Natural Resources
Vol.07 No.11(2016), Article ID:72448,11 pages
10.4236/nr.2016.711052
Thermochemical Heat Storage Performance in the Gas/Liquid-Solid Reactions of SrCl2 with NH3
Kazuki Kuwata1, Soichirou Masuda1, Noriyuki Kobayashi1*, Takuya Fuse2, Toru Okamura2
1Department of Chemical Engineering, Nagoya University, Nagoya, Japan
2Denso Corporation, Kariya, Japan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 24, 2016; Accepted: November 27, 2016; Published: November 30, 2016
ABSTRACT
Thermochemical heat storage is a promising technology for improving energy efficiency through the utilization of low-grade waste heat. The formation of a SrCl2 ammine complex was selected as the reaction system for the purpose of this study. Discharge characteristics were evaluated in a packed bed reactor for both the gas-solid reaction and the liquid-solid reaction. The average power of the gas-solid reaction was influenced by the pressure of the supplied ammonia gas, with greater powers being recorded at higher ammonia pressure. For the liquid-solid reaction, the obtained average power was comparable to that obtained for the gas-solid reaction at 0.2 MPa. Moreover, the lower heat transfer resistance in the reactor was observed, which was likely caused by the presence of liquid ammonia in the system. Finally, the short-term durability of the liquid-solid reaction system was demonstrated over 10 stable charge/discharge cycles.
Keywords:
Thermochemical Heat Storage, SrCl2 Ammine Complex Formation, Gas/Liquid-Solid Reaction

1. Introduction
The efficient use of energy is extremely important due to the ongoing depletion of energy resources and also in the context of environmental protection. For example, the utilization of surplus heat is crucial to improve the energy efficiency of a range of processes, as the majority of excess energy is exhausted as heat. In this context, thermal energy storage techniques have been employed for heat management, with examples including utilization of the latent heat of water-ice and organic materials [1] [2] [3] [4] , and the heat of chemical reactions (i.e., thermochemical heat storage) with metal hydroxides [5] [6] [7] [8] and some metal salts [9] [10] [11] [12] . In particular, heat storage using the gas-solid reaction between a condensable gas as the working fluid and a metal salt or metal oxide has been studied in detail because of its excellent heat storage capacity and its wide temperature range.
More specifically, the utilization of low-grade heat (i.e., <100˚C) is particularly important, as this type of heat is lost to the atmospheric surroundings from various industrial processes and automotive uses, and as such, it accounts for the majority of heat waste. Thus, the storage and reuse of low-grade heat for a range of heating purposes could benefit from such processes.
We herein investigate the formation of a SrCl2 ammine complex (see Equations (1)- (3) below) as a potential thermochemical heat storage system, due to its ability to store the heat <100˚C, and its relatively high heat of reaction compared to other ammine/ water reactions [13] [14] . Figure 1 shows the flow of ammonia during the charge/ discharge operation of this system.
Charge operation
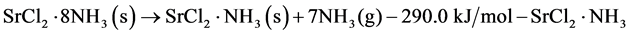 (1)
(1)
Discharge operation (gas-solid)
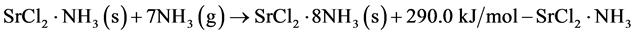 (2)
(2)
Discharge operation (liquid-solid)
 (3)
(3)
The thermochemical heat storage system employed herein consists of a reactor and an evaporator/condenser, the latter of which is responsible for the supply and storage of the working fluid. The charging operation involves the endothermic gas-solid reaction shown in Equation (1), which charges heat to the reactor, and where the released ammonia vapor is transported to the condenser. In this study, both a liquid-solid reaction and a gas-solid reaction are employed for heat discharge. In terms of the discharge operation using the gas-solid reaction shown in Equation (2), the ammonia vapor is transported from the evaporator to the reactor, where an exothermic reaction subsequently occurs. For the system of interest, the gas-solid reaction is expected to exhibit a larger heat of reaction than the liquid-solid reaction. In contrast, for the liquid-solid reaction shown in Equation (3), liquid ammonia is simply supplied to the reactor without the evaporation stage.
Figure 1. Ammonia flow during the charge and discharge operations.
