Open Journal of Ophthalmology
Vol.4 No.2(2014), Article ID:45875,10 pages DOI:10.4236/ojoph.2014.42009
Dynamic Contour Tonometry, Tono-Pen XL®, and Goldmann Applanation Tonometry in Comparison to Intracameral Intraocular Pressure (IOP) Measurements in Patients with Corneal Pathologies
Matthias Neuburger¹, Juliane Großwendt¹, Sonja Lautebach¹, Philip Maier¹, Florian Birnbaum¹, Daniel Böhringer¹, Jens Funk², Jens Friedrich Jordan¹, Thomas Reinhard¹
1Eye Center, Albert-Ludwigs-University, Freiburg, Germany
2University Eye Hospital, Zürich, Switzerland
Email: matthias.neuburger@uniklinik-freiburg.de
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 13 March 2014; revised 15 April 2014; accepted 6 May 2014
ABSTRACT
Purpose: The accuracy of Goldmann applanation tonometry (GAT) has been shown to depend on several biomechanical properties of the cornea. Newer tonometry devices (e.g., the Dynamic Contour Tonometer PASCAL® [DCT] and the Tono-Pen® XL [TP]) have been designed to accurately measure intraocular pressure (IOP) independent of corneal thickness (CCT) and pathology. This study investigates the influence of corneal pathologies on the accuracy of these IOP measuring devices, and compares this accuracy to that of direct intracameral IOP measurement. Methods: 8 eyes of 8 patients suffering from corneal pathologies scheduled for penetrating keratoplasty, and 10 eyes of 10 patients scheduled for cataract surgery (control group) were examined. Before surgery, the anterior chamber was cannulated at the temporal corneal limbus. In a closed system, the intraocular pressure (IOP) was directly set to 10, 20, and 30 mmHg with a manometric water column. Intraocular pressure measurements taken by GAT, DCT, and TP were compared to intracameral measurements obtained by a precision reference pressure sensor. Results: Control group: All three methods showed good agreement with the intracameral readings (mean deviation of all three devices, −0.9 mmHg). Group with corneal pathologies: The TP yielded the most exact IOP values in the group with corneal pathologies when taking all diagnoses into account. The mean deviations from the intracameral IOP measurements were −0.9 mmHg ± 3.2 mmHg (mean ± SD) for TP, −2.9 mmHg ± 3.3 mmHg for GAT, and −5.0 mmHg ± 7.9 mmHg for DCT. For bullous keratopathy, the most exact IOP readings were obtained by the TP (mean deviation −0.2 mmHg ± 3.5 mmHg). The TP and GAT devices underestimated IOP in the patients with Fuchs’ endothelial dystrophy; all 3 devices underestimated adjusted IOP after keratoplasty. DCT showed the greatest deviations from adjusted IOP in the case of non-herpetic scars. In the control group, none of the devices showed a statistically relevant dependency on CCT. Nevertheless, in the group with corneal pathologies, only TP showed no dependency on CCT. Conclusion: Our results suggest that the Tono-Pen XL® is the most accurate measurement device to determine IOP in patients with corneal pathologies, especially in patients suffering from corneal edema (bullous keratopathy). GAT yielded surprisingly exact IOP values in patients suffering from irregular corneal surface. DCT showed a high degree of deviation from the adjusted IOP, and should not be used to determine IOP in corneas with the disorders listed here.
Keywords:Keratoplasty, IOP Measurement, Goldmann Applanation Tonometry, Dynamic Contour Tonometry, Tono-Pen XL®

1. Objective
For more than 50 years, Goldmann Applanation Tonometry (GAT) has been the gold standard for measuring intraocular pressure (IOP) [1] . However, several biomechanical limitations of this method have been characterized. These include the need for mathematical adjustment of the obtained values for central corneal thickness, uncertain accuracy in the presence of corneal scarring and corneal curvature, and the known underestimation of IOP in eyes with corneal edema [2] -[7] . Therefore, we still lack an IOP measurement device that yields reproducible and exact IOP values in patients suffering from corneal pathologies like Fuchs’ endothelial dystrophy, or for patients who have undergone penetrating keratoplasty [8] [9] .
Several new devices aiming for correct IOP measurement that is less dependent on the cornea’s biomechanical properties have been developed. Two of these devices are the Dynamic Contour Tonometer (PASCAL®, DCT; Ziemer Ophthalmic Systems AG, Port, Switzerland) and the Tono-Pen XL® (TP; Reichert, Depew, New York, United States).
