Open Journal of Blood Diseases
Vol.3 No.3A(2013), Article ID:38510,6 pages DOI:10.4236/ojbd.2013.33A004
Oxidative Stress and Antioxidant Status in Acute and Chronic Myeloid Leukemia Patients
![]()
1Sri Sathya Sai Institute of Higher Learning, Anantapur, India; 2Nizam’s Institute of Medical Sciences, Hyderabad, India.
Email: *rajeswarichandrashekaru@sssihl.edu.in
Copyright © 2013 Ullagaddi Rajeshwari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 1st, 2013; revised August 1st, 2013; accepted August 8th, 2013
Keywords: Chronic and Acute Myeloid Leukemia; Oxidative Stress; Antioxidants; Malondialdehyde; Protein Carbonyls; Non-Enzymatic Antioxidants
ABSTRACT
Oxidative stress, a pervasive condition of increased number of reactive oxygen species, is now recognized to be prominent feature of various diseases and their progression. The relationship between antioxidants and levels of well-known markers of oxidative stress, measured as lipid peroxides and oxidized proteins reflect health indices. The aim of this study is to evaluate the extent of oxidative stress and antioxidant status in acute and chronic myeloid leukemia patients. The present study included 60 patients selected using standard questionnaire based on age, family history, Body Mass Index (BMI), dietary intake, with no other complications and 30 age and sex-matched healthy subjects. The median age of myeloid leukemia patients was 43 years and that of controls was 42 years. Out of 60 myeloid leukemia patients, 30 were in acute and 30 were in chronic state. Oxidative stress and antioxidant status were evaluated in the patients and in the controls by assessing standard oxidative stress markers viz. plasma and erythrocyte lipid peroxide levels in terms of malondialdehyde and oxidized proteins as protein carbonyls whereas antioxidant status was assessed in terms of serum non enzymatic antioxidant levels. There was a significant increase (p < 0.01) in plasma and erythrocyte lipid peroxidation and protein oxidation in acute and chronic myeloid leukemia patients as compared to healthy subjects. Antioxidant status as indicated by the levels of non-enzymatic antioxidants viz. erythrocyte reduced glutathione (GSH), serum β carotene, vitamin A & C and ceruloplasmin was found to be significantly decreased (p < 0.01) in both the leukemia patients as compared to healthy participants. However, chronic myeloid leukemia patients had significantly (p < 0.05) higher oxidative stress and lower antioxidant status as compared to acute myeloid leukemia patients.
1. Introduction
The substances that can damage DNA and cause cancer are known as free radical (FR) producers. Free radicals induce changes in the sequence of DNA viz. deletions, gene amplification, rearrangement and mutations, which cause the activation of various protooncogenes and/or tumor suppressor genes. Free radicals, the by-products of normal metabolism increase inflammation and exposure to exogenous sources including nitrogen oxide pollutants, smoking, certain drugs (e.g., acetaminophen, bleomycin) and radiations can induce cancer-causing mutations, oxidize lipids and proteins, and alter signal transduction pathways that enhance cancer risk [1]. Reactive oxygen species may also arise through the metabolism of certain drugs, like anthracyclic antineoplastic agents (doxorubicin), pesticides and solvents [2].
Oxidative stress is defined as a pervasive condition of increased production and/or inadequate removal of ROS [3]. Lipid peroxidation is evaluated in terms of malondialdehyde (MDA) levels. Protein carbonyl (PC) is a product of irreversible non-enzymatic oxidation of protein [4]. Oxidative stress is now recognized to be a prominent feature of many acute and chronic diseases, and even cancer and leukemia [5]. Defense mechanisms of the body play an important role in the form of antioxidants and therefore, minimize the damage, adapting itself to the stressful situations. Antioxidants are compounds that dispose, scavenge, and suppress the formation of ROS, or oppose their actions and play a major role in the prevention of various diseases including cancer and their clinical manifestations [4,6].
Myeloid Leukemia
Leukemia is a disease of the white blood cells (WBC). There is a higher incidence of leukemia in people exposed to intense radiation. Some types of cytotoxic drugs can also lead to an increased risk. The peak incidence is between 40 and 60 years with the median age being 53 years [7]. Acute myeloid leukemia (AML) is a type of cancer that starts in the cells that normally develop into different types of blood cells. AML is also called as acute myelogenous leukemia, acute granulocyctic leukemia and acute non-lymphocytic leukemia [8].
Chronic myelagenous leukemia (CML) is also referred to as chronic myeloid, chronic myelocytic and chronic granulocytic leukemia. A number of diseases as well as cancers and leukemia viz. acute lymphocytic leukemia (ALL), acute nonlymphocytic leukemia (ANLL) have been reported to have significantly increased levels of various reactive oxygen species (ROS) such as superoxide radicals, H2O2, and decreased levels of enzymatic and non enzymatic antioxidants compared to healthy individuals [8].