Therefore, although the heat of reaction is small, the selection of this liquid-solid reaction is likely to be particularly applicable in extremely cold conditions, where the evaporator performance is limited due to heat exchange inhibition through frost formation or through a low saturated ammonia vapor pressure. In addition, presence of liquid ammonia on the heat exchange surface or in the reactant interparticle voids might improve heat transfer performance in the reactor. Although several previous studies report the use of water as the working fluid in liquid-solid reactions [15] [16] , few studies report the use of liquid ammonia.
We therefore chose to evaluate the discharge characteristics of both reactions using gaseous and liquid ammonia, where the experimental apparatus consisted of a packed bed reactor. In addition, reaction cycling was carried out in the liquid-solid reaction system to evaluate its short-term durability.
2. Experimental
2.1. Materials
Anhydrous SrCl2 powder (purity >98.0%) was obtained from Kanto Kagaku, Japan. The median diameter (dp50) of the SrCl2 particles was 100 µm. Prior to use, the powder was dried at 200˚C for 12h in an electric furnace.
2.2. Experimental Apparatus
Figure 2 shows a schematic representation of the experimental apparatus employed herein, which consisted of a reactor, an ammonia condenser, an ammonia cylinder, a vacuum pump, and a liquid level indicator. The various components of the setup were connected by a range of tubes for the transport of ammonia. In addition, flows of water connected to thermostat baths were connected to the reactor and the ammonia condenser.
Photographic images of the top view of the reactor and the stainless steel mesh and plate can be seen in Figure 3. The SrCl2 particles were deposited on a heat exchange
Figure 2. Schematic representation of the experimental apparatus (left) and s cross section of the reactor (right).
Figure 3. Photographic images of the top view of the SrCl2- filled reactor (left) and the stainless steel mesh and plate (right).
plate (100 mm × 100 mm) with a thickness of 1 mm, onto which were placed a stainless steel mesh and a stainless steel plate. The purposes of the stainless steel mesh and plate were to prevent the outflow of SrCl2 particles and to limit particle scattering through curtailing the momentum of the liquid ammonia flow. The heating medium (i.e., water) flowed under the heat exchange plate, and the inlet and outlet water temperatures were measured during using a platinum resistance temperature detector. The heat generated by the reactant bed was transferred to the water through the heat exchange plate, resulting in an increase in water temperature during discharge.
2.3. Evaluation of Discharge Characteristics
The discharge characteristics of the liquid-solid reaction and the gas-solid reaction were evaluated. The two exothermic reactions of interest are shown in Equations (4) and (5) below. The coordination change of 0 to 8 ammonia molecules, which isn’t strictly based on the practical conditions, was selected to create uniform initial packed bed conditions.
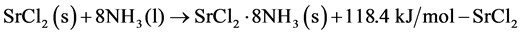 (4)
(4)
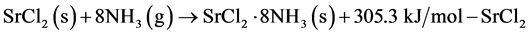 (5)
(5)
In this process, the condensation of ammonia was carried out prior to beginning the discharge operation of the liquid-gas reaction. Ammonia was supplied from the NH3 cylinder at the pressure beyond equilibrium pressure and was condensed in the isotherm condenser and the liquid level indicator. The successful condensation of ammonia was confirmed visually. Subsequently, the valve above the liquid level indicator was closed. The liquid NH3 level in the indicator was 13 mL, which is equivalent to the quantity of SrCl2 employed to fill the reactor. Following condensation, the liquid NH3 was supplied to the reactor by opening the valve below the liquid level indicator, which triggered the start of the liquid-solid reaction. For the gas-solid reaction, gaseous NH3 was supplied directly from the NH3 cylinder to the reactor. The various experimental conditions employed are outlined in Table 1.
The average power, Qaverage, as defined in Equation (6), was based on the measured water temperature (see nomenclature list for definitions).
Table 1. Experimental conditions for discharge characteristic evaluation.
*Mass of SrCl2: 9.6 g; Thickness of packed bed: 1 mm; Flow rate of heat transfer water: 0.8 L/min.
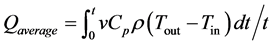 (6)
(6)
Furthermore, the characteristic heat transfer coefficient, U, was determined to evaluate the heat transfer resistance in the reactor, and was calculated from the experimental results as outlined in Equation (7).
