Open Journal of Anesthesiology
Vol.2 No.2(2012), Article ID:18552,7 pages DOI:10.4236/ojanes.2012.22008
Serotonin Influences the Endogenous Opiate Peptides in the Rat Spinal Cord to Participates in Pain Modulation
![]()
1College of Pharmacy, Xinxiang Medical University, Xinxiang, China; 2Subei People’s Hospital of Jiangsu Province, Yangzhou University, Yangzhou, China.
Email: *bcd2009@126.com
Received December 18th, 2011; revised January 4th, 2012; accepted January 15th, 2012
Keywords: Serotonin; Endogenous Opiate Peptide; Spinal Cord; Antinociception
ABSTRACT
Spinal cord is a necessary pathway that transfers the body nociceptive inputs to the brain. Endogenous opiate peptides have been proven to participate in the nociceptive process at spinal level. It has reported that serotonin (5-HT, 5-hydroxytryptamine) in spinal cord plays a role in pan modulation, which can be blocked by opiate receptor antagonists. The present study was designed to investigate the interaction between 5-HT and endogenous opiate peptides at rat spinal level effecting on pain modulation. The results showed that 1) pain stimulation increased not only leucine-enkephalin (L-Ek), β-endorphin (β-Ep) and dynorphin A1-13 (DynA1-13) concentrations but also 5-HT and 5-hydorxyindoleace acid (5-HIAA, the 5-HT main metabolic product) concentrations in spinal cord significantly; 2) 5-HT could increase L-Ek, β-Ep and DynA1-13 concentrations in spinal cord in a dose-dependent manner, whereas cypotolamine (a 5-HT receptor antagonist) decreased L-Ek, β-Ep and DynA1-13 concentrations in spinal cord. The data suggested that 5-HT antinociceptive role might be involved in the endogenous opiate peptide system through 5-HT receptors at spinal level.
1. Introduction
Serotonin (5-HT, 5-Hydroxytryptamine) is a monoamine neurotransmitter. In animals including humans, serotonin is synthesized from the amino acid L-tryptophan by a short metabolic pathway consisting of two enzymes: tryptophan hydroxylase (TPH) and amino acid decarboxylase (DDC). The TPH-mediated reaction is the rate-limiting step in the pathway. TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a brain-specific isoform [1]. Serotonin is mainly metabolized to 5-hydorxyindoleace acid (5- HIAA), which involves first oxidation by monoamine oxidase (MAO) to the corresponding aldehyde, and then it is followed by oxidation by aldehyde dehydrogenase to 5-HIAA, the indole acetic acid derivative. Serotonin is synthesized in serotonergic neurons in central nervous system where it has various functions of the regulation of mood, appetite, sleep, memory and learning, as well as muscle contraction. Many experiments have discovered that serotonin in spinal cord plays an important role in pan modulation. Intrathecal sildenafil effectively attenuated the pain evoked by formalin injection, in which serotonin receptors may be involved in the antinociceptive action of sildenafil at the spinal level [2]. The spinal serotonin system plays an important role in the mode of action of spinal cord stimulation involving the activation of descending serotonergic pathways that may inhibit spinal nociceptive processing partially via a GABAergic link [3]. A loss or decrease in the descending inhibitory serotonin system upon the spinal processing of nociceptive information appears to occur following spinal nerve injury, and this kind of decrease in the descending inhibitory serotonin system is proposed to be involved in the development of central sensitization and ultimately to the nerve injury-induced neuropathic pain [4].
Since Hughes et al. purified and identified L-Ek and M-Ek in 1975 [5], the endorphin had been confirmed in 1976 [6] and dynorphin in 1979 [7]. Endogenous opiate peptides include three series—enkephalin, endorphin and dynorphin [8], which have been proven to participate in the pain modulation in the spinal cord [9-13].
The spinal cord is a necessary pathway that transfers the body nociceptive inputs to the brain, in which it has been proven the relationship between serotonin and endogenous opiate peptide system relating with pain modulation. Serotonin in the spinal cord has an analgesic role, which can be blocked by an opiate receptor antagonist-naloxone [14]. β-Endorphin produces analgesia at spinal level via an opiate receptor-mediated interaction with spinal monoaminergic nerve terminals [15]. Selective degeneration of spinal cord serotoninergic pathway can influence the role of morphine regulates pain [16]. Spinal system participate in the development of copulatory analgesia, which is mediated by descending serotonergic fibers, although intrinsic spinal systems would involve both opiate and GABA interneurons [17]. However, there is not direct evidence that interaction between the serotonin and endogenous opiate peptides at spinal level relating with pain modulation. The communication was designed to investigate the interaction between serotonin and endogenous opiate peptide in spinal cord effecting on the pain modulation.