The purpose of this study was to investigate the accuracy of GAT and these new devices compared to direct intracameral IOP readings in a prospective in vivo study in patients suffering from corneal pathologies.
2. Materials and Methods
8 patients (a total of 8 eyes) scheduled for penetrating keratoplasty due to corneal pathology were included in this study. Four patients were experiencing bullous keratopathy after cataract surgery; one patient had non-herpetic scars; one patient had Fuchs’ endothelial dystrophy; and two patients needed to undergo keratoplasty again due to graft failure or corneal scarring. All eyes included in this group were pseudophakic. In a control group, 10 patients without corneal pathology who were scheduled for cataract surgery were included.
The indications for penetrating keratoplasty and cataract surgery were independent from participation in this study. Exclusion criteria included any history of ocular surgery in the 4 weeks prior to penetrating keratoplasty or cataract surgery, or any active intraocular inflammation. Patients scheduled for penetrating keratoplasty had to be pseudophakic to participate in the study. The presence of any corneal pathology was an exclusion criterion for the control group.
Before surgery, central corneal thickness (CCT) was measured with an ultrasonic pachymeter (PachetteTM, DGH Technology Inc., Exton, Pennsylvania, United States).
For penetrating keratoplasty, patients were anesthetized with general anesthesia. Cataract surgery was performed either in general anesthesia or in retrobulbar anesthesia (injection of 5 ml mepivacaine [Scandicain] 2% 15 minutes prior to surgery; no oculopression was performed).
All IOP measurements were performed before surgery by one of the authors (MN). With the aid of a laser water scale, the tubing system used to manometrically determine the IOP was adjusted to the height of each patient’s anterior chamber before the measurement was taken.
The experimental setting for adjusting the IOP has been described elsewhere in detail [10] . The anterior chamber was cannulated at the temporal corneal limbus with a cannula connected to an adjustable water column and a pressure sensor used as reference (the DCT tip was directly connected with the water column, Figure 1). The IOP was directly calibrated by changing the height of the water column in relation to the anterior chamber (open system) to three different IOP levels (10, 20, and 30 mmHg). After each IOP level was reached, the system was closed, and three consecutive measurements (DCT, GAT, Tono-Pen, and simultaneously with the reference sensor) were taken (closed system). The maintenance of a tension-free position for the cannula was maintained with high priority. This tension-free position was reached by using sterile adhesive tape (Steri-StripTM Adhesive Skin Closures; 3M Deutschland, Neuss, Germany) and sterile pads to fix the cannula (Figure 2).
Measurement with the DCT (the measurement principle for the DCT has been described in detail elsewhere [11] ) was performed with a modified Perkins tonometer, allowing performance of dynamic contour tonometry measurement on a patients in the supine position, with a DCT tip mounted on the body of a Perkins tonometer

Figure 1. Cannula (left) to be placed in the patient’s anterior chamber. A: Reference sensor for direct intracameral IOP reading. IOP was directly calibrated by changing the height of the water column in relation to the anterior chamber (open system) to the respective IOP level (10, 20, and 30 mmHg). After each IOP level was reached, the system was closed by turning the three way cocks (B and C), and three consecutive measurements (DCT, GAT, TP, and simultaneously with the reference sensor) were taken (closed system).
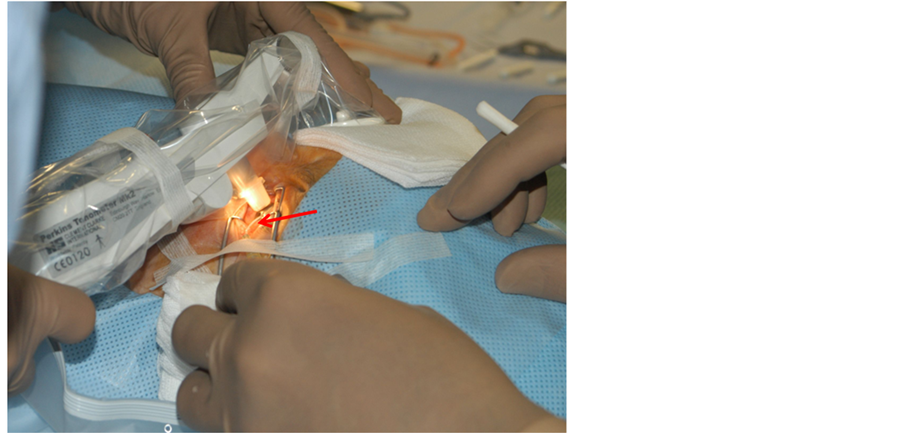
Figure 2. Dynamic contour tonometer measurement with a modified Perkins tonometer. The intracameral cannula (red arrow) is fixed with sterile tapes to ensure its tension-free position during the measurement.