2. Materials and Methods
Study population: The patients were selected from Nizam’s Institute of Medical Sciences (NIMS) Hyderabad, India. Sixty diagnosed myeloid leukemia (ML) patients were chosen for the study under the supervision of a medical oncologist. Out of 60 myeloid leukemia patients, 30 were of acute myeloid leukemia and 30 were of chronic myeloid leukemia. Thirty age and sex-matched healthy subjects were included in the study as controls (15 females, 15 males; mean age 42.2 ± 7.6 years) (Table 1). There were no dietary or supportive antioxidant medications given to patients and control subjects as well which could affect the status of free radicals and antioxidants. Informed consent was obtained from all the individuals included in the study. The present investigations were approved by the Institutional Ethical Committee for biomedical research. Blood samples were collected from the patients in Nizam’s Institute of Medical Sciences (NIMS) Hyderabad, India by the trained technician under the supervision of oncologist. At the time of blood collection, there were no evidences of infections, tissue injuries or inflammatory manifestations in the patients as well as in the controls.
Biochemical Analyses
Blood samples taken from the patients and healthy subjects following an overnight fasting (avoiding the possible influence of dietary factors on the levels of the biochemical parameters) into EDTA (5%) containing vials were used for the collection of plasma and erythrocytes, and vials without any anti-coagulant were used for the collection of serum.
Lipid peroxidation in plasma [9] and erythrocytes [10], reduced glutathione (GSH) in erythrocytes [11], protein oxidation [12], vitamin A and β-carotene [13], vitamin C [14] and ceruloplasmin [15] in serum were estimated. The findings were expressed as mean ± standard error. The differences were compared using Student’s t-test. A value of p < 0.05 was considered to be statistically significant (SPSS version 11).
