Natural Resources
Vol.05 No.15(2014), Article ID:52848,8 pages
10.4236/nr.2014.515081
Degradation of Oxo-Degradable-Polyethylene and Polylactic Acid Films Embodied in the Substrate of the Edible Fungus Pleurotus ostreatus
Dalia Santa Cruz-Navarro, Rosa María Espinosa-Valdemar, Margarita Beltrán-Villavicencio, Alethia Vázquez-Morillas, Maribel Velasco-Pérez
Universidad Autónoma Metropolitana-Azcapotzalco, México D.F., México
Email: alethia@correo.azc.uam.mx
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 October 2014; revised 20 November 2014; accepted 3 December 2014
ABSTRACT
Degradable plastic mulch is being used to overcome the negative environmental impacts of burning and landfilling agricultural plastic waste. In this study P. ostreatus was used to model the capacity of a vegetal species to degrade conventional and degradable plastic films. Plastics studied were oxo-degradable polyethylene (OXO-PE), UV-irradiated oxo-degradable polyethylene (UV- OXO-PE), polylactic acid (PLA) and conventional polyethylene (C-PE). The cultivation of P. ostreatus resulted in a reduction in the median of weight (78.2% - 80.2%) and volume (56.1% - 60.1%) of the substrate (wheat straw). Degradation of the plastics embodied was evidenced by a reduction in the median of the elongation at break (OXO-PE 475% to 109%, UV-OXO-PE 23% to 8%, PLA 596% to 398% and C-PE 505% to 304%) and an increase in the median of the carbonyl index (OXO-PE 0.062 to 0.114, UV-OXO-PE 0.098 to 0.145 and PLA 0.024 to 0.034). The Kruskal-Wallis test found no statistical difference (p = 0.384) between the medians of the biological efficiency for substrates containing plastics and the substrate without plastic. In conclusion, plastics embodied in the substrates used for cultivation of P. ostreatus are degraded and the degradation of these plastics does not affect the short term growth of P. ostreatus.
Keywords:
Plastic Degradation, P. ostreatus, Mechanical Properties, Carbonyl Index,
Biological Efficiency

1. Introduction
Current lifestyle would be difficult to imagine without plastic; this is the most common multipurpose material [1] . The industry of plastics has grown continuously since the 1950s, and global plastic production was 288 million tons in 2012 [1] . The development of this industry has caused an increase in plastic waste. Only in the European Union post-consumer plastic waste generated was 25.2 million tons in 2012. Packing represents the 62.2% of plastic waste and applications like building and construction, and electrical and electronic items represent from 6% to 5% each [1] .
Plastics are used in agriculture. Mulching was among the first methods employed to modify the microclimate of crops, and polyethylene film was first used in a greenhouse by Professor Emmert at the University of Kentucky [2] . Later, Emmert investigated the use of mulch and row covers [2] . Films occupy the largest volume of plastics used in agriculture, and polyethylene films are widely used for mulching [2] [3] . Plastic mulch is used to increase yield crop, prevent water losses and protect crops from severe weather, insects and birds [2] . In Mexico, the average waste generation of agro plastics from 2006 to 2012 was 313,130 tons/year [3] .
The most common end of life scenarios for plastic mulch is burning on-site and disposition in a landfill [4] . These scenarios have negative environmental impacts attached; burning plastic produces the air pollutant dioxin and landfill is at the bottom of the waste management hierarchy. In the search of solutions, degradable plastic films are used to reduce the negative impacts of plastic mulch; PLA and OXO-PE are two of these materials [5] .
PLA is a thermoplastic produced mostly from the fermentation of starch; under controlled conditions it is biodegradable [6] . Oxo-degradable plastics are conventional plastics modified with pro-oxidant additives made of cobalt, manganese and iron compounds or polyunsaturated molecules [7] . These additives promote the fragmentation of plastics under exposition to UV radiation and temperature [8] .