(Figure 2). This instrument was developed by Ziemer Ophthalmic Systems AG (Port, Switzerland). During the measurement, lubricant eye drops (Corneregel® Fluid Eye drops; Mann Pharma) were used to ensure a normal corneal surface and optimal quality for the DCT measurements. If no audible pulsation was present, the measurement of the IOP was aborted and not included in the statistical analysis. The DCT provides an internal quality assessment of the measurement, with Grade 1 representing the best and Grade 5 representing the worst quality. Only measurement quality 1 to 3 was accepted. If it was not possible to gain a measurement with a quality grade of 3 or better in a patient after three attempts, the patient was excluded from the study.
The Tono-Pen® XL (TP; the way it works has been detailed elsewhere [12] ) performs four independent IOP readings and provides a reliability index for each single measurement. Measurements were only used if the scatter index was below 5%. Three of the measurements with an index below 5% were taken, and the average of these measurements was calculated.
Three consecutive measurements with GAT were performed using the Perkins tonometer (HAAG-STREIT AG, Koeniz, Switzerland); the average of these three measurements was calculated.
In the following description, the term DCT measurement refers to the measurement with the handheld DCT; the term GAT refers to the measurement with the Perkins tonometer; the term TP refers to measurements taken with the Tono-Pen® XL; and the reference point is the DCT tip integrated into the tube system to obtain intracameral measurements.
All patients signed informed consent before entering the study. The study was performed in accordance with the Declaration of Helsinki and was approved by the institutional ethics committee of the Medical Department of the University of Freiburg.
Statistical analysis: We calculated the measurement errors from the difference between the intracameral reference pressure and the respective measurement result. We computed average measurement errors for each device and reference pressure separately. We plotted measurement errors against reference pressure for each device, and performed a regression analysis by means of a linear model. Intercept and slope of the regression lines were used to rank the devices for validity. The regression and the dependency of the measurement results on CCT were tested for statistical significance by means of analysis of variance (ANOVA). All computations were performed with the R programme (www.r-project.org).
3. Results
Control group (Figure 3): In the control group of 10 patients scheduled for cataract surgery, all three devices showed good agreement with the adjusted IOP as measured by the reference sensor. The mean deviation for all adjusted IOP levels was −0.9 mmHg ± 5.5 mmHg (mean ± SD) for DCT; −0.9 mmHg ± 3.4 mmHg for GAT; and −0.9 mmHg ± 2.5 mmHg for TP. Intercept and slope of the regression line for each device can be found in Table 1.
Group with corneal pathologies (Figure 3): When taking into account all examined patients, the TP yielded the most exact IOP values in the group with corneal pathologies; we found a mean deviation of −0.9 mmHg ± 3.2 mmHg. Mean deviation was −2.9 mmHg ± 3.3 mmHg for GAT and −5.0 mmHg ± 7.9 mmHg for DCT. Intercept and slope of the regression line for each device are displayed in Table 1.
Table 1. Intercept and slope of the regression lines of each device in the respective group (DCT: Dynamic contour tonometry; GAT: Goldmann applanation tonometry; TP: Tono-Pen).
