World Journal of Cardiovascular Diseases
Vol.3 No.4A(2013), Article ID:34417,12 pages DOI:10.4236/wjcd.2013.34A004
Coronary artery bypass grafting in diabetic patients: Should we still use the saphenous vein graft? A review of literature in the past 15 years
![]()
1Dipartimento Cardio-Nefro-Polmonare, UO Di Cardiochirurgia, Azienda Ospedaliero-Universitaria di Parma, Parma, Italy
2Sezione di Cardiochirurgia, Dipartimento di Medicina Clinica e Sperimentale, Università degli Studi di Parma, Parma, Italy
Email: alberto.molardi@gmail.com
Copyright © 2013 Alberto Molardi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 23 May 2013; revised 23 June 2013; accepted 5 July 2013
Keywords: Total Arterial Revascularization; Coronary Artery Bypass Grafting; Diabetes; Review
ABSTRACT
The burden of diseases associated with diabetes mellitus is dramatic: adults with diabetes mellitus are 2 to 4 times more likely to have cardiovascular diseases than those without it, and at least 65% will die because of diabetes complications. The revascularization strategy in these types of patients included percutaneous coronary interventions with bare metal stents or medicated stents and surgical coronary artery bypass grafting (CABG), but it is well known that in the diabetic patient with two or more vessel disease, the surgical strategy allows the best midand longterm results. Moreover, benefits of CABG surgery are limited by life expectancy of the most common type of graft, the saphenous vein (SV). Nearly 40 years after the introduction of bypass surgery, the rate of vein graft failure remains at high levels. Several arterial conduits had been studied as alternative conduits to SV: the Right Internal Thoracic Artery (RITA), the Radial Artery (RA), the Gastroepiploic Artery (GEA) and the Inferior Epigastric Artery (IEA), 40 years ago. The aim of our article is to review the scientific literature of the past 15 years to answer this question: are we ready to treat the diabetic patient, with a completely arterial revascularization, avoiding the use of the great saphenous vein grafts?
1. INTRODUCTION
The burden of diseases associated with diabetes mellitus is dramatic: adults with diabetes mellitus are 2 to 4 times more likely to have cardiovascular diseases than those without it, and at least 65% will die because of diabetes complications [1].
The national burden of cardiovascular diseases caused by diabetes mellitus is increasing, at an unprecedented rate in all western countries [1,2].
Cardiovascular disease in diabetics is clinically challenging because they tend to be extensive with multivessel involvement [3,4].
It is why surgical revascularization has been reported to be well suited for diabetic patients [5,6].
Typically, the elderly are the patients waiting for coronary artery bypass grafting (CABG) surgery with diabetes mellitus. Female gender is increasing in the diabetic population. Diabetic patients often have a history of hypertension and myocardial infarction. Frequently, they have manifestations of congestive heart disease, resulting in NYHA classification III-IV. It has been shown that diabetic patients have a smaller vessel diameter [7], and statistically they have three-vessel disease more frequently and a lower left ventricular ejection fraction [8, 9].
The revascularization strategy in these types of patients included percutaneous coronary interventions with bare metal stents or medicated stents and surgical coronay artery bypass grafting, but it’s well known that in the diabetic patient with two or more vessel disease, the surgical strategy allows the best midand long-term results [10].
Moreover, the benefits of CABG surgery remain limited by the life expectancy of the most common type of graft, the saphenous vein. Nearly 40 years after the introduction of bypass surgery, the rate of vein graft failure remains at high levels [11].
The introduction of the left internal thoracic artery (LITA) graft radically changed the long term patency and survival of patients who underwent CABG [12]. This conduit has shown a remarkable patency rate in several long-term series all around the world. So the introduction of the first arterial conduit has opened to the research of others arterial conduits which could be used with the same results of the LITA.
Several arterial conduits had been studied as alternative ones to saphenous vein (SV): the right internal thoracic artery (RITA), the Radial Artery (RA), the gastroepiploic artery (GEA) and the inferior epigastric artery (IEA) [13].