Once the crops have been harvested, the remaining agricultural waste can be composted or digested anaerobically to produce energy. In Mexico, agricultural waste is also used as substrate for the growth of edible fungus. Cultivation of edible mushrooms in Mexico started in 1933 [9] . In 2011, Mexico was the first producer and exporter of edible mushrooms in Latin America and the number 13th worldwide, producing 62,000 tons of mushrooms and generating 25,000 jobs [10] . In 2000, more than 280,000 tons of agricultural wastes were used in the cultivation of edible mushrooms [11] . Also, edible mushrooms are known for their nutritional value [12] .
The production of P. ostreatus has been extensively studied to find the substrate that maximizes the mass of mushroom generated by mass of substrate used in cultivation, called biological efficiency (BE). Examples of some studies and the BE achieved are shown in Table 1. As can be observed, this mushroom has been used to treat biodegradable wastes, i.e. agricultural residues with a high content of cellulose and lignin.
Table 1. BE of P. ostreatus for different substrates.
Due to incomplete degradation of the mulching film, agricultural waste used to grow edible fungus can contain small fragments of plastics. In this context, the aim of this work was to assess the degradation of C-PE, OXO-PE and PLA plastic film waste embodied in the substrate used for cultivation of the eatable fungus Pleurotus ostreatus and to evaluate the effect of plastic films in the cultivation of this fungus. This species is selected because it has been extensively studied due to its high capacity to use complex molecules such as cellulose and lignin as a nutrient source, requires very low infrastructure for cultivation and grows relatively fast.
2. Material and Methods
This section presents the seed and substrate used for cultivation, the experimental design, the methods followed for inoculation and harvesting of the fungus, the determination of the degradation of substrate and plastic films and the evaluation of the effects of plastic film on the cultivation on P. ostreatus.
2.1. Seed and Substrate
The seed used for cultivation was sorghum with invasion of inoculum of P. ostreatus; the seeds were obtained from PRODISET with the product code BGAT. The substrate used was wheat straw (WS); which is commonly employed in commercial cultivation of P. ostreatus. Prior to substrate preparation, WS was hydrated for 24 h with tap water, drained to remove excess water, and then sterilised in an autoclave at a pressure of 1.1 kg/m3 and a temperature of 121˚C for 15 min. Sterilisation was carried out to eliminate other microorganisms which could compete with the fungus.
The humidity content of the sterilised WS was determined experimentally. Three samples of 100 g of sterilised WS (wet weight) were dried in an oven at 60˚C for 24 h. Then, these samples were allowed to cool to room temperature in a desiccator. Finally, samples were weighted (dry weight). The humidity content was calculated by dividing the difference of the wet and dry weight by the wet weight.
2.2. Experimental Design
An experimental design with four treatments, a control and five replicates was used (Table 2). The treatment combinations were as follows: WS with inoculum mixed with strips of 1) OXO-PE, 2) UV-OXO-PE, 3) PLA and 4) C-PE. The control 5) consisted of WS with inoculum and without strips of plastic. In all cases, plastic strips measured 1.5 cm × 10 cm.
Treatment 2) UV-OXO-PE simulated the process of natural plastic aging in a homemade accelerated intemperism test UV chamber [20] . Strips of oxo-degradable plastic were irradiated at a UV-A wavelength of 340 nm at 60˚C until their elongation at break was less than 40%.
Experiments were carried out in the laboratory of Sustainable Technologies of the Universidad AutónomaMetropolitana-Azcapotzalco. Temperature and humidity during the cultivation of P. ostreatus were controlled.
2.3. Cultivation of P. ostreatus
For each experimental unit one kg of sterilized WS was weighted and mixed with 10 g of the relevant type of plastic (≈1% wet weight). Also, 50 g of seed were weighted (≈5% wet weight). Then, a container with a volume of 7.9 L was lined with a thermo-resistant paper bag (40 cm × 60 cm). Consecutive layers of substrate (WS + plastic) and seeds were placed in the container. The bag was closed, taken out of the container and transferred to the spawning chamber.