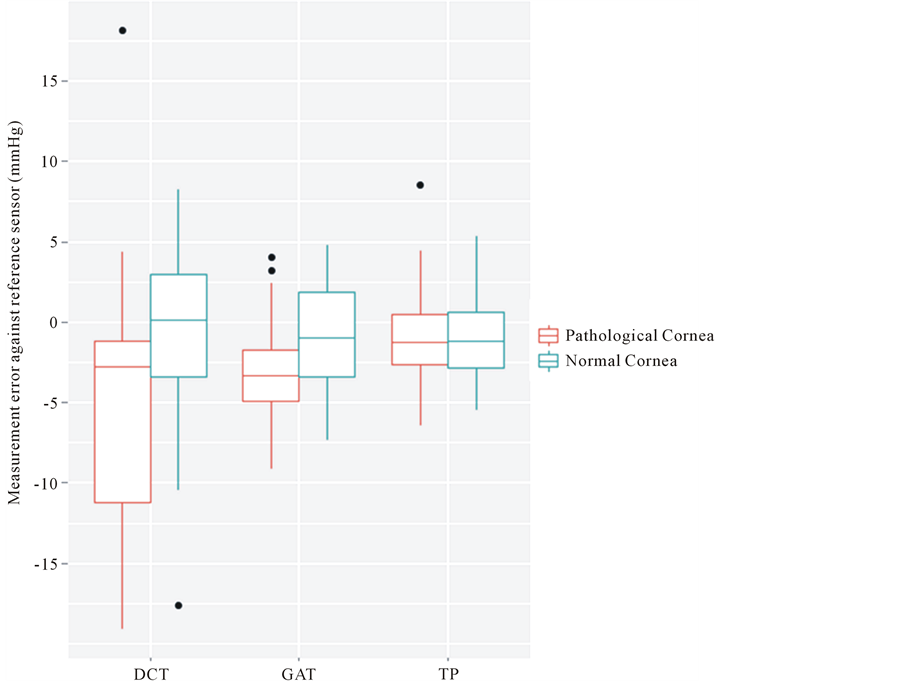
Figure 3. Measurement error of dynamic contour tonometry (DCT), Goldmann applanation tonometry (GAT), and Tono-Pen XL (TP) against the reference sensor in the group with corneal pathologies (red) and the cataract group (blue). X-axis: respective device. Y-axis: measurement error in mmHg. Tono-Pen showed the best agreement with the adjusted IOP, while GAT and especially DCT underestimated the IOP. Dynamic contour tonometry also showed the greatest scattering of all three devices.
Bullous keratopathy (Figure 4): In this group, all 3 devices underestimated the adjusted IOP. We found a mean deviation of −0.2 mmHg ± 3.5 mmHg for TP, −4.0 mmHg ± 3.2 mmHg for GAT, and −6.5 mmHg ± 5.1 mmHg for DCT, respectively. This underestimation of the adjusted IOP increased with increasing IOP during measurements obtained with GAT and DCT.
Fuchs’ endothelial dystrophy (Figure 4): Both TP and GAT underestimated the IOP (mean deviation −2.3 mmHg ± 0.4 mmHg (TP) and −1.6 mmHg ± 1.7 mmHg (GAT)). DCT showed a greater scattering in comparison with the other devices (mean deviation +0.1 mmHg ± 3.7 mmHg).
Post-keratoplasty (Figure 4): All three devices underestimated the adjusted IOP with a great degree of scattering, especially in the instance of DCT. Mean deviations were −0.5 mmHg ± 3.2 mmHg (TP), −1.9 mmHg ± 4.1 mmHg (GAT), and −0.6 mmHg ± 11.4 mmHg (DCT).
Non-herpetic scars (Figure 4): All three devices underestimated the adjusted IOP, with the DCT showing the greatest deviation and scattering. Mean deviations were −3.6 mmHg ± 1.8 mmHg (TP), −1.9 mmHg ± 2.8 mmHg (GAT), and −13.1 mmHg ± 5.9 mmHg (DCT).
Central corneal thickness (CCT) (Figure 5): Mean CCT was 559 µm (±19.5 µm) in the control group and 616 µm (±28.4 µm) in the group with corneal pathologies. None of the measurement devices showed a statistically relevant dependency on the CCT in the control group. In the group with corneal pathologies, the DCT and the GAT showed a statistically significant negative correlation with the CCT (DCT: t = −2.3, P = 0.03; GAT: t = −2.2, P = 0.04). In this group, only the Tono-Pen showed no statistically relevant dependency on CCT (t = −0.0244, P = 0.99).