2. Materials and Methods
2.1. Animals
Adult male Sprague-Dawley rats weighing 180 - 220 g were used in all experiments (Animal Center of Yangzhou University, Yangzhou, Jiangsu, China). Animals were housed in a colony room under controlled temperature, humidity and a 12 hours light/dark cycle (light on at 6:00 am), with food and water available ad libitum. All procedures were conducted according to the guidelines of the International Association for the Study of Pain [18] and approved by the Animal Care and Use Committee of Yangzhou University.
2.2. Materials
Leucine-enkephalin (L-Ek), β-endorphin (β-Ep) and dynorphin A1-13 (DynA1-13) were obtained from Peninsula Laboratories, San Carlos, CA, USA; 125Iodine was from Amersham Pharmacia, Buckinghamshire, UK; Serotonin, 5-HIAA (the 5-HT main metabolizing product), cypoheptadine (5-HT receptor antagonist) and other chemical reagents were from Sigma Co., St. Louis, MO, USA.
Rabbit anti-rat L-Ek, β-Ep or DynA1-13 serum was made by Department of Neurobiology, Second Military Medical University, Shanghai, China. The specificity of each kind of antiserum was more than 99% reactivity with its corresponding antigen and less than 1% reactivity with other similar peptides. The effective dilution of the antiserum was 1: 20,000 - 80,000 for radioimmunoassay.
2.3. Surgery
Under pentobarbital sodium (35 mg/kg, intraperitoneal injection) anesthesia, the rat was implanted a chronic intrathecal catheter (PE-10, 12 cm in length, 0.6 cm outer diameter) extending into the lumbar enlargement of the spinal cord for intrathecal injection (ith). All operations were carried out under aseptic conditions and the animals were allowed to recover for at least 14 days after the surgery.
2.4. Spinal Cord Administration
Ten μl of artificial cerebral spinal fluid (ACSF, containing 0.1 M NaCl, 1.0 mM KH2PO4, 4.0 mM KCl, 2.0 mM MgSO4, 2.0 mM CaCl2, 2.1 mM NaHCO3 and 8.0 mM Glucose), which could dissolve the drug, was gently injected into the lumbar enlargement of the spinal cord through the chronic intrathecal catheter over 10 min.
2.5. Pain Stimulation
All animals were tested under the condition of free activity in the small cages (30 cm in diameter, 25 cm in height) from 8:00 to 10:00 am. We used the potassium iontophoresis inducing tail-flick served as pain stimulus. The small wet cotton with the potassium iontophoresis was set on the skin of the tail. The cotton was exposed to direct electrical current, and the anode led the potassium iontophoresis to permeate the skin of the tail. If the current was strong enough, the permeated potassium iontophoresis resulted in the animal feeling the pain stimulation. The intensity of current at the moment of the response was recorded as the pain threshold, which was expressed as mA (WQ-9E Pain Threshold Measurer, Shanghai, China). Through the positive electrodes producing the direct electrical current was generated from Pain Threshold Measurer to induce the potassium iontophoresis into the animal tail skin and result in acute pain. The intensity was fixed to 1.2 - 1.4 × pain threshold (0.6 - 0.7 mA) for 1 min.
2.6. Prepare of Tissue Sample
After the decapitation, the lumber spinal cords were taken out and put into the liquid nitrogen. After weighing, the tissues were homogenized in 1.0 ml of 0.1 M acetic acid at 4˚C. Two hours later, the same volume of 0.1 M sodium hydroxide was mixed in the homogenate. Using the centrifugation at 10,000 g at 4˚C for 20 min, the supernatants were withdrawn and stored at –80˚C for assay.
2.7. Radioimmunoassay for Peptide Measurement
The L-Ek, β-Ep and DynA1-13 concentrations were determined with specific rabbit antiserum. The peptides were labeled 125Iodine using the chloramines-T method and iodinated peptides were purified by Sephadex G-50. The assay sensitivities for the L-Ek, β-Ep or DynA1-13 were 3.0, 1.2 and 6.3 pg/tube and intraand inter-assay coefficients of variation were less than 5.1% and 8.0%, respectively.