3. Results
The present study conducted to assess oxidative stress in patients with AML and CML i.e. lipid peroxidation in terms of MDA in erythrocyte and plasma, protein carbonyl (PC) levels in the serum, non enzymatic antioxidant status of the patients generated very useful data. Erythrocyte and plasma MDA, and serum PC levels were found to be significantly increased (p < 0.01) in AML and CML patients as compared to healthy subjects (Figures 1-3). GSH in erythrocytes (p < 0.01), β-carotene, vitamin A, ceruloplasmin and vitamin C in serum were found to be significantly decreased (p < 0.01) in both AML and CML patients as compared to healthy subjects. However, CML patients had higher MDA and PC and lesser antioxidants than that of AML patients (Table 2).
4. Discussion
There is strong evidence that oxidative stress is a central issue in the processes of ageing, transformation or death of living cells and thus leads to many pathological processes among which is the induction of cancers. Oxidative stress originates basically from an acute or chronic imbalance between the production of reactive oxygen and nitrogen species and antioxidant capacities of living cells and organisms [1].
Free radicals play an important role in the pathophysiology of myeloid leukemia, and that high levels of
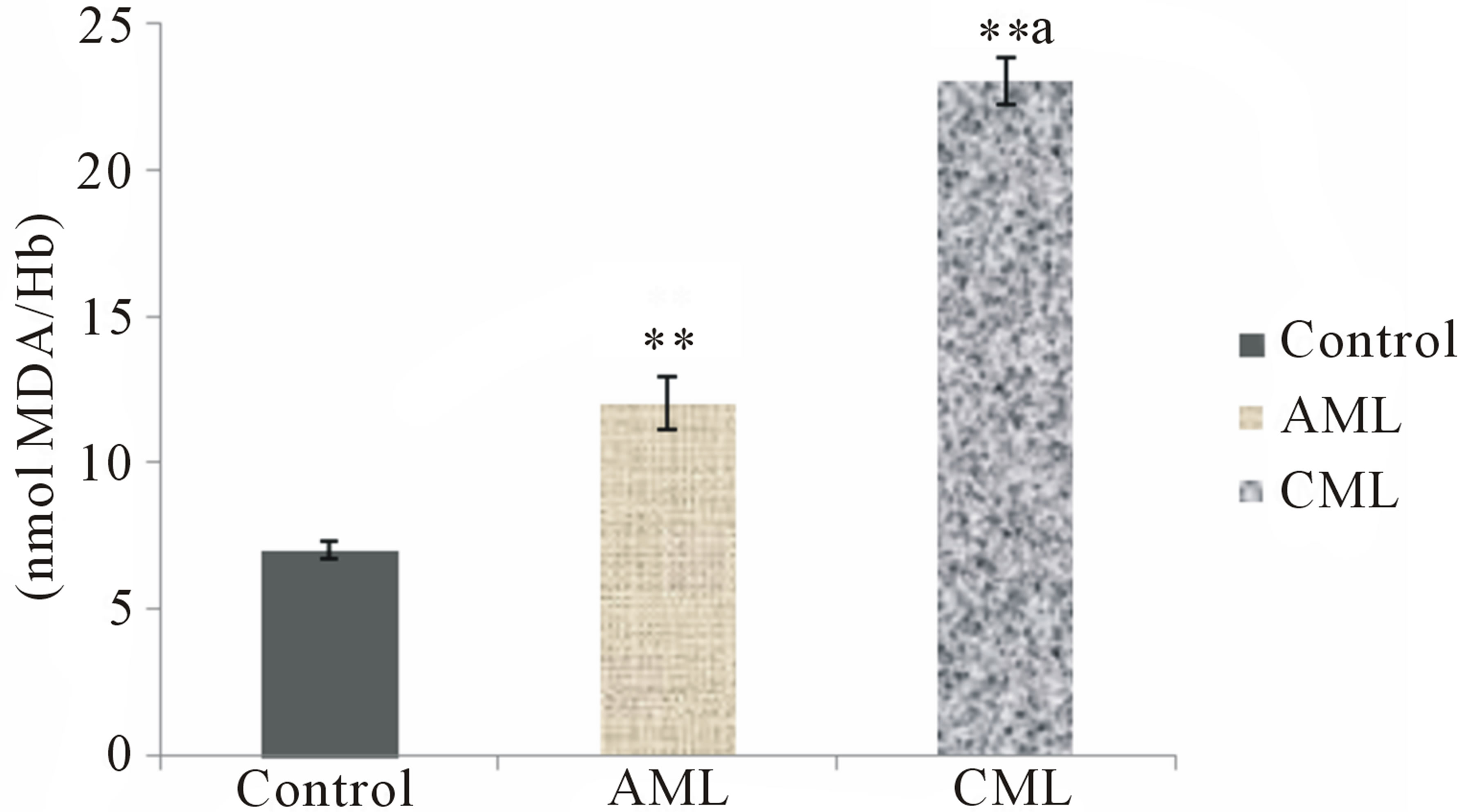
Figure 1. Erythrocyte lipid peroxidation in healthy controls, acute and chronic myeloid leukemia patients; Values are mean ± SEM of 30 patients/subjects in each group; **Statistically significant (p < 0.01) in comparison to healthy controls; aStatistically significant (p < 0.05) in comparison to AML.
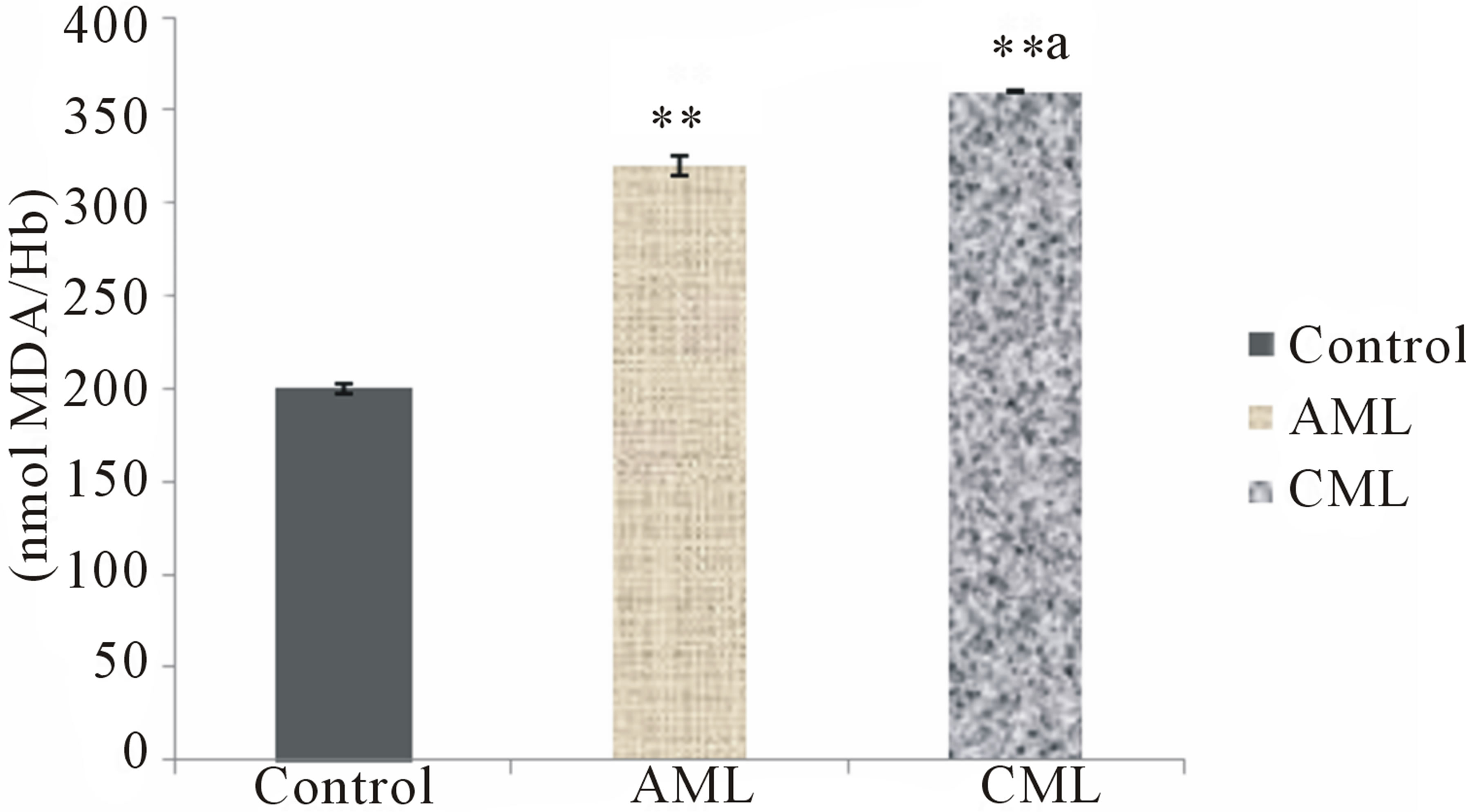
Figure 2. Plasma lipid peroxidation in healthy controls, acute and chronic myeloid leukemia patients; Values are mean ± SEM of 30 patients/subjects in each group; **Statistically significant (p < 0.01) in comparison to healthy controls; aStatistically significant (p < 0.05) in comparison to AML.