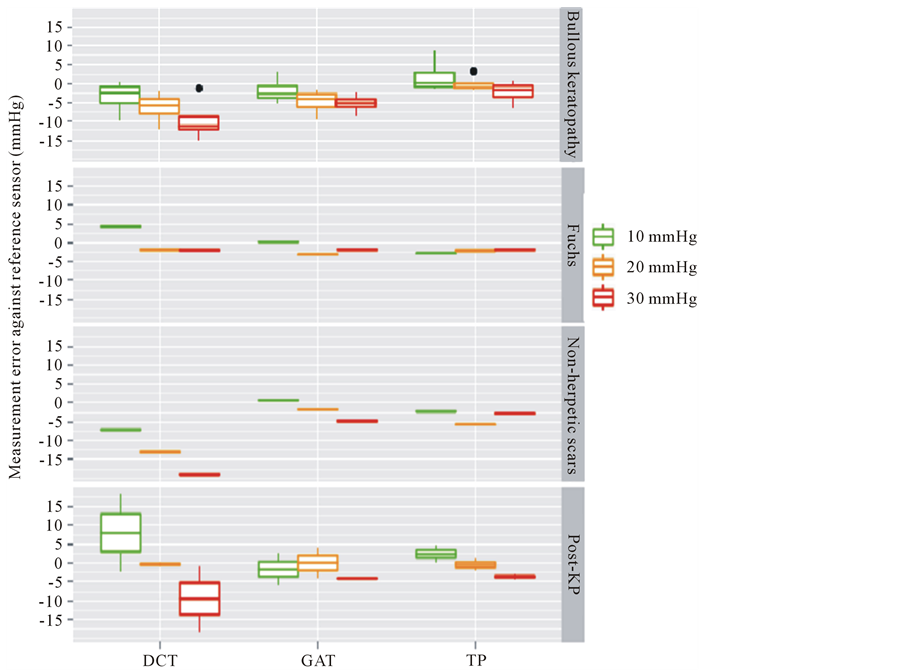
Figure 4. The measurement error of dynamic contour tonometry (DCT), Goldmann applanation tonometry (GAT), and Tono-Pen (TP) against the reference sensor for the respective diagnosis. X-axis: respective device. Y-axis: measurement error in mmHg. Green, yellow, red box: adjusted IOP level (10, 20, 30 mmHg, respectively). DCT and GAT underestimate the adjusted pressure in patients suffering from bullous keratopathy, while all three devices yield quite accurate measurement results in Fuchs’ endothelial dystrophy. The TP and GAT yield exact IOP values on an irregular cornea surface (as with non-herpetic scars, and postkeratoplasty), while DCT underestimates the IOP at increasing IOP (non-herpetic scars) or shows a high degree of scattering (post-keratoplasty).
Table 1 shows the intercept and slops for each measurement device according to diagnosis. No statistically relevant dependency on astigmatism or axial length was found in any of the devices.
4. Discussion
The gold standard for measuring IOP, Goldmann Applanation Tonometry (GAT), is known to depend on CCT and several other corneal properties (edema, tear film, astigmatism) [1] -[4] . A device that can yield exact and reproducible IOP values is still necessary, especially in patients suffering from corneal pathologies such as bullous keratopathy, Fuchs’ endothelial dystrophy, or corneal scaring [7] -[9] . Accurate IOP measurement remains difficult after penetrating keratoplasty, but is important nonetheless, as elevated IOP values negatively affect graft survival [13] .
In recent years, several methods to measure IOP independent from corneal properties have been developed. Dynamic Contour Tonometry (PASCAL®, DCT) is based on the physics of Pascal’s law. This device is said to measure IOP independent from CCT or other biomechanical corneal properties; this has been confirmed in several clinical trials [10] [14] [15] . After LASIK eye surgery, DCT showed no change in IOP measurement, while GAT revealed lower IOP levels [16] . Nevertheless, there have been recent reports that CCT and corneal edema may influence DCT measurements [17] [18] . Considering this method’s measurement principles, the cornea’s
 (a) (b)
(a) (b)
Figure 5. Measurement error of dynamic contour tonometry (handheld, filled circle), Goldmann applanation tonometry (Perkins, open circle), and Tono-Pen (Tono-pen, filled square) against the reference sensor depending on CCT in the cataract group (a) and the group with corneal pathologies (b). X-axis: Central corneal thickness (CCT) in µm. Y-axis: Measurement error in mmHg. In the cataract group (a), none of the devices showed a statistically relevant dependency on CCT, while in the keratoplasty group only the TP showed no statistically relevant dependency on CCT. In this group, GAT and especially DCT underestimated the adjusted IOP at increasing CCT.
viscoelastic properties should also be taken into account as another potential influencing factor. After penetrating keratoplasty, Viestenz found that DCT yielded reproducible IOP values that were higher in comparison to those for GAT, and were disturbed by postoperative intrastromal sutures [19] .
The Tono-Pen® XL (TP) is a handheld tonometer operating on the same principle as the Mackay-Marg tonometer [12] . The accuracy of the TP is still a matter of discussion. Several authors have reported that its measurements are not dependent on CCT, while others did find a positive correlation [20] [21] . When comparing TP to GAT, some authors found the Tono-Pen to underestimate or overestimate the IOP, with a particular trend toward overestimation at higher IOP values [20] [22] [23] . In patients who had undergone penetrating keratoplasty, both Shemesh and Rao found the TP to show good agreement with IOP values yielded by GAT [24] [25] .