2.8. High Performance Liquid Chromatograph for Neurotransmitter Measurement
Serotonin and 5-HIAA concentrations were measured by Waters Breeze high performance liquid chromatograph (HPLC) system with 2465 electrochemical detection (ECD) and 1525 HPLC pump. The hydrogen peroxide was detected electrochemically with a platinum electrode set at 2 mV (vs Ag/AgCl). The 2.1 × 150 mm 3.5 μm Waters SymmetryShieldTM RP18 analytical column was protected by a 2.1 × 10 mm 3.5 μm Waters SymmetryShield™ Sentry™ Guard column. The mobile phase was 0.01 M sodium bicarbonate containing 11% methanol, 0.58 mM sodium octyl sulfate (SOS) and 0.02 mM ethyenediaminetetraacetic acid (EDTA). The pH was adjusted to 2.9 using hydrochloric acid, and then filtered by 0.22 μm membranes. The flow rate was fixed at 0.25 ml/min. The 25-μl solution of the standard or sample was injected into the column directly.
2.9. Statistical Analysis
Data were expressed as mean ± standard error of the mean (S.E.M.), which the repeated experiment time was the animal number of each group, and were analyzed between groups by the analysis of variance (ANOVA) and χ2 test. P < 0.05 was considered statistically significant.
3. Results
3.1. Pain Stimulation Increased Endogenous Opiate Peptide and Serotonin Concentrations in the Spinal Cord
Giving the animal 1min pain stimulation, L-Ek concentration in the spinal cord was increased from 8.4 ± 2.1 pg/mg (tissue weight) to 35.5 ± 5.6 pg/mg in 5 min (P < 0.001) and 17.6 ± 4.3 pg/mg in 10 min (P < 0.05) after the stimulation (Figure 1(a)); β-Ep concentration in the spinal cord was increased from 10.6 ± 2.7 pg/mg (tissue weight) to 48.9 ± 8.2 pg/mg in 5 min (P < 0.001) and 35.4 ± 6.5 pg/mg in 10 min (P < 0.01) after the stimulation (Figure 1(b)); DynA1-13 concentration in the spinal cord was increased from 6.5 ± 1.8 pg/mg (tissue weight) to 18.7 ± 4.3 pg/mg in 5 min (P < 0.001) and 11.4 ± 2.1 pg/mg in 10 min (P < 0.01) after the stimulation Figure 1(c)); Serotonin concentration in the spinal cord was increased from 7.8 ± 2.0 pg/mg (tissue weight) to 42.5 ± 10.2 pg/mg in 5 min (P < 0.001) and 29.8 ± 7.3 pg/mg in 10 min (P < 0.01) after the stimulation (Figure 1(d)); And 5-HIAA concentration in the spinal cord was increased from 12.3 ± 3.8 pg/mg (tissue weight) to 67.8 ± 14.3 pg/mg in 5 min (P < 0.001) and 39.7 ± 12.4 pg/mg in 10 min (P < 0.01) after the stimulation (Figure 1(e)). In control group, L-Ek, β-Ep, DynA1-13, serotonin and 5-HIAA concentrations did not changed.
3.2. Serotonin Increased Endogenous Opiate Peptide Concentrations in the Spinal Cord in a Dose-Dependent Manner
Administration of 20 ng serotonin into the spinal cord increased L-Ek concentration in the spinal cord from 8.4 ± 2.1 pg/mg (tissue weight) to 33.8 ± 5.9 pg/mg in 5 min (P < 0.001) and 23.4 ± 4.6 pg/mg in 10 min (P < 0.001); Administration of 10 ng serotonin into the spinal cord increased L-Ek concentration in the spinal cord from 8.4 ± 2.1 pg/mg (tissue weight) to 18.7 ± 3.6 pg/mg in 5 min (P < 0.01) and 11.7 ± 3.7 pg/mg in 10 min; In control group, L-Ek concentration did not changed (Figure 2(a)).
Administration of 20 ng serotonin into the spinal cord increased β-Ep concentration in the spinal cord from 10.6 ± 2.7 pg/mg (tissue weight) to 47.9 ± 9.7 pg/mg in 5 min (P < 0.001) and 27.5 ± 7.4 pg/mg in 10 min (P < 0.001); Administration of 10 ng serotonin into the spinal cord increased β-Ep concentration in the spinal cord from 10.6 ± 2.7 pg/mg (tissue weight) to 26.5 ± 6.6 pg/mg in 5 min (P < 0.001) and 17.6 ± 5.2 pg/mg in 10 min; In control group, β-Ep concentration did not changed (Figure 2(b)).