The aim of our article is to review the scientific literature of the past 15 years to answer to this question: are we ready to treat the diabetic patient with a completely arterial revascularization, avoiding the use of the great saphenous vein grafts?
2. MATERIALS AND METHODS
Our search strategy was: Medline 2002 to 2013 using pubmed interface (total arterial) and (CABG) and (diabetes).
More than 35 articles were found; from these 24 articles were identified as providing the best evidence to answer the question (Table 1).
3. RESULTS
3.1. The Bilateral Internal Mammary Artery (BIMA) and the Multiple Internal Mammary Artery (MIMA)
RITA possesses the same molecular and cellular characteristics that contribute to a unique resistance to atherosclerosis and extremely high long term patency rates as the LITA. The vasoactivity of this arterial conduit has also well characterized: the RITA produces significantly more prostaciclin, a vasodilator and platelet inhibitor, than the saphenous vein and as well as the LITA. The harvest technique is similar to the LITA and it has been proposed as a skeletonized or pedicled graft for the proximal right coronary artery (RCA) or as a skeletonized or pedicled free graft for the branches of the left coronary artery (LCA) as a Y graft anastomosed to the LITA or an another arterial graft as the radial artery (RA) or a venous graft [14].
Both conduits used as a direct graft or a Y graft seem to have a positive effect on the outcome of diabetic patient undergoing CABG procedures.
The practice of using multiple arterial conduits for CABG is supported by reports showing their early operative morbidity and mortality results to be equivalent or better than for CABG with a single arterial graft [15-17].
Formica, et al. and Dorman, et al. founded a 12 to 15 years improvement in the late outcome of patients who underwent CABG with the use of BIMA [2,18].
Apparently, none of the surgical techniques utilized to perform operations demostrated a superiority in terms of graft patency, in-hospital and late mortality and morbidity [2,19-21].
LITA and RITA have been used as peduncolated or skeletonized graft, with direct anastomosis on the target vessels or in composite grafts (Y graft), used to perform the revascularization of a single coronary branch or more coronary branches [19,21] but the type of utilized surgical technique didn’t influence the outcomes of the surgical therapy.
Dorman, et al., Rankin, et al., Nasso, et al., Parsa, et al., and Loker, et al. emphasized how the use of a second arterial graft is sufficient to improve the long term quality of life of the patient and their rate of cardiac related mortality and morbidity [2,19-22].
In all these studies, the incidence of surgical wound infections and mediastinitis is comparable to non-diabetic patients.
Only one study has reported data in contrast to those listed above: Saito, et al. [23] compared the use of SIMA versus BIMA in a large sample of 7702 cases with a propensity matched analysis and he found that the use of BIMA did not affect either short-term survival as postoperative mortality was low in both groups or overall morbidity despite higher incidence of deep sternal infection. This study has the limitation of a 30 days post-intervention follow up and the finding of an higher rate of wound infection is not reported in other propensity matched studies [2,22,24,25].
3.2. The Radial Artery (RA)
The use of a radial artery as a conduit for coronary artery bypass grafting was first described by Carpentier in 1973 [26]. Early patency rates were poor and the interest in the use of this conduit faded. The radial artery possesses a pronounced medial layer and is highly vasoreactive. Cosmetic concerns have also been cited as a deterrent for radial artery usage.
Surgeons’ interest in radial artery as a graft for CABG has a known revival in the last 20 - 25 years with the improvements of the harvesting techniques [27] and the identification of the pharmacological agents to prevent the graft spasm [13,28].
The long-term outcome after CABG depends on graft patency. Previous angiographic observational studies have shown that the RA achieved excellent short- (96% - 100%), mid- (94% - 97%), and long-term graft patency (84% - 96%) when used as either an aortocoronary bypass or a composite graft [13,29-31].