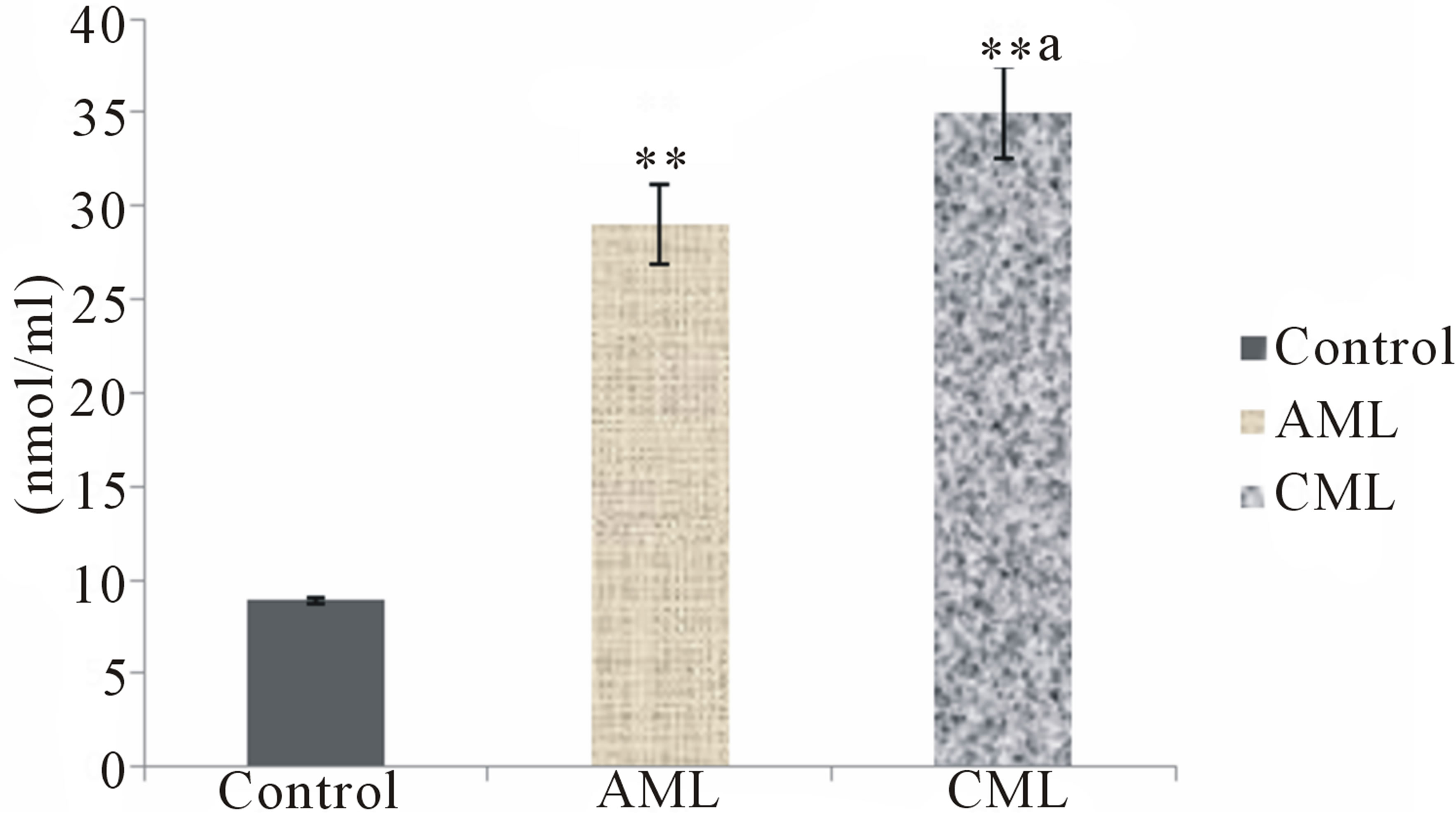
Figure 3. Protein oxidation in healthy controls, acute and chronic myeloid leukemia patients; Values are mean ± SEM of 30 patients/subjects in each group; **Statistically significant (p < 0.01) in comparison to healthy controls; aStatistically significant (p < 0.05) in comparison to AML.
free radicals may cause oxidative stress in haematopoietic cells if the antioxidant defense system is not potential. Oxidative stress may occur in patients with leukemia due to the higher number of mature and immature myeloid series cells as well as other unknown factors. Malondialdehyde (MDA) which is a stable end product of free radical induced-lipid peroxidation was used as a surrogate marker for oxidative damage to tissues. Antioxidants, which control the oxidative stress state, represent a major line of defense regulating overall true state of health. The relationship between the levels of antioxidants and the levels of well-known markers of oxidative stress that are measured as lipid peroxides and oxidized proteins reflect better health indices and postures [4].