In or study, we wanted to examine which of the three devices for measuring IOP (GAT, TP, DCT) yielded the most exact IOP values in patients with corneal pathologies. We therefore used a closed system that allowed for adjustment of the IOP of each patient manometrically to 10, 20, and 30 mmHg, respectively, and for control of this adjusted IOP through a reference sensor. At each pressure level, we measured the IOP using each of the three devices.
This study was performed in 8 patients scheduled for penetrating keratoplasty due to bullous keratopathy (n = 4), Fuchs’ endothelial dystrophy (n = 1), non-herpetic scars (n = 1); or after keratoplasty (n = 2), as well as in 10 patients scheduled for cataract surgery who served as the control group.
In the control group, we found all three devices to yield exact IOP values. Each device showed a mean deviation from the IOP value of −0.9 mmHg. These results indicate the reliability and accuracy of our measurement configuration. None of the three devices in the control group showed a statistically relevant dependency on CCT. For the DCT, this is in accordance with most studies; the literature has generally reported that DCT is independent of CCT [10] [26] . Nevertheless, most of the authors report a positive correlation for GAT and TP with CCT [2] [27] . Our 10-patient control group was small; our results therefore allow no obvious conclusion concerning the relationship of GAT and TP and CCT with respect to measuring IOP.
In the group with corneal pathologies, the TP yielded the most accurate IOP measurements for all three devices, followed in accuracy by GAT and DCT. For the entire group of patients with corneal pathologies, the IOP measurement results for TP were just as accurate as those for the control group. Several other studies have reported similar results, with the TP yielding exact IOP values after penetrating keratoplasty [24] [25] . Nevertheless, these groups compared TP measurements of IOP to IOP values obtained by GAT, and not to direct intracameral readings. The TP not only yielded the lowest mean deviation over all diagnoses (−0.9 mmHg, compared with −2.9 mmHg [GAT] and −5.0 mmHg [DCT]), but also the lowest standard deviation (−3.2 mmHg [TP], −3.3 mmHg [GAT], −8.0 mmHg [DCT]), indicating the reliability of the measurement. The DCT in particular had a very high standard deviation for its various measurements; the results for this device showed a high degree of scattering around the adjusted IOP.
Despite the small number of patients, a statistically significant negative correlation existed between the CCT and the IOP (GAT) and especially the IOP (DCT) measurement results in the group with corneal pathologies for all diagnoses. Most patients in the study group suffered from pathologies that led to edematous swelling of the cornea (Fuchs’ endothelial dystrophy, bullous keratopathy, graft failure). Indeed, mean CCT in the group with corneal pathologies was significantly higher than in the control group (559 µm vs. 616 µm; P < 0.05). Our results are therefore in accordance with Simon and also Oh, who recorded significantly lower IOP (GAT) measurement results in edematous corneas [5] [6] . Francis reported the same negative correlation between CCT and IOP (DCT) measurement results on edematous corneas [17] . Tono-Pen measurements showed no statistically relevant correlation between the IOP measurement results and the CCT; this is in accordance with the accurate IOP measurement results delivered by this device in our study, especially in the bullous keratopathy group.
Due to the small number of patients in the respective group suffering from Fuchs’ endothelial dystrophy, nonherpetic scars, bullous keratopathy, and post-keratoplasty, an interpretation of our results with respect to each individual diagnosis is difficult (Figure 4). Nevertheless, especially in the group with bullous keratopathy, TP yielded the most accurate measurement results, whereas GAT and, to an even greater degree, DCT underestimated the IOP at all adjusted pressure levels, especially with increasing IOP. In the group of patients with nonherpetic scars or who had undergone keratoplasty, we found that GAT measurement results were surprisingly accurate despite the irregular corneal surface. Tono-Pen also yielded quite exact IOP values, whereas the DCT showed a great variance. All three devices yielded relatively accurate IOP values on Fuchs’ endothelial dystrophy.
One possible explanation for the accuracy of the IOP measurement results obtained by TP may be the small contact area of this device with the cornea, and the minor distortion of the cornea during measurement. Possible biomechanical properties of the cornea, such as edema or a higher degree of stiffness due to scarring, therefore may have less influence on the measurement procedure. The higher resistance against distortion caused by corneal scarring, or the lower resistance due to corneal edema, might explain the worse results obtained by GAT and especially by DCT in our study.