Table 2. Substrates used for the cultivation of P. ostreatus.
The chamber was kept dark during spawning. On the third day in the spawning chamber, openings of 5 cm of length in the bag were cut with a sterile razor. Bags were transferred to the luminous chamber for fruiting when the substrate was fully invaded by the inoculum. Temperature and humidity during invasion, spawning and fruiting were recorded with a USB data logger. Finally, fruiting bodies were harvested manually when they were totally developed. Then, their weight was measured and recorded as fresh weight.
2.4. Determination of the Degradation of the Substrate
The degradation of the substrate was estimated with the reduction on dry weight and volume. Weight reduction was calculated as the difference between the dry weight of the substrate at the time of sowing and dry weight of the substrate at the end of the experiment. The volume reduction of the substrate was calculated as the difference between the wet volume of the substrate at the time of sowing and the wet volume of the substrate at the end of the experiment.
2.5. Evaluation of the Degradability of Plastics
Plastics were recovered from the substrate and washed with deionised water once the experiment was finished. The degradation of plastics was estimated through the tensile test and the determination of the carbonyl index.
2.5.1. Tensile Test
The tensile test was done in accordance to the ASTM D882-10 standard test method for tensile properties of thin plastic sheeting [21] . A vice action grip for thin film, model TG3415 by Lloyds instruments, was used to perform this test. For each type of plastic, 15 strips recovered from the substrates and 15 unused strips were analysed. The elongation at break was calculated with the Software NEXIGEN Plus.
2.5.2. Carbonyl Index
When plastics with pro-oxidant additives are degraded, carbonyl molecules are produced. Changes in the chemical composition of plastics can be measured with the carbonyl index, which is calculated dividing the height of the peak of the FT-IR spectrum at 1751 cm−1 (C-O and C=O bonds) between the height of the peak at 1895 cm−1 (C-C bonds).This analysis was done with a Nicolet iS10 FT-IR spectrometer by Thermo Scientific. For each type of plastic, four unused plastic strips and four plastic strips recovered from the substrates and cleaned with deionised water were analysed.
2.6. Evaluation of the Effect of Plastics on the Cultivation of P. ostreatus
The BE was used to evaluate the effect of plastics on the cultivation of P. ostreatus. BE was calculated with the following equation [22] :
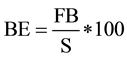
where: BE is biological efficiency, FB is fresh weight if the fruiting body and S is dry weight of the substrate.
2.7. Data Analysis
Experimental data was analysed statistically with the software SPSS version 18. Data was screened to verify that the assumptions for parametric tests of a normal distribution (p > 0.05 in Shapiro-Wilk test) and equal variances (p > 0.05 in Levene test) were fulfilled. Since not all data sets fulfilled both conditions, the median of data sets was compared with the non parametric Kruskal-Wallis test.
3. Results and Discussion
3.1. Growth of P. ostreatus
According to the distributor of the seeds of P. ostreatus, optimal environmental conditions for spawning are temperatures of 25˚C - 28˚C, high CO2 concentration and low humidity; while temperatures of 12˚C - 18˚C, relative humidity (RH) of 85%, and low CO2 (<800 ppm) are required for the fruiting phase. Temperatures from 17˚C to 21˚C and RH from 55% to 90% were registered in the spawning chamber; while temperatures from 21˚C to 29˚C and RH from 97.5% to 100% were registered in the fruiting chamber.
The experimental units remained a total of 35 days in the spawning chamber. The invasion of P. ostreatus reached a maximum of 75% in all substrates. Fruiting occurred on the 38th day for substrates OXO-PE, PLA, C-PE and WS-I and on the 40th day for substrate UV-OXO-PE. To illustrate the development of the mushroom Figure 1 shows the first harvest of C-PE and UV-OXO-PE substrates.