In the present study, significantly increased lipid peroxidation in erythrocytes and plasma (measured in terms of the levels of MDA) was observed in patients with AML and CML as compared with healthy volunteers. This could be attributed to the increased formation or inadequate clearance of free radicals by the cellular antioxidants. The present observations are in agreement with other reports on hematological malignancies, including various human cancers [16].
It has been suggested that ROS exert their cytotoxic effect by carbonylating proteins, leading to a loss of protein function. Protein dysfunction is considered to be a widespread marker of severe oxidative stress, damage, and disease. ROS also cause peroxidation of membrane phospholipids, changing the permeability of the cellular membrane, increasing membrane fluidity and rigidity, and in some cases, increasing the risk of membrane rupture. Our findings are in broad agreement with an earlier study carried out on CML patients that accumulation of ROS may result in significantly increased lipid peroxidation and protein damage at the cellular and molecular levels [17].
Many antioxidants are micronutrients or depend on micronutrients for their activity. These include vitamins A and E as well as trace elements such as zinc, magnesium and selenium, which act as cofactors for antioxidant enzymes [18]. Antioxidants have been shown to inhibit both the initiation and promotion in carcinogenesis as well as counteract cell immortalization and transformation [19].The actions of different antioxidants show different patterns during neoplastic transformation, and tumour, cancer or leukaemic cells, which exhibit abnormal activities of the antioxidant enzymes as well as the concentrations of non-enzymatic antioxidants, when compared with their appropriate normal cells [20]. GR is a glutathione regenerating enzyme that permits the conversion of oxidized glutathione (GSSG) to reduced glutathione (GSH) by the oxidation of NADH to NAD+ [21]. Glutathione reductase is a secondary antioxidant enzyme that helps in the detoxification of reactive oxygen species by decreasing peroxide levels or by maintaining a steady state supply of metabolic intermediates like GSH [22].
Vitamin C also known as acid, is a water-soluble antioxidant, which prevents oxidative damage to the cell membrane induced by aqueous radicals [23]. Vitamin C is a powerful antioxidant, and acts as a scavenger of ROS to prevent, or at least alleviate the deleterious effects caused by ROS [24]. It works synergistically with vitamin E to quench free radicals and also regenerates the reduced form of vitamin E [25]. Ascorbic acid, in the defense system, protects metabolic processes against H2O2 and other toxic derivatives of oxygen. Acting as a chain breaking antioxidant, it impairs with the formation of free radicals in the process of formation of intercellular substances through the body, including collagen, bone matrix and tooth dentine [26].
Carotenoids have received considerable attention for their possible clinical use in diseases associated with reactive oxygen species such as cancer [27]. Carotenoids are found to decrease oxidative damage to DNA [28]. β- carotene (provitamin A) is assumed to stimulate the im-
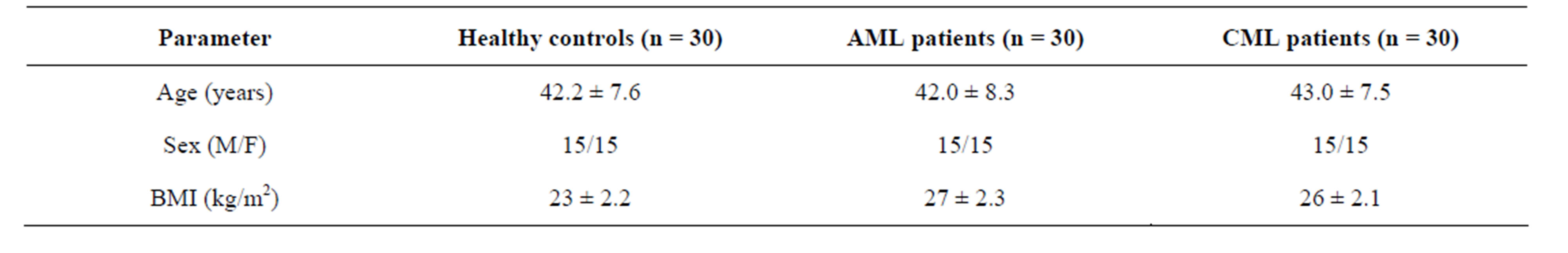
Table 1. Demographic characteristics of healthy controls, AML and CML patients.