Administration of 20 ng serotonin into the spinal cord increased DynA1-13 concentration in the spinal cord from 6.5 ± 1.8 pg/mg (tissue weight) to 29.4 ± 6.7 pg/mg in 5 min (P < 0.001) and 18.9 ± 5.7 pg/mg in 10 min (P < 0.001); Administration of 10 ng serotonin into the spinal cord increased DynA1-13 concentration in the spinal cord from 6.5 ± 1.8 pg/mg (tissue weight) to 16.7 ± 3.5 pg/mg in 5 min (P < 0.001) and 10.7 ± 3.9 pg/mg in 10 min; In control group, DynA1-13 concentration did not changed (Figure 2(c)).
3.3. Serotonin Receptor Antagonist Decreased the Endogenous Opiate Peptide Concentrations in the Spinal Cord in a Dose-Dependent Manner
Administration of 100 ng cypotolamine (a serotonin receptor antagonist) into the spinal cord decreased L-Ek concentration in the spinal cord from 8.4 ± 2.1 pg/mg (tissue weight) to 1.8 ± 0.7 pg/mg in 5 min (P < 0.001), 2.9 ± 1.3 pg/mg in 10 min (P < 0.001) and 5.1 ± 1.3 pg/mg in 20 min (P < 0.01); Administration of 50 ng cypotolamine into the spinal cord decreased L-Ek concentration in the spinal cord from 8.4 ± 2.1 pg/mg (tissue weight) to 3.4 ± 1.1 pg/mg in 5 min (P < 0.001), 5.5 ± 1.2 pg/mg in 10 min (P < 0.05) and 7.7 ± 2.0 pg/mg in 20 min; In control group, L-Ek concentration did not changed (Figure 3(a)).
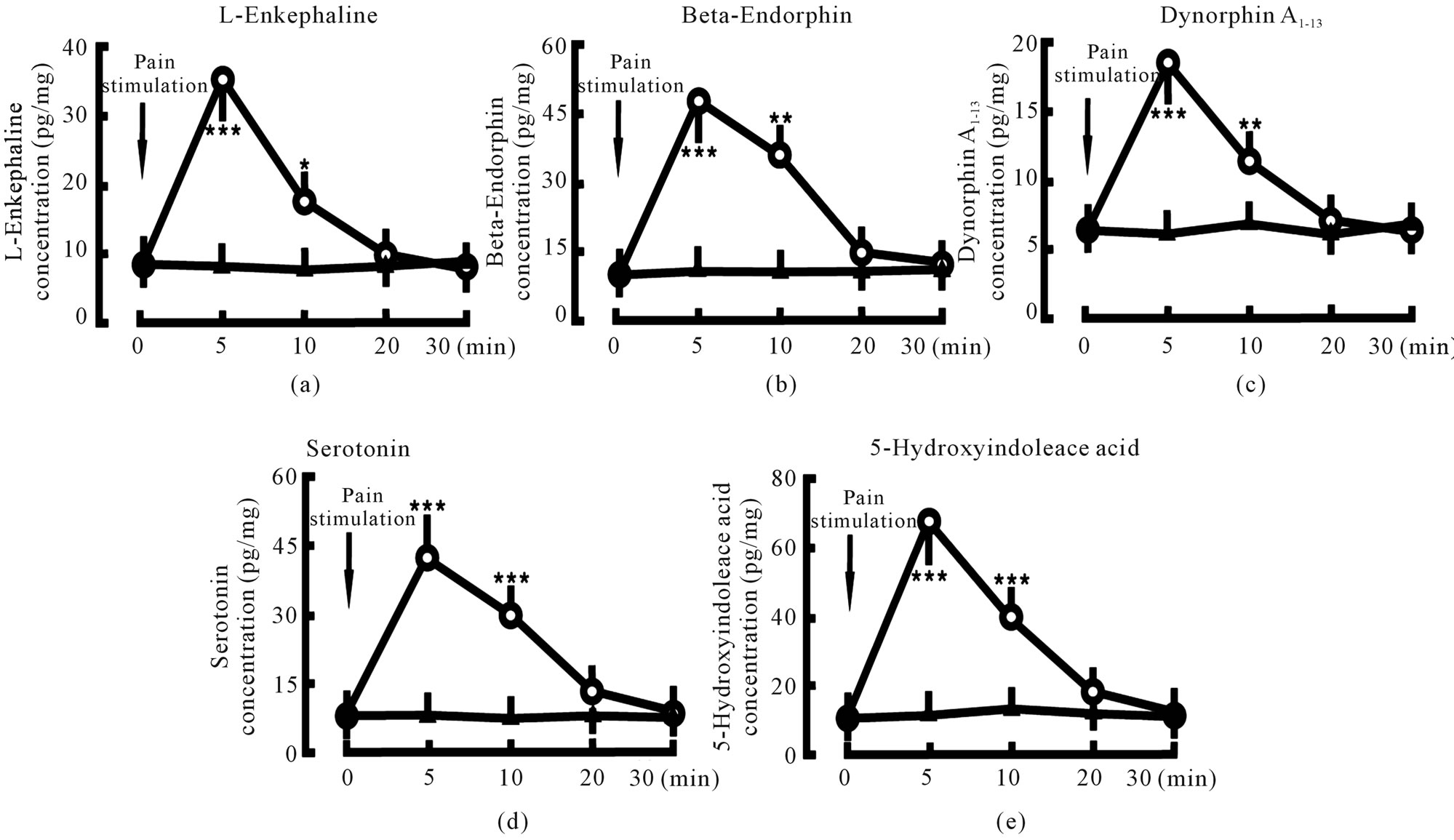