 (7)
(7)
where Q is the momentary power, based on the measured water temperature. Tfl is the temperature of the heat transfer water, which was calculated as a logarithmic mean of the inlet and outlet temperatures of the heat transfer water. Moreover, A is the area of the heat transfer surface (i.e., 100 mm2), and Tr is the characteristic temperature of the reactant packed bed, which was determined by measuring the temperature using a thermocouple at 0.5 mm above the heat exchange plate, as indicated in Figure 2. The characteristic heat transfer coefficient is the parameter that reflects the heat resistances 1) through the packed bed, 2) between the packed bed and the heat transfer plate surface, 3) the heat transfer plate, and 4) the thermal boundary layer at the bottom of the heat transfer plate. The heat resistances at 3) and 4) are same under all the experimental conditions, therefore, the change in this parameter implies heat resistance changes at 1) and 2).
2.4. Cycling Capabilities of the Liquid-Solid Reaction
We then aimed to evaluate the short-term cycling capabilities of the discharge operation of the liquid-solid reaction, as outlined in Equation (3).
Following the condensation of NH3, the discharge and charge operations were repeated 10 times as per the reaction conditions outlined in Table 2. Each reaction cycle was operated as follows. During discharge, liquid NH3 was supplied to the reactor from the liquid level indicator, allowing the liquid-solid reaction to commence. Following the exothermic reaction, the reactor was heated to 80˚C using the thermostat bath, allowing the endothermic reaction to take place. The desorbed NH3 then transferred to the condenser and liquid level indicator and was condensed. This charge operation took place
Table 2. Experimental conditions for reaction cycling.
over 6 h, and was completed by closing the valve between the reactor and the condenser, and by subsequently cooling the reactor to 5˚C. After precooling, the heat release operation was repeated once more. The condenser temperature was maintained at 5˚C for the duration of this experiment. It should be noted that as the initial reactant was SrCl2, the exothermic reaction shown in Equation (3) takes place upon the supply of liquid NH3. This initial reaction was defined as cycle 0, while the subsequent exothermic reaction shown in Equation (1) was defined as cycle 1.
The average power, Qaverage, defined by Equation (6), and the conversion ratio, X, defined by Equation (8), were calculated
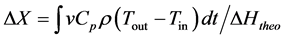 (8)
(8)
3. Results and Discussion
3.1. Comparison of the Liquid-Solid and Gas-Solid Reactions
The variation in average power of the liquid-solid and gas-solid reactions with time over a range of pressures is outlined in Figure 4. As shown, for the gas-solid reactions at both 5˚C and 15˚C, an increase in pressure resulted in an increase in the average power, due to the difference in equilibrium temperature of the reaction. Gaseous ammonia should be supplied at higher pressure for higher performance. The average power of the liquid-solid reaction was generally lower than that of the gas-solid reactions at 0.4 MPa or 0.6 MPa. However, the average power of the liquid-solid reaction was comparable with that of the 0.2 MPa gas-solid reaction it was slightly higher at 15˚C, while at 5˚C, it became higher after 200 s. Thus, the liquid-solid reaction can potentially become effective at low temperatures.
We then examined variations in the characteristic heat transfer coefficient, U, with time (Figure 5). Comparable results were obtained for all gas-solid reactions, and the average value obtained over 400 s was U = 165 W/m2/K at 15˚C and U = 161 W/m2/K at 5˚C. However, a larger heat transfer coefficient was obtained for the liquid-solid reaction at both 15 and 5˚C, giving an average value of U = 302W/m2/K and U = 304 W/m2/K over 400 s respectively. This indicates the decreased thermal resistance imparted by the liquid ammonia.
Indeed, this can be explained by the pictorial representation shown in Figure 6. In the gas-solid reaction, the heat of reaction transfers through the reactant particle prior

Figure 4. Variation in the average power Qave with pressure over time (a) 15˚C and (b) 5˚C.

Figure 5. Variation in the characteristic heat transfer coefficient U over time (a) 15˚C and (b) 5˚C.

Figure 6. Heat transfer models for the two different reaction types: (a) gas-solid reaction; and (b) liquid-solid reaction.
to reaching the heat transfer plate (Figure 6(a)). While in the liquid-solid reaction, as the ammonia is expected to remain in its pure form prior to the reaction with SrCl2, and due to the insolubility of this salt in ammonia [17] , the reactant particles come into contact with pure liquid ammonia during the reaction (Figure 6(b)). Thus, the presence of liquid ammonia on the heat exchange surface or in the interparticle voids results in additional heat migration pathways and decreased thermal resistance. Decrease in the heat transfer coefficient over time in the liquid-solid reaction likely indicates the reduction of liquid ammonia with progress of the reaction. This enhancement of thermal conductivity in the reactor might be influenced by the distribution of the liquid present, therefore, the discharge performance of the liquid-solid reaction can be improved by devising a liquid supply method.