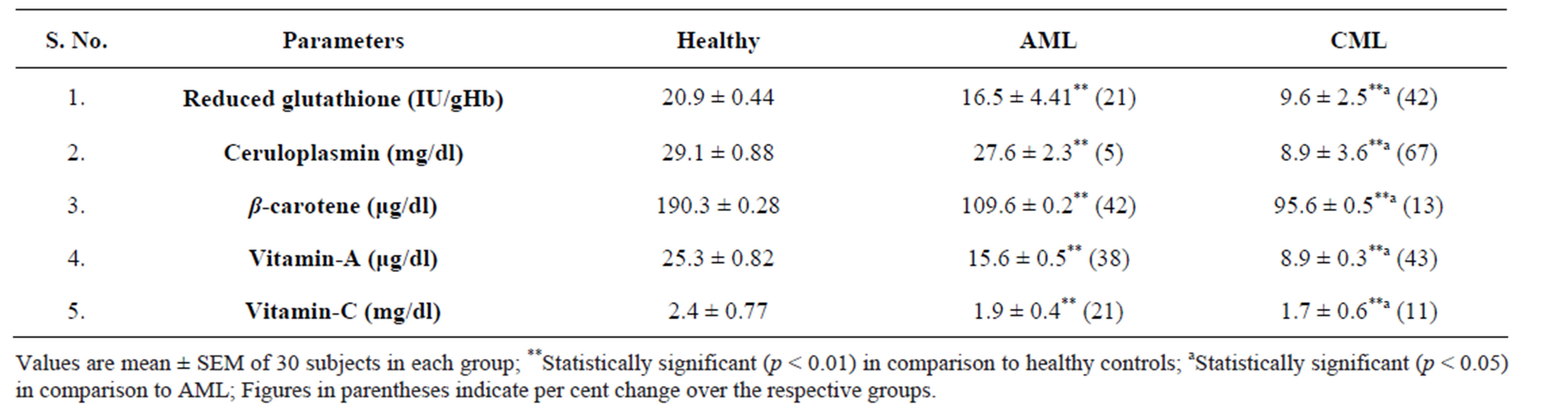
Table 2. Non-enzymatic antioxidants in healthy controls, acute and chronic myeloid leukemia patients.
mune system and cancer suppressor genes as well as deregulate oncogenes and block tumor angiogenesis. Therefore, carotenoids have been regarded to be of value not only as effective nutrients for the eyes, but also as antioxidants [29]. β-carotene has great capacity to remove the singlet oxygen and hence possesses antioxidant and antiproliferative properties and anticancer activity in humans [30]. Studies have demonstrated that β-carotene and vitamin A protect lipid molecules, low-density lipoproteins, proteins, and DNA against free radical attack, playing an essential role in the protection against diseases [31].
Ceruloplasmin (Cp) is a copper–containing ferroxidase that functions as an antioxidant in part by oxidizing toxic ferrous iron to nontoxic ferric iron [32]. Ferrous iron (Fe2+) is highly damaging because of its ability to generate toxic free radicals, particularly superoxide and hydroxyl radicals. Cp also serves as a general antioxidant by catalyzing the destruction of oxygen radicals and can bind to and inhibit neutrophil myeloperoxidase oxidant activity. Cp is a highly effective antioxidant that can prevent oxidative damage to lipids, DNA and proteins [33].
In our study, it was observed that the levels of nonenzymatic antioxidants were significantly (p < 0.01) decreased in ML patients as compared to healthy controls. These findings suggest that alterations in antioxidant defenses, which normally protect biological tissues from ROS, may be related to leukemia progression. Alternatively, it is possible that the cancer process itself causes the observed dysfunction of the antioxidant system.
Cellular non-enzymatic antioxidants are also known as free radical scavengers that protect cells against toxic free radicals. GSH is the chief constituent of the thiol pool and a vital intracellular scavenger of free radicals. Therefore, decreased GSH levels may reflect a depletion of non-enzymatic antioxidant reserve counterparts [20]. In this study, a significant depletion of GSH and other non enzymatic antioxidants was observed in patients with AML and CML as compared with healthy volunteers. The low levels of non enzymatic antioxidants in patients with ML provides some evidence that free radical generation in the haematopoietic cells is high compared to their normal counterparts.
Free radicals therefore, have the potential to lead additional mutations that could contribute to the progression of ML. Other studies have shown that the levels of lipid peroxidation increased continuously with disease progression and the aging process, whereas a defective nonenzymatic antioxidant defense system was found as the disease progressed and this is evidenced by the higher levels of lipid peroxidation in plasma and erythrocytes and protein carbonyls and lesser antioxidants in CML than that of AML in the present investigation. Although reactive species are well recognized for playing a dual role—deleterious and beneficial, excessive accumulation of ROS leads to antioxidant depletion and dysfunction [34].
5. Conclusion
In conclusion, high levels of plasma and erythrocyte malondialdehyde and protein carbonyls and poor antioxidant status in acute and chronic myeloid leukemia patients as compared to healthy subjects confirm oxidative stress in the patients. Higher oxidative stress and lower antioxidant status in chronic myeloid leukemia (CML) patients reflect higher magnitude of oxidative stress in chronic myeloid leukemia than acute myeloid leukemia (AML) indicating a possible link between decreased antioxidant levels and increased cellular alterations due to oxidative damage, supporting the possibility of the persistence of oxidative stress in CML. More studies are necessary to confirm whether these alterations are the cause or the consequence of carcinogenesis.
REFERENCES
- B. Halliwell and J. M. C. Gutteridge, “Free Radicals in Biology and Medicine,” 3rd Edition, Oxford University Press, Oxford, 1999.