Figure 1. Effect of pain stimulation on endogenous opiate peptide and serotonin concentrations in spinal cord. Pain stimulation denotes the beginning of pain stimulation. Pain stimulation group (○, n = 8): the animal was given 1 min pain stimulation; Control group (▲, n = 8): the animal was given the sham treatment. N indicates the animal number in each group. The data are expressed as mean ± standard error mean (SEM). *P < 0.05, **P < 0.01 and ***P < 0.001 are used for the comparison of the change of L-enkephaline, β-endorphin, dynorphin A1-13, serotonin or 5-hydroxyindoleace acid concentration from pain stimulation group and control group.
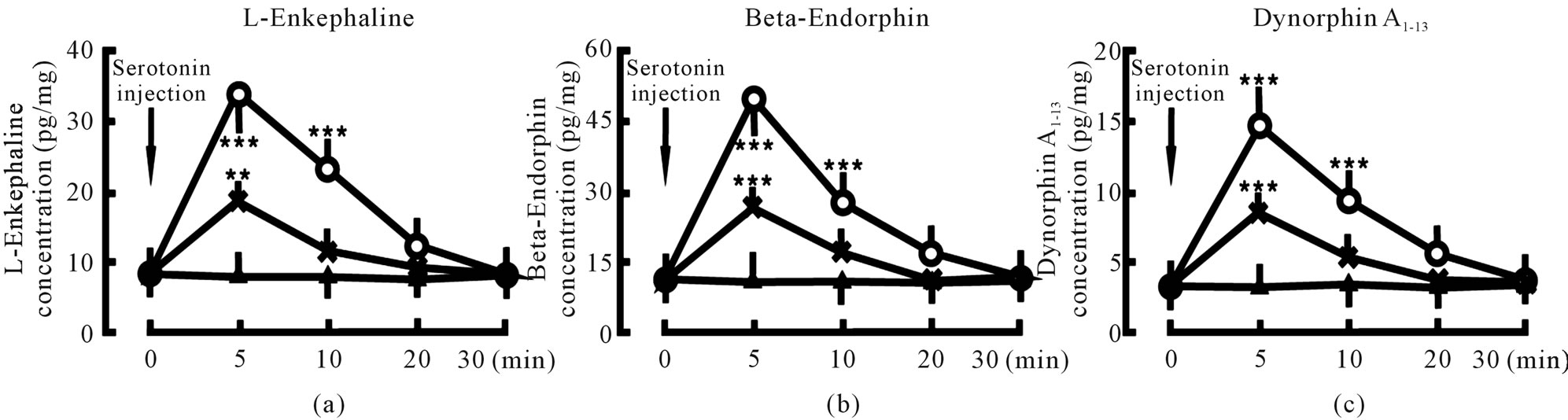
Figure 2. Effect of serotonin on endogenous opiate peptide concentration in spinal cord. Serotonin injection denotes the beginning of serotonin administration. Serotonin 20 ng group (○, n = 8): the animal was given 20 ng serotonin into the spinal cord; Serotonin 10 ng group (×, n = 8): the animal was given 10 ng serotonin into the spinal cord; Control group (▲, n = 8): the animal was not given serotonin into the spinal cord. The data are expressed as mean ± standard error mean (SEM). **P < 0.01 and ***P < 0.001 are used for the comparison of the change of L-enkephaline, β-endorphin or dynorphin A1-13 concentration from serotonin 20 ng group or serotonin 10 ng group and control group.