P. ostreatus developed with morphological abnormalities during the first two weeks of fruiting; the stalk was unusually long (around 15 cm) and twisted, and the pileus (or cap) did not open completely. These abnormalities were caused by a high concentration of CO2 in the fruiting chamber. Hence, the chamber was aired opening a window for a couple of hours every day. P. ostreatus developed normally after the chamber was aired. The fruiting phase lasted in total 43 days. Each experimental unit was harvested from 3 to 4 times.
3.2. Degradation of the Substrate
Reduction in the median of dry weight of all substrates was from 78.2% to 80.2% (Figure 2); while reduction in the median of volume was from 56.1% to 60.1% (Figure 3). Also, the Kruskal-Wallis test found no statistical difference in the medians of reduction of dry weight (p = 0.117) and reduction of volume (p = 0.420) for all substrates. Since there is no statistical difference between the median of the experimental units without plastic (WS-I) and the medians of substrates with plastic, the observed reduction in weight and volume in the substrates was due to their degradation by the action of P. ostreatus.

Figure 1. First harvest for the substrates (a) C-PE; (b) UV-OXO-PE.
Figure 2. Box plot for percent of dry weight reduction for the substrates OXO-PE, UV-OXO-PE, PLA, C-PE and WS-I.
Figure 3. Box plot for percent of volume reduction for the substrates OXO-PE, UV-OXO-PE, PLA, C-PE and WS-I.
3.3. Evaluation of the Degradation of Plastics
The degradation of plastics was evaluated through a visual inspection, the tensile test and the carbonyl index. Figure 4 shows a comparison of the physical state of the plastics recovered from the substrate after the cultivation of P. ostreatus. The strips of UV-OXO-PE were fractured (Figure 4(b)).
3.3.1. Tensile Test
The tensile test was carried out at a crosshead speed of 50 cm∙min−1 and a load of 2 N for all samples with exception of the PLA strips recovered from the substrates. The load used for PLA samples was reduced to 1 N since the break point could not be determined applying a load of 2 N.
A box plot with the results of the tensile test for unused plastics and plastics recovered from the substrate for each type of plastic is shown in Figure 5. In all cases, the median of the percent elongation at break of the plastics recovered from the substrates is considerably smaller than the median of the unused plastics. Also, UV- OXO-PE samples have the smallest percent elongation at break. Since these samples went through the ageing process, it was expected that elongation at break values would be lower.
The Kruskal-Wallis test found that the medians of the percent elongation at break are different for unused plastics and plastics recovered from the substrates for OXO-PE (p = 0.000), UV-OXO-PE (p = 0.000), PLA (p = 0.000) and C-PE (p = 0.027). This test shows than plastics in the substrates went through a degradation process; evidenced by a reduction in the elongation at break for all types of plastics [23] .
Considering than the degraded PLA samples were analysed with half the load of other samples, the difference in percentage between the medians of unused plastics and plastics recovered from substrates decreases as follows PLA > OXO-PE > UV-OXO-PE > C-PE.
3.3.2. Carbonyl Index
Box plots for the experimental data of the carbonyl index for unused plastics and plastics recovered from the substrates are shown in Figure 6. Although the median of the carbonyl index was smaller for the C-PE strips recovered from the substrate than for the unused C-PE strips, the Kruskal-Wallis test showed that there was no statistical difference between these samples (p = 1.000). These results could be explained by the variability observed in the carbonyl index of the C-PE strips; values ranged from 0.015 to 0.15.
According to the Kruskal-Wallis test the medians of the carbonyl index of the unused plastics and the plastics recovered from the substrates are statistically different (p < 0.05) for OXO-PE, UV-OXO-PE and PLA; showing that these plastics were degraded during the cultivation of the fungus. The difference between the medians of the carbonyl index for unused plastics and plastics recovered from the substrate decreases as follows: OXO-PE (0.53 units) > UV-OXO-PE (0.047 units) > PLA (0.010 units). Since the mechanism for degradation of PLA is hydrolysis, the carbonyl index does not measures accurately the degradation of this plastic.