- B. Frei, “Reactive Oxygen Species and Antioxidant Vitamins: Mechanisms of Action,” The American Journal of Medicine, Vol. 97, No. 3, 1994, pp. S5-S13. http://dx.doi.org/10.1016/0002-9343(94)90292-5
- M. Irshad and P. S. Chaudhuri, “Oxidant-Antioxidant System: Role and Significance in Human Body,” Indian Journal of Experimental Biology, Vol. 40, No. 11, 2002, pp. 1233-1239.
- R. Dalle-Donne, R. Rossi, R. Colombo, D. Giustarini and A. Milazani, “Biomarkers of Oxidative Stress in Human Disease,” Clinical Chemistry, Vol. 52, No. 4, 2006, pp. 601-623. http://dx.doi.org/10.1373/clinchem.2005.061408
- F. Galli, M. Piroddi, C. Annetti, et al., “Oxidative Stress and Reactive Oxygen Species,” Contributions to Nephrology, Vol. 149, 2005, pp. 240-260. http://dx.doi.org/10.1159/000085686
- H. Uzun, D. Konukoglu, R. Gelisgen, K. Zengin and M. Taskin, “Plasma Protein Carbonyl and Thiol Stress before and after Laproscopic Gastric Banding in Morbidly Obese Patients,” Obesity Surgery, Vol. 17, No. 10, 2007, pp. 1367- 1373. http://dx.doi.org/10.1007/s11695-007-9242-8
- S. Faderl, M. Talpaz, Z. Estrov, S. O’Brien, R. Kurzrock and H. M. Kantarjian, “The Biology of Chronic Myeloid Leukemia,” The New England Journal of Medicine, Vol. 341, 1999, pp. 164-172. http://dx.doi.org/10.1056/NEJM199907153410306
- G. S. Devi, M. H. Prasad, I. Saraswathi, D. Raghu, D. N. Rao and P. P. Reddy, “Free Radicals Antioxidant Enzymes and Lipid Peroxidation in Different Types of Leukemias,” Clinica Chimica Acta, Vol. 293, No. 1-2, 2000, pp. 53-62. http://dx.doi.org/10.1016/S0009-8981(99)00222-3
- J. Beuge and S. D. Aust, “Microsomal Lipid Peroxidation,” Methods in Enzymology, Academic Press, New York, 1978, pp. 302-316.
- J. Stocks and T. L. Dormandy, “The Antioxidative of Human Red Cell Lipids Induced by Hydrogen Peroxides,” British Journal of Haematology, Vol. 20, 1971, pp. 95-111. http://dx.doi.org/10.1111/j.1365-2141.1971.tb00790.x
- E. Beutler, O. Duron and B. M. Kelley, “Improved Method for the Determination of Blood Glutathione,” The Journal of Laboratory and Clinical Medicine, Vol. 61, 1963, pp. 882-890.
- R. L. Levine, D. Garland, C. N. Oliver, A. Amici, I. Climent, A. Lenz, B. Ahn, S. Shaltiel and E. R. Stadtman, “Determination of Carbonyl Content in Oxidatively Modified Proteins,” Methods in Enzymology, Vol. 186, 1990, pp. 464-478. http://dx.doi.org/10.1016/0076-6879(90)86141-H
- J. B. Henry, “Clinical Diagnosis and Management by Laboratory Methods,” 17th Edition, W.B Sounders Company, Philadelphia, 1984, pp. 505-589.
- J. H. Roe, “Standard Methods in Clinical Chemistry,” In: D. Seigson, Ed., Vol. 2, Academic Process, New York, 1961, p. 35.
- N. Raghuramulu, N. K. Madhavan and K. Sundarm, Eds., “A Manual of Laboratory Techniques,” NIN, Hyderabad, 1983, pp. 140-142,319-320.
- A. E. Hammouda, S. F. Soliman, K. A. Tolba, Z. A. ElKabbany and M. S. Makhlouf, “Plasma Concentrations of Lipid Peroxidation Products in Children with Acute Lymphoblastic Leukemia,” Clinical Chemistry, Vol. 38, No. 4, 1992, pp. 594-595.
- R. K. Singh, A. K. Tripathi, P. Tripathi, S. Singh, R. Singh and R. Ahmad, “Studies on Biomarkers for Oxidative Stress in Patients with Chronic Myeloid Leukemia,” Hematology/Oncology and Stem Cell Therapy, Vol. 2, No. 1, 2009, pp. 285-288.
- R. Ahmad, A. K. Tripathi, P. Tripathi, R. Singh, S. Singh and R. K. Singh, “Studies on Lipid Peroxidation and NonEnzymatic Antioxidant Status as Indices of Oxidative in Patients with Chronic Myeloid Leukemia,” Singapore Medical Journal, Vol. 51, No. 2, 2010, pp. 110-115.
- Y. Sun, “Free Radicals, Antioxidant Enzymes and Carcinogenesis,” Free Radical Biology & Medicine, Vol. 8, 1990, pp. 583-599. http://dx.doi.org/10.1016/0891-5849(90)90156-D
- G. Wu, Y. Z. Fang, S. Yang, J. R. Lupton and N. D. Turner, “Glutathione Metabolism and Its Implications for Health,” Journal of Nutrition, Vol. 134, No. 3, 2004, pp. 489-492.