Administration of 100 ng cypotolamine into the spinal cord decreased β-Ep concentration in the spinal cord from 10.6 ± 2.7 pg/mg (tissue weight) to 3.2 ± 1.0 pg/mg in 5 min (P < 0.001), 5.9 ± 1.1 pg/mg in 10 min (P < 0.001) and 8.7 ± 2.1 pg/mg in 20min (P < 0.01); Administration of 50 ng cypotolamine into the spinal cord decreased β-Ep concentration in the spinal cord from 10.6 ± 2.7 pg/mg (tissue weight) to 5.6 ± 1.2 pg/mg in 5 min (P < 0.001), 7.3 ± 1.3 pg/mg in 10 min (P < 0.01) and 9.1 ± 2.2 pg/mg in 20 min; In control group, β-Ep concentration did not changed (Figure 3(b)).
Administration of 100 ng cypotolamine into the spinal cord decreased DynA1-13 concentration in the spinal cord from 6.5 ± 1.8 pg/mg (tissue weight) to 1.2 ± 0.4 pg/mg

Figure 3. Effect of serotonin receptor inhibitor—cypotolamine on endogenous opiate peptide concentration in spinal cord. Cypotolamine injection denotes the beginning of cypotolamine administration. Cypotolamine 100 ng group (○, n = 8): the animal was given 100 ng cypotolamine into the spinal cord; Cypotolamine 50 ng group (×, n = 8): the animal was given 50 ng cypotolamine into the spinal cord; Control group (▲, n = 8): the animal was not given cypotolamine into the spinal cord. The data are expressed as mean ± standard error mean (SEM). *P < 0.05, **P < 0.01 and ***P < 0.001 are used for the comparison of the change of L-enkephaline, β-endorphin or dynorphin A1-13 concentration from cypotolamine 100 ng group or cypotolamine 50 ng group and control group.
in 5 min (P < 0.001), 2.9 ± 1.3 pg/mg in 10 min (P < 0.001) and 4.2 ± 1.4 pg/mg in 20 min (P < 0.01); Administration of 50 ng cypotolamine into the spinal cord decreased DynA1-13 concentration in the spinal cord from 6.5 ± 1.8 pg/mg (tissue weight) to 3.1 ± 1.0 pg/mg in 5 min (P < 0.001), 4.8 ± 1.3 pg/mg in 10 min (P < 0.01) and 5.8 ± 1.4 pg/mg in 20 min; In control group, DynA1-13 concentration did not changed (Figure 3(c)).
4. Discussions
Serotonin is an important neurotransmitter to regulate the pain process in the spinal cord through 5-HT receptor. 5-HT1/2A/2C and 5-HT1/2C receptors increases the descending facilitation mechanisms induced by incision in the ipsilateral paw; 5-HT2A/3 receptors contribute to descending pronociceptive pathways conveyed by lamina X spinal neurons [19]. The descending serotonergic pathways and spinal 5-HT7 receptors play a crucial role in the antinociceptive effects [20].
Endogenous opiate peptide system is involved in the serotonin effecting pain modulation. Endogenous opiate peptide may partly act as a necessary mediator for the serotonin-induced suppression on the spinal transmission of nociceptive input [21,22]. Some studies have pointed that endogenous opiate peptide is involved in serotoninproduced antinociception at the spinal level using the tail-flick assay, which effect may be mediated through different types of opiate receptors [14]. Combination of serotonin and δ-selective opiates is more effective in suppressing noxiously evoked activity than combinations with μ-selective opiates [23]. The present study showed that 1) pain stimulation increased not only L-Ek, β-Ep, DynA1-13 concentrations but also serotonin and 5-HIAA concentrations in the spinal cord significantly; 2) serotonin increased L-Ek, β-Ep and DynA1-13 concentrations in the spinal cord in a dose-dependent manner; 3) cypotolamine, a serotonin receptor antagonist decreased L-Ek, β-Ep and DynA1-13 concentrations in the spinal cord. The data suggested that the antinociceptive role of serotonin at spinal level was relating with the endogenous opiate peptide system through serotonin receptors. Our previous study has shown that pain stimulation can influence the endogenous opiate peptide and serotonin system in the nucleus raphe magnus [24].
Chronic nerve injury evoked hypernociception may be contributed by genetic differences of descending serotonergic inhibitory control [25]. Opiates mediates their stimulatory effects on stimulate prolactin release, at least in part, through a serotonergic mechanism in adult rats [26]. Serotonin may influence the gene expression or peptide synthesis process to act the endogenous opiate peptide system in spinal cord. However, it needs to be studied in the near further.