The small number of patients in the study group was a major important limitation of our study. This small number makes statistically significant statements regarding the respective diagnoses difficult. Nevertheless, when taking all diagnoses into account, the results concerning the high accuracy of TP and its independence from CCT measurements as well as the dependance of GAT and DCT from CCT measurements were all statistically significant. Regarding DCT, the measurement procedure with the modified Perkins tonometer was problematic, as the device had to be held in a stable position over the supine patient for several seconds. It was therefore sometimes complicated to obtain measurement results of adequate quality. Nevertheless, as only measurement results with a quality grade of 3 or better were provided in the statistical analysis, the difficulties that occurred during the measurement procedure could not have played a role in the interpretation of the poor quality of the measurement results obtained by this device.
In summary, our results suggest that the Tono-Pen XL® is the most accurate measurement device for the determination of IOP in patients with corneal pathologies, especially in patients suffering from corneal edema (bullous keratopathy), probably due to its relatively small contact area with the cornea. These results are in accordance with several other groups reporting on its accuracy compared to GAT after penetrating keratoplasty. To our knowledge, our study is the first to reveal this accuracy when comparing the obtained IOP values to direct intracameral readings.
GAT yielded surprisingly exact IOP values in patients with an irregular corneal surface (non-herpetic scars, post-keratoplasty), and underestimated IOP in patients suffering from corneal edema. Apart from Fuchs’ endothelial dystrophy, DCT showed great deviations from the adjusted IOP in all patient groups and should not be used to determine IOP in corneas with any pathologies or disorders.
References
- Goldmann, H. and Schmidt, T. (1957) Applanation Tonometry. Ophthalmologica, 134, 221-242.
- Ehlers, N., Bramsen, T. and Sperling, S. (1975) Applanation Tonometry and Central Corneal Thickness. Acta Ophthalmol (Copenh), 53, 34-43.
- Whitacre, M.M. and Stein, R. (1993) Sources of Error with Use of Goldmann-Type Tonometers. Survey of Ophthalmology, 38, 1-30.
- Whitacre, M.M., Stein, R.A. and Hassanein, K. (1993) The Effect of Corneal Thickness on Applanation Tonometry. American Journal of Ophthalmology, 115, 592-596.
- Oh, J., Yoo, C., Kim, Y.Y., Kim, H. and Song, J. (2009) The Effect of Contact Lens-Induced Corneal Edema on Goldmann Applanation Tonometry and Dynamic Contour Tonometry. Graefe’s Archive for Clinical and Experimental Ophthalmology, 247, 371-375.
- Simon, G., Small, R.H., Ren, Q. and Parel, J.M. (1993) Effect of Corneal Hydration on Goldmann Applanation Tonometry and Corneal Topography. Refractive Corneal Surgery, 9, 110-117.
- Reinhard, T. and Sundmacher, R. (1999) Determining Real IOP Values. Journal of Cataract & Refractive Surgery, 25, 157-158. http://dx.doi.org/10.1016/S0886-3350(99)80106-0
- Madjlessi, F., Marx, W., Reinhard, T., Althaus, C. and Sundmacher, R. (2000) Impression and Applanation Tonometry in Irregular corneas. Comparison with Intraocular Needle Tonometry. Ophthalmologe, 97, 478-481.
- Marx, W., Madjlessi, F., Reinhard, T., Althaus, C. and Sundmacher, R. (1999) More than 4 Years’ Experience with Electronic Intraocular Needle Tonometry. Ophthalmologe, 96, 498-502. http://dx.doi.org/10.1007/s003470050444
- Boehm, A.G., Weber, A., Pillunat, L.E., Koch, R. and Spoerl, E. (2008) Dynamic Contour Tonometry in Comparison to Intracameral IOP Measurements. Investigative Ophthalmology & Visual Science, 49, 2472-2477.
- Kniestedt, C. and Kanngiesser, H.E. (2006) Dynamic Contour Tonometry. Ophthalmologe, 103, 713-721. http://dx.doi.org/10.1007/s00347-006-1387-7
- Frenkel, R.E., Hong, Y.J. and Shin, D.H. (1988) Comparison of the Tono-Pen to the Goldmann Applanation Tonometer. Archives of Ophthalmology, 106, 750-753.
- Reinhard, T., Kallmann, C., Cepin, A., Godehardt, E. and Sundmacher, R. (1997) The Influence of Glaucoma History on Graft Survival after Penetrating Keratoplasty. Graefe’s Archive for Clinical and Experimental Ophthalmology, 235, 553-557. http://dx.doi.org/10.1007/BF00947083
- Barleon, L., Hoffmann, E.M., Berres, M., Pfeiffer, N. and Grus, F.H. (2006) Comparison of Dynamic Contour Tonometry and Goldmann Applanation Tonometry in Glaucoma Patients and Healthy Subjects. American Journal of Ophthalmology, 142, 583-590.