3.4. Evaluation of the Effect of Plastics on the Cultivation of P. ostreatus
The medians of the BE of P. ostreatus for the different substrates vary from 25.8% to 35.2% (Figure 7). The low BE values obtained were attributed to the problems in establishing the optimum temperature and relative humidity in the spawning and fruiting chambers [24] [25] . The Kruskal-Wallis test found no statistical difference between the medians of the BE for different substrates (p = 0.384); showing that neither plastics nor their degradation products affect negatively the growth of P. ostreatus.
4. Conclusions
This study aimed to evaluate degradation of different types of plastic films embodied in the substrate of P. ostreatus and the effects of plastics in the development of this fungus. The results presented indicate that: plastics are partially degraded when embodied in the substrates used for cultivation of P. ostreatus; this was demonstrated by a decrease in the elongation at break and the carbonyl index of plastics recovered from the substrates; plastic samples that contained oxo-additives were degraded in the substrates regardless of previous UV irradiation (simulation of natural aging); P. ostreatus considerably reduces the mass and volume of the substrate; low concentration of plastics in the substrate does not affect negatively the growth of P. ostreatus.

Figure 4. Plastic strips recovered after the cultivation of P. ostreatus. (a) OXO-PE; (b) UV-OXO-PE; (c) PLA; (d) C-PE.
Figure 5. Box plot for percent elongation at break for unused plastic strips (control) and plastic strips recovered from the substrates. The plastics tested were OXO-PE, UV-OXO-PE, PLA and C-PE.
Figure 6. Box plot for carbonyl index for unused plastic strips (controls) and plastic strips recovered from the substrates. The plastics tested were OXO-PE, UV-OXO-PE, PLA and C-PE.
Figure 7. Box plot for percent BE for the substrates OXO-PE, UV-OXO-PE, PLA, C-PE and WS-I.
This work serves as a reference to model the capacity of vegetal species to degrade conventional and new plastic materials used in agriculture as well as the effects of degradation of plastics in crop yield when the agricultural waste is used as a substrate.
References
- Association of Plastics Manufacturers, Plastics Europe (2013) Plastic―The Facts 2013. An Analysis of European Latest Plastics Production, Demand and Waste Data.
- Kasirajan, S. and Ngouajio, M. (2012) Polyethylene and Biodegradable Mulches for Agricultural Applications: A Review. Agronomy for Sustainable Development, 32, 501-529. http://dx.doi.org/10.1007/s13593-011-0068-3
- National Ecology Institute, Department of the Environment and Natural Resources, National Institute of Ecology and Climate Change (2012) Basic Diagnosis of the Integrated Waste Management. Extended Version.
- Washington State University (2013) Using Biodegradable Plastics as Agricultural Mulches. Washington State University Extension Fact Sheet (FS103W).
- Johnson, G. (2012) Evaluation of Biodegradable Plastic Mulches for Watermelon Production in Delaware. University of Delaware, Delaware.
- Bastioli, C. (2001) Global Status of the Production of Biobased Packaging Materials. Starch/Stärke, 53, 351-355. http://dx.doi.org/10.1002/1521-379X(200108)53:8<351::AID-STAR351>3.0.CO;2-R
- Ojeda, T.F.M., Dalmolin, E., Forte, M.M.C., Jacques, R.J.S., Bento, F.M. and Camargo, F.A.O. (2009) Abiotic and Biotic Degradation of Oxo-Biodegradable Polyethylenes. Polymer Degradation and Stability, 94, 965-970. http://dx.doi.org/10.1016/j.polymdegradstab.2009.03.011
- Chiellini, E., Corti, A., D’Antone, S. and Baciu, R. (2006) Oxo-Biodegradable Carbon Backbone Polymers―Oxidative Degradation of Polyethylene under Accelerated Test Conditions. Polymer Degradation and Stability, 91, 2739-2747. http://dx.doi.org/10.1016/j.polymdegradstab.2006.03.022
- Martínez-Carrera, D., Leben, R., Morales, P., Sobal, M. and Larqué-Saavedra, A. (1991) History of the Commercial Cultures of Edible Mushrooms in Mexico. Science and Development, 96, 33-43.