In conclusion, the present study makes it clear that 1) the spinal cord releases endogenous opiate peptides and serotonin during pain process; 2) serotonin enhances, whereas the serotonin receptor antagonist inhibits the spinal cord release of endogenous opiate peptides. The data indicated that the antinociceptive role of serotonin at spinal level was relating with endogenous opiate peptide system via serotonin receptors.
5. Acknowledgements
This work was supported by Xinxiang Medical University, Subei People’s Hospital of Jiangsu Province and grants from National Natural Science Foundation of China (81100956/H0912).
REFERENCES
- D. J. Walther, J. U. Peter, S. Bashammakh, H. Hörtnagl, M. Voits, H. Fink and M. Bader, “Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform,” Science, Vol. 299, 2003, p. 76. doi:10.1126/science.1078197
- H. G. Lee, W. M. Kim, C. H. Park and M. H. Yoon, “Roles of Adenosine and Serotonin Receptors on the Antinociception of Sildenafil in the Spinal Cord of Rats,” Yonsei Medical Journal, Vol. 51, No. 6, 2010, pp. 960- 964. doi:10.3349/ymj.2010.51.6.960
- Z. Song, C. Ultenius, B. A. Meyerson and B. Linderoth, “Pain Relief by Spinal Cord Stimulation Involves Serotonergic Mechanisms: An Experimental Study in a Rat Model of Mononeuropathy,” Pain, Vol. 147, No. 1, 2009, pp. 241-248. doi:10.1016/j.pain.2009.09.020
- F.-Y. Liu, X.-X. Qu, X. Ding, J. Cai, H. Jiang, Y. Wan, J.-S. Han and G.-G. Xing, “Decrease in the Descending Inhibitory 5-HT System in Rats with Spinal Nerve Ligation,” Brain Research, Vol. 1330, 2010, pp. 45-60. doi:10.1016/j.brainres.2010.03.010
- J. Hughes, T. W. Smith, H. W. Kosterlitz, L. A. Fothergill, B. A. Morgan and H. R. Morris, “Identification of Two Related Pentapeptides from the Brain with Potent Opiate Agonist Activity,” Nature, Vol. 258, 1975, pp. 577-580. doi:10.1038/258577a0
- L. H. Lazarus, N. Ling and R. Guillemin, “β-Lipotropin as a Prohormone for the Morphinomimetic Peptides Endorphins and Enkephalins,” Proceedings of the National Academy of Sciences, Vol. 73, No. 6, 1976, pp. 2156-2159. doi:10.1073/pnas.73.6.2156
- A. Goldstenin, S. Tachibana, L. I. Lowney, M. Hunkapiller and L. Hood, “Dynorphin-(1-13), an Extraordinarily Potent Opioid Peptide,” Proceedings of the National Academy of Sciences of the USA, Vol. 76, No. 12, 1979, pp. 6666-6670. doi:10.1073/pnas.76.12.6666
- M. J. Millan and A. Herz, “The Endocrinology of the Opioids,” International Review of Neurobiology, Vol. 26, 1985, pp. 1-83. doi:10.1016/S0074-7742(08)60072-0
- R. J. Bodnar, “Endogenous Opiates and Behavior: 2009,” Peptides, Vol. 31, No. 12, 2010, pp. 2325-2359. doi:10.1016/j.peptides.2010.09.016
- A. Herz and M. J. Millan, “Opioids and Opioid Receptors Mediating Antinociception at Various Levels of the Neuraxis,” Physiol Bohemoslov, Vol. 39, No. 5, 1990, pp. 395-401.
- J. P. Rosenfeld, “Interacting Brain Stem Components of Opiate-Activated, Descending, Pain-Inhibitory Systems,” Neuroscience & Biobehavioral Reviews, Vol. 18, No. 3, 1994, pp. 403-409. doi:10.1016/0149-7634(94)90053-1
- J. A. Stamford, “Descending Control of Pain,” British Journal of Anaesthesia, Vol. 75, 1995, pp. 217-227.