- J. S. Giftson, S. Jayanthi and N. Nalini, “Chemopreventive Efficacy of Gallic Acid, an Antioxidant and Anticarcinogenic Polyphenol, against 1, 2-Dimethyl Hydrazine Induced Rat Colon Carcinogenesis,” Investigational New Drugs, Vol. 28, 2010, pp. 251-259.
- V. Sreedharan, K. K. Venkatachalam and N. Namasivayam, “Effect of Morin on Tissue Lipiperoxidation and Antioxidant Status in 1,2-Dimethylhydrazine Induced Experimental Colon Carcinogenesis,” Investigational New Drugs, Vol. 27, 2009, pp. 21-30.
- V. Sudhahar, S. A. Kumar, P. Varalakshmi and R. Sundarapandiyan, “Mitigating Role of Lupeol and Lupeol Linoleate on Hepatic Lipemic-Oxidative Injury and Lipoprotein Peroxidation in Experimental Hypercholesterolemia,” Molecular and Cellular Biochemistry, Vol. 295, No. 1-2, 2007, pp. 189-198. http://dx.doi.org/10.1007/s11010-006-9288-2
- C. H. Foyer and G. Noctor, “Oxidant and Antioxidant Signaling in Plants: A Re-Evaluation of the Concept of Oxidative Stress in a Physiological Context,” Plant, Cell and Environment, Vol. 28, 2005, pp. 1056-1071. http://dx.doi.org/10.1111/j.1365-3040.2005.01327.x
- V. Arulmozhi, M. Krishnaveni, K. Karthishwaran, G. Dhamodharan and S. Mirunalini, “Antioxidant and Antihyperlipidemic Effect of Solanum nigrum Fruit Extract on the Experimental Model against Chronic Ethanol Toxicity,” Pharmacognosy Magazine, Vol. 6, No. 21, 2010, pp. 42-50. http://dx.doi.org/10.4103/0973-1296.59965
- P. Veeru, M. P. Kishor and M. Meenakshi, “Screening of Medicinal Plant Extracts for Antioxidant Activity,” Journal of Medicinal Plants Research, Vol. 3, 2009, pp. 608- 612.
- S. Goto, K. Kogure, K. Abe, Y. Kimata, K. Kitahama, E. Yamashita and H. Terada, “Efficient Radical Trapping at the Surface and Inside the Phospholipid Membrane Is Responsible for Highly Potent Antiperoxidative Activity of the Carotenoid Astaxanthin,” Biochimica et Biophysica Acta, Vol. 1512, No. 2, 2001, pp. 251-258. http://dx.doi.org/10.1016/S0005-2736(01)00326-1
- A. R. Collins and V. Harrington, “Antioxidants: Not the Only Reason to Eat Fruit and Vegetables,” Phytochemistry Reviews, Vol. 1, No. 2, 2002, pp. 167-174. http://dx.doi.org/10.1023/A:1022514432233
- S. B. Nissen, A. Tjonneland, C. Stripp, A. Olsen, J. Christensen, K. Overvad, L. O. Dragsted and B. Thomson, “Intake of Vitamin A, C and E from Diets and Supplements and Breast Cancer in Post Menopausal Women,” Cancer Causes Control, Vol. 14, No. 8, 2003, pp. 695-704. http://dx.doi.org/10.1023/A:1026377521890
- K. Dahan, M. Fennel and N. B. Kumar, “Lycopene in the Prevention of Prostrate Cancer,” Journal of Social Integration and Oncology, Vol. 6, 2008, pp. 29-36.
- D. S. Oliveira, A. L. Lobato, S. M. R. Ribeiro, N. M. C. Santana, J. B. P. Chaves and H. M. P. Ana, “Carotenoids and Vitamin C during Handling and Distribution of Guava (Psidium guajava L.), Mango (Mangifera indica L.), and Papaya (Carica papaya L.) at Commercial Restaurants,” Journal of Agricultural and Food Chemistry, Vol. 58, No. 10, 2010, pp. 6166-6172. http://dx.doi.org/10.1021/jf903734x
- J. C. Wiggins, M. Goyal, B. L. Wharram and R. C. Wiggins, “Antioxidant Ceruloplasmin Is Expressed by Glomerular Parietal Epithelial Cells and Secreted into Urine in Association with Glomerular Aging and High-Calorie Diet,” Journal of American Society of Nephrology, Vol. 17, 2006, pp. 1382-1387.
- Y. S. Park, K. Suzuki, S. Mumby, N. Taniguchi and J. M. C. Gutteridge, “Antioxidant Binding of Ceruloplasmin to Myeloperoxidase: Myeloperoxidase Is Inhibited, but Oxidase, Peroxidase and Immunoreactive Properties of Ceruloplasmin Remain Intact,” Free Radical Research, Vol. 33, 2000, pp. 261-265.
- M. Sattler, S. Verma, G. Shrikhande, et al., “The BCR/ABL Tryrosine Kinase Induces Production of Reactive Oxygen Species in Hematopoietic Cells,” The Journal of Biological Chemistry, Vol. 275, No. 32, 2000, pp. 24273-24278.
NOTES
*Corresponding author.