The thermal conductivity in the reactor was evaluated roughly using the limited measuring point of the packed bed in this experiment. Therefore, detailed research on the mechanism of the liquid-solid reaction and the reactor design will be conducted in the future.
3.2. Cycling Performance of the Liquid-Solid Discharge Operation
We then examined the cycling performance of the liquid-solid discharge operation, where the reactions were cycled 10 times. Figure 7 shows the variation in the average power and conversion ratio, where a clear trend was observed for both measurements, despite small differences being detected over the 10 cycles. More specifically, the peak value of the average power fluctuated between 14 and 16 W, while the conversion ratio fluctuated between 0.6 and 0.7 after 400 s. Interestingly, no apparent degradation was observed in the short term for this system. Figure 8 shows photographic images of the SrCl2 packed bed particles after evaluation of the 10th cycle performance, where scattering of the reactant particles from the heat exchange surface and agglomeration between the particles were observed. Scattering was likely caused by the uncontrolled momentum of the supplied liquid NH3, while agglomeration was assumed to be caused
Figure 7. Variation in the average power Qave and the conversion ratio ∆X over 10 dis- charge operations for the liquid-solid reaction.
Figure 8. Packed SrCl2 particle beds following the 10 reaction cycles for the liquid-solid reaction.
by increased adhesion between the particles due to the presence of liquid NH3. We therefore expect that the varying physical contact between the heat exchange surface, the packed bed, and the liquid ammonia led to the fluctuation in results. As this could potentially prevent reliable cycling over the long term, alternative routes to liquid ammonia supply or particle fixing should be examined.
However, in the context of the short-term durability of the proposed system, no apparent degradation was observed, with comparable performances being obtained in each discharge operation.
4. Conclusions
In the context of thermochemical heat storage, we herein used the formation of a SrCl2 ammine complex as the reaction system of interest to evaluate the discharge characteristics of both the gas-solid reaction and the liquid-solid reaction. Furthermore, we examined the short-term durability of the liquid-solid reaction in terms of reaction cyclability. From the experimental results obtained, the following conclusions could be made:
1) For the gas-solid reactions, an increase in pressure resulted in an increase in the average power, likely due to the difference in equilibrium temperature of the reaction.
2) The average power of the liquid-solid reaction was generally lower than that of the gas-solid reaction carried out at either 0.4 or 0.6 MPa. However, in terms of the low pressure (i.e., 0.2 MPa) gas-solid reaction, comparable results were obtained to the liquid-solid reaction.
3) For the liquid-solid reaction, the lower thermal resistance in the reactor compared to the gas-solid reaction was observed, probably due to the presence of liquid ammonia during the reaction.
4) The short-term durability of the liquid-solid reaction system was confirmed following successful reaction cycling (i.e., over 10 cycles).
We expect that these results will aid in the improvement of energy efficiency through the utilization of waste heat, and will thus contribute to new advances in thermochemical heat storage.
Cite this paper
Kuwata, K., Masuda, S., Kobayashi, N., Fuse, T. and Okamura, T. (2016) Thermochemical Heat Storage Performance in the Gas/Liquid-Solid Reactions of SrCl2 with NH3. Natural Resources, 7, 655-665. http://dx.doi.org/10.4236/nr.2016.711052
References
- 1. Carbonell, D., Phillippen, D., Haller, M.Y. and Frank, E. (2015) Modelling of an Ice Storage Based on a De-Icing Concept for Solar Heating Applications. Solar Energy, 121, 2-16.
https:/doi.org/10.1016/j.solener.2015.06.051 - 2. Mahmood, M.M., Fariborz, H., Jeff, M. and Paul, S. (2015) Heat and Cold Storage Using Phase Change Materials in Domestic Refrigeration Systems: The State-of-the-Art Review. Energy and Buildings, 14, 615-628.
- 3. Abdel, I.N.K. and Fatizma, Z.T. (2015) Experimental Investigation of Latent Heat Storage in a Coil in PCM Storage Unit. Journal of Energy Storage, 5, 177-186.