- Z.-Q. Zhao, “Neural Mechanism Underlying Acupuncture Analgesia,” Progress in Neurobiology, Vol. 85, No. 4, 2008, pp. 355-375. doi:10.1016/j.pneurobio.2008.05.004
- S.-W. Yang, Z.-H. Zhang, R. Wang, Y.-F. Xie, J.-T. Qiao and N. Dafny, “Norepinephrine and Serotonin-Induced Antinociception Are Blocked by Naloxone with Different Dosages,” Brain Research Bulletin, Vol. 35, No. 2, 1994, pp. 113-117. doi:10.1016/0361-9230(94)90090-6
- T. Crisp, J. L. Stafinsky, J. E. Hess and M. Uram, “Spinal β-Endorphin Analgesia Involves an Interaction with Local Monoaminergic Systems,” European Journal of Pharmacology, Vol. 160, No. 2, 1989, pp. 211-217. doi:10.1016/0014-2999(89)90493-7
- M. O. Carruba, E. Nisoli, V. Garosi, P. Sacerdote, A. E. Panerai and M. da Prada, “Catecholamine and Serotonin Depletion from Rat Spinal Cord: Effects on Morphine and Footshock Induced Analgesia,” Pharmacological Research, Vol. 25, No. 2, 1992, pp. 187-194. doi:10.1016/1043-6618(92)91387-V
- G. González-Mariscal, P. Gómora and C. Beyer, “Participation of Opiatergic, GABAergic, and Serotonergic Systems in the Expression of Copulatory Analgesia in Male Rats,” Pharmacology Biochemistry and Behavior, Vol. 49, No. 2, 1994, pp. 303-307. doi:10.1016/0091-3057(94)90425-1
- M. Zimmermann, “Ethical Guidelines for Investigations of Experimental Pain in Conscious Animal,” Pain, Vol. 16, No. 2, 1983, pp. 109-110. doi:10.1016/0304-3959(83)90201-4
- J. W. Silveira, Q. M. Dias, E. A. Del Bel and W. A. Prado, “Serotonin Receptors Are Involved in the Spinal Mediation of Descending Facilitation of Surgical Incision-Induced Increase of Fos-Like Immunoreactivity in Rats,” Molecular Pain, Vol. 6, 2010, p. 17. doi:10.1186/1744-8069-6-17
- O. Yanarates, A. Dogrul, V. Yildirim, A. Sahin, A. Sizlan, M. Seyrek, O. Akgül, O. Kozak, E. Kurt and U. Aypar, “Spinal 5-HT7 Receptors Play an Important Role in the Antinociceptive and Antihyperalgesic Effects of Tramadol and Its Metabolite, O-Desmethyltramadol, via Activation of Descending Serotonergic Pathways,” Anesthesiology, Vol. 112, No. 3, 2010, pp. 696-710. doi:10.1097/ALN.0b013e3181cd7920
- Y.-J. Li, Z.-H. Zhang, J.-Y. Chen and J.-T. Qiao, “Effects of Intrathecal Naloxone and Atropine on the Nociceptive Suppression Induced by Norepinephrine and Serotonin at the Spinal Level in Rats,” Brain Research, Vol. 666, No. 1, 1994, pp. 113-116. doi:10.1016/0006-8993(94)90290-9
- A. H. Lichtman and M. S. Fanselow, “Opioid and Nonopioid Conditional Analgesia: The Role of Spinal Opioid, Noradrenergic, and Serotonergic Systems,” Behavioral Neuroscience, Vol. 105, No. 5, 1991, pp. 687-698. doi:10.1037/0735-7044.105.5.687
- K. Nakatani, L. M. Kitahata, Y. Harada, K. Omote, J. G. Collins and O. Yuge, “Interaction between Opiate Subtypes and Serotonin in Suppressing Noxiously Evoked Activity of WDR Neurons,” Nihon Masui Igakai, Vol. 44, No. 6, 1995, pp. 795-799.
- J. Yang, H. F. Yuan, J. G. Chu, Y. Yang, H. T. Xu, G. Wang, W. Y. Liu and B. C. Lin, “Arginine Vasopressin Antinociception in the Rat Nucleus Raphe Magnus Is Involved in the Endogenous Opiate Peptide and Serotonin System,” Peptides, Vol. 30, No. 7, 2009, pp. 1355-1361. doi:10.1016/j.peptides.2009.03.014
- N. Wijnvoord, B. Albuquerque, A. Häussler, T. Myrczek, L. Popp and I. Tegeder, “Inter-Strain Differences of Serotonergic Inhibitory Pain Control in Inbred Mice,” Molecular Pain, Vol. 6, 2010, p. 70. doi:10.1186/1744-8069-6-70
- L. A. Bero and C. M. Kuhn, “Role of Serotonin in Opiate-Induced Prolactin Secretion and Antinociception in the Developing Rat,” Journal of Pharmacology Exprimental Therapeutics, Vol. 240, No. 3, 1987, pp. 831-836.
NOTES
*Corresponding author.

