Spit, R. G. Kraines and R. H. Holman, “Measurement of Patient Outcome in Arthritis,” Arthritis & Rheumatism, Vol. 23, No. 2, 1980, pp. 137-145. doi:10.1002/art.1780230202
- A. Finckh, M. H. Liang, C. M. van Herckenrode, et al., “Long-Term Impact of Early Treatment on Radiographic Progression in Rheumatoid Arthritis: A Meta-Analysis,” Arthritis & Rheumatism, Vol. 55, No. 6, 2006, pp. 864-872. doi:10.1002/art.22353
- H. Kallberg, L. Padyukov, R. M. Plenge, et al., “Gene-Gene and Gene-Environment Interactions Involving HLA-DRB1, PTPN22, and Smoking in Two Subsets of Rheumatoid Arthritis,” The American Journal of Human Genetics, Vol. 80, No. 5, 2007, pp. 867-875.
- L. Klareskog, P. Stolt, K. Lundberg, et al., “A New Model for an Etiology of Rheumatoid Arthritis: Smoking May Trigger HLA-DR (shared epitope)—Restricted Immune Reactions to Autoantigens Modified by Citrullination,” Arthritis & Rheumatism, Vol. 54, No. 1, 2006, pp. 38-46. doi:10.1002/art.21575
- M. Pedersen, S. Jacobsen, P. Garred, et al., “Strong Combined Gene-Environment Effects in Anti-Cyclic Citrullinated Peptide-Positive Rheumatoid Arthritis: A Nationwide Case-Control Study in Denmark,” Arthritis & Rheumatism, Vol. 56, No. 5, 2007, pp. 1446-1453. doi:10.1002/art.22597
- M. M. Nielen, D. van Schaardenburg, H. W. Reesink, et al., “Specific Autoantibodies Precede the Symptoms of Rheumatoid Arthritis: A Study of Serial Measurements in Blood Donors,” Arthritis & Rheumatism, Vol. 50, No. 2, 2004, pp. 380-386. doi:10.1002/art.20018
- S. Rantapaa-Dahlqvist, B. A. de Jong, E. Berglin, et al., “Antibodies against Cyclic Citrullinated Peptide and Arheumatoid Factor Predict the Development of Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 48, No. 10, 2003, pp. 2741-2749.
- M. K. Koivula, M. Heliovaara, J. Ramberg, et al., “Autoantibodies Binding to Citrullinated Telopeptide of Type II Collagen and to Cyclic Citrullinated Peptides Predict Synergistically the Development of Seropositive Rheumatoid Arthritis,” Annals of the Rheumatic Diseases, Vol. 66, No. 11, 2007, pp. 1450-1455. doi:10.1136/ard.2006.062919
- S. Nijenhuis, A. J. W. Zendman, E. R. Vossenaar, G. J. M. Pruijn, W. J. van Venrooij, “Autoantibodies to Citrullinated Proteins in Rheumatoid Arthritis: Clinical Performance and Biochemical Aspects of an RA-Specific Marker,” Clinica Chimica Acta, Vol. 350, No. 1-2, 2004, pp. 17-34. doi:10.1016/j.cccn.2004.07.016
- G. Harauz, N. Ishiyama, C. M. Hill, I. R. Bates, D. S. Libich and C. Fares, “Myelin Basic Protein—Diverse Conformational States of an Intrinsically Unstructured Protein and Its Roles in Myelin Assembly and Multiple Sclerosis,” Micron, Vol. 35, No. 7, 2004, pp. 503-542. doi:10.1016/j.micron.2004.04.005
- E. R. Vossenaar, A. J. W. Zendman, W. J. van Venrooij and G. J. M. Pruijn, “PAD, a Growing Family of Citrullinating Enzymes: Genes, Features and Involvement in Disease,” BioEssays, Vol. 25, No. 11, 2003, pp. 1106-1118.