- 4. Francis, A., Neil, H., Philip, E. and Mervyn, S. (2010) A Review of Materials, Heat Transfer and Phase Change Problem Formulation for Latent Heat Thermal Energy Storage Systems (LHTESS). Renewable and Sustainable Energy Reviews, 14, 615-628.
https:/doi.org/10.1016/j.rser.2009.10.015 - 5. Schmidt, M., Szczukowski, C., Robkopf, C., Linder, M. and Worner, A. (2014) Experimental Results of 10 kW High Temperature Thermochemical Storage Reactor Based on Calcium Hydroxide. Applied Thermal Engineering, 62, 553-559.
https:/doi.org/10.1016/j.applthermaleng.2013.09.020 - 6. Ogura, H., Miyazaki, M., Matsuda, H. and Hasatani, M.(1992) Numerical Analysis of Heat Transfer in Particle-Bed Reactor with Fins in Chemical Heat Pump Using Ca(OH)2/CaO Reaction. Kagaku Kogaku Ronbunshu, 18, 669-676.
https:/doi.org/10.1252/kakoronbunshu.18.669 - 7. Schaube, F., Koch, L., Worner, A. and Muller-Steinhagen, H. (2012) A Thermodynamic and Kinetic Study of the De- and Rehydration of Ca(OH)2 at High H2O Partial Pressures for Thermo-Chemical Heat Storage. Thermochemica Acta, 538, 9-20.
https:/doi.org/10.1016/j.tca.2012.03.003 - 8. Zamengo, M., Ryu, J. and Kato, Y. (2014) Thermochemical Performance of Magnesium Hydroxide-Expanded Graphite Pellets for Chemical Heat Pump. Applied Thermal Engineering, 64, 339-347.
https:/doi.org/10.1016/j.applthermaleng.2013.12.036 - 9. Molenda, M., Stengler, J., Linder, M. and Worner, A. (2013) Reversible Hydration Behavior of CaCl2 at High H2O Partial Pressures for Thermochemical Energy Storage. Thermochemica Acta, 560, 76-81.
https:/doi.org/10.1016/j.tca.2013.03.020 - 10. Ogura, H., Mikami, H. and Suzuki, H. (2015) Enhancement of Refrigeration Cold-Heat Production by Thermal Driven Chemical Heat Pump. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers, 32, 373-380.
- 11. Sakamoto, Y. and Yamamoto, H. (2014) Performance of Thermal Energy Storage Unit Using Solid Ammoniated Salt (CaCl2-NH3 System). Natural Resources, 5, 337-342.
https:/doi.org/10.4236/nr.2014.58031 - 12. Cot-Gores, J., Castell, A. and Cabeza, L.F. (2012) Thermochemical Energy Storage and Conversion: A-State-of-the-Art Review of the Experimental Research under Practical Conditions. Renewable and Sustainable Energy Reviews, 16, 5207-5224.
https:/doi.org/10.1016/j.rser.2012.04.007 - 13. Masuda, S., Kobayashi, N., Okamura, T. and Fuse, T. (2012) Heat Release Performance at Forming Ammine Complex with SrCl2. Proceedings of the 2012 JSRAE Annual Conference, 249-250.
- 14. Liu, C.Y. and Aika, K. (2004) Ammonia Absorption on Alkaline Earth Halides as Ammonia Separation and Storage Procedure. Bulletin of the Chemical Society of Japan, 77, 123-131.
https:/doi.org/10.1246/bcsj.77.123 - 15. Kito, T., Osaka, Y., Kuwata, K., Kobayashi, N., Huang, H. and He, Z. (2014) Study on Performance of Chemical Heat Storage System for Direct Steam Generation. Journal of Renewable and Sustainable Energy, 6, 023101.
https:/doi.org/10.1063/1.4868029 - 16. Niwa, A. and Kobayashi, N. (2015) Calcium Bromide Hydration for Heat Storage Systems. Cogent Engineering, 2, 1064218.
https:/doi.org/10.1080/23311916.2015.1064218 - 17. Barron, A.R. (2016) Liquid Ammonia as a Solvent.
http://cnx.org/content/m33060/latest/
Nomenclature
Qaverage Average heat output (W)
t Time (s)
Cp Heat capacity (kJ/kg/K)
T Temperature (˚C)
U Characteristic heat transfer coefficient (W/m2/K)
A Area of the heating surface (m2)
Greek letters
v Flow rate (m3/s)
ρ Density (kg/m3)
X Conversion ratio (-)
Htheo Theoretical heat of reaction (kJ)
Subscripts
in inlet
out outlet
r reactant
fl heat transfer fluid
Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact nr@scirp.org