- Pache, M., Wilmsmeyer, S., Lautebach, S. and Funk, J. (2005) Dynamic Contour Tonometry versus Goldmann Applanation Tonometry: A Comparative Study. Graefe’s Archive for Clinical and Experimental Ophthalmology, 243, 763- 767. http://dx.doi.org/10.1007/s00417-005-1124-y
- Pepose, J.S., Feigenbaum, S.K., Qazi, M.A., Sanderson, J.P. and Roberts, C.J. (2007) Changes in Corneal Biomechanics and Intraocular Pressure Following LASIK Using Static, Dynamic, and Noncontact Tonometry. American Journal of Ophthalmology, 143, 39-47. http://dx.doi.org/10.1016/j.ajo.2006.09.036
- Francis, B.A., Hsieh, A., Lai, M., Chopra, V., Pena, F., Azen, S. and Varma, R. (2007) Effects of Corneal Thickness, Corneal Curvature, and Intraocular Pressure Level on Goldmann Applanation Tonometry and Dynamic Contour Tonometry. Ophthalmology, 114, 20-26. http://dx.doi.org/10.1016/j.ophtha.2006.06.047
- Viestenz, A., Langenbucher, A. and Viestenz, A. (2006) Reproducibility of Dynamic Contour Tonometry. Comparison with TonoPenXL and Goldmann Applanation Tonometry—A Clinical Study on 323 Normal Eyes. Klinische Monatsblätter für Augenheilkunde, 223, 813-819.
- Viestenz, A., Langenbucher, A., Seitz, B. and Viestenz, A. (2006) Evaluation of Dynamic Contour Tonometry in Penetrating Keratoplasties. Ophthalmologe, 103, 773-776. http://dx.doi.org/10.1007/s00347-006-1395-7
- Amaral, W.O.G., Teixeira, R.M.B., Alencar, L.M., Cronemberger, S. and Calixto, N. (2006) Central and Peripheral Corneal Thickness: Influence on the Iop Measurement by Tonopen. Arquivos Brasileiros de Oftalmologia, 69, 41-45. http://dx.doi.org/10.1590/S0004-27492006000100009
- Bhan, A., Browning, A.C., Shah, S., Hamilton, R., Dave, D. and Dua, H.S. (2002) Effect of Corneal Thickness on Intraocular Pressure Measurements with the Pneumotonometer, Goldmann Applanation Tonometer, and Tono-Pen. Investigative Ophthalmology & Visual Science, 43, 1389-1392.
- Midelfart, A. and Wigers, A. (1994) Clinical Comparison of the ProTon and Tono-Pen Tonometers with the Goldmann Applanation Tonometer. British Journal of Ophthalmology, 78, 895-898.
- Iester, M., Mermoud, A., Achache, F. and Roy, S. (2001) New Tonopen XL: Comparison with the Goldmann Tonometer. Eye, 15, 52-58. http://dx.doi.org/10.1038/eye.2001.13
- Rao, V.J., Gnanaraj, L., Mitchell, K.W. and Figueiredo, F.C. (2001) Clinical Comparison of Ocular Blood Flow Tonometer, Tonopen, and Goldmann Applanation Tonometer for Measuring Intraocular Pressure in Postkeratoplasty Eyes. Cornea, 20, 834-838. http://dx.doi.org/10.1097/00003226-200111000-00011
- Shemesh, G., Waisbourd, M., Varssano, D., Michaeli, A., Lazar, M. and Kurtz, S. (2009) Measurements of Intraocular Pressure by Goldmann Tonometry, Tonopen XL, and the Transpalpebral Tonometer, TGDc-01, after Penetrating Keratoplasty: A Comparativye Study. Cornea, 28, 724-727. http://dx.doi.org/10.1097/ICO.0b013e3181930be8
- Kaufmann, C., Bachmann, L.M. and Thiel, M.A. (2003) Intraocular Pressure Measurements Using Dynamic Contour Tonometry after Laser in Situ Keratomileusis. Investigative Ophthalmology & Visual Science, 44, 3790-3794. http://dx.doi.org/10.1167/iovs.02-0946
- Kohlhaas, M., Boehm, A.G., Spoerl, E., Pürsten, A., Grein, H.J. and Pillunat, L.E. (2006) Effect of Central Corneal Thickness, Corneal Curvature, and Axial Length on Applanation Tonometry. Archives of Ophthalmology, 124, 471- 476. http://dx.doi.org/10.1001/archopht.124.4.471