- Cruz, A. (2013) Mexico Is the Leading Producer of Mushrooms in Latin America and It Has Still a Lot of Potential. The Chronic. Mexico City.
- Martínez-Carrera, D., Larqué, A., Aliphat, M., Aguilar, A., Bonilla, M. and Martínez, W. (2000) The Biotechnology of Edible Mushrooms for the Mexican Food Safety and Sovereignty. II National Forum of Food Safety and Sovereignty, Mexican Academy of Sciences-CONACYT, Mexico City, 193-207.
- Bautista, M., Alanís, M.G., González, E. and García, C.L. (1998) Chemical Composition of Three Different Mexican Oyster Mushrooms Strains (Pleurotus ostreatus). Archivos Latinoamericanos de Nutricion, 48.
- Martínez-Carrera, D., Morales, P. and Sobal, M. (1990) Pleurotus ostreatus Cultures on Cane Bagasse Enriched with Coffee Pulp or Barley Straw. Micología Neotropical Aplicada, 3, 49-52.
- De León-Chocooj, R. (1990) Culture of the Edible Mushrooms from the Genus Pleurotus on Water Hyacinth and the Determination of the Heavy Metal on the Collected Samples [MsC]. UNAM, Mexico City.
- Mata, G. and Gaitán, H.R. (1995) Pleurotus Culture on Sugar Cane Leaves. Mexican Magazine of Mycology, 11, 17- 22.
- Bautista, J.M., Franco, B.L., Martínez, S.G., Gamiño, S.Z. and Ocaña, C.R. (1998) Nutritional Value of the Oyster Mushrooms (Pleurotus ostreatus) Grown on Avocado and Pineapple Wastes. University Act, 8.
- Pérez, R. and Mata, G. (2005) Culture and Selection of Pleurotus ostreatus and P. pulmonarius Strains on Pine Shavings: The Obtaining of New Strains and Their Production Evaluation. Mexican Magazine of Mycology, 20, 53-59.
- García, N., Bermúdez, R.C., Gross, P. and Hernández, M. (2006) Pleurotus sp. Strains Culture on Coffee Pulp. Mexican Magazine of Mycology, 23, 99-101.
- Romero, O., Huerta, M., Damián, M.Á. and Macías, A. (2010) Evaluation of the Productive Capacity of Pleurotus ostreatus on Dehydrated Banana Leaves (Musa paradisiaca L., cv. Roatan) over Other Agricultural Substrates. Costa Rican Agronomy, 34, 53-63.
- Cano Blanco, M., León Galicia, A., Rabell Contreras, M.F., Vázquez Morillas, A. and Osada Velázquez, M.H. (2011) Performance Assessment of a Self-Constructed UV Chamber for Plastic Degradation. Global Plastics Environmental Conference, Society of Plastics Engineers Environmental Division, Atlanta.
- ASTM D882-10 (2010) Standard Test Method for Tensile Properties of Thin Plastic Sheeting.
- Belewu, M.A. and Belewu, K.Y. (2005) Cultivation of Mushroom (Volvariella volvacea) on Banana Leaves. African Journal of Biotechnology, 4, 1401-1403.
- Coda, E., Pujol, O. and Aradilla Zapata, D. (2007) Degradation of Waste Plastic Materials. Chemical Engineering, 448, 186-190.
- Wang, D., Sakoda, A. and Suzuki, M. (2001) Biological Efficiency and Nutritional Value of Pleurotus ostreatus Cultivated on Spent Beer Grain. Bioresource Technology, 78, 293-300. http://dx.doi.org/10.1016/S0960-8524(01)00002-5
- Barba Chávez, J.M.A., López Cruz, J.I. and Castañeda de León, V.T. (2006) The Culture of Edible Oyster Mushrooms as an Industrial Development Process in Mexico. AGT, Mexico City.