Patency rates of the RA have exceeded those of SV grafts at all time points and are comparable to other arterial grafts. Many reports have shown better outcomes of the RA compared with the SV [30,32-43], though some
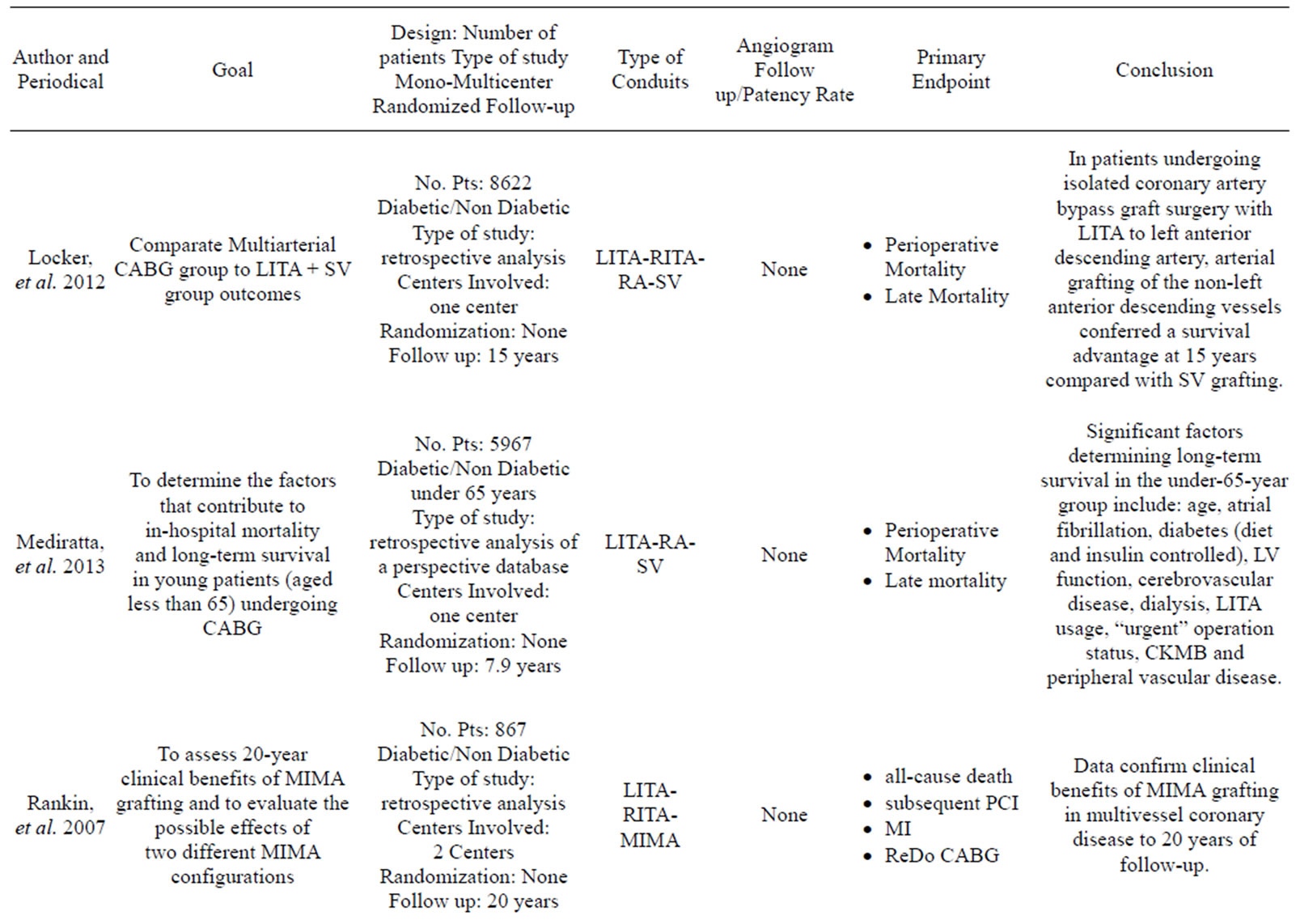
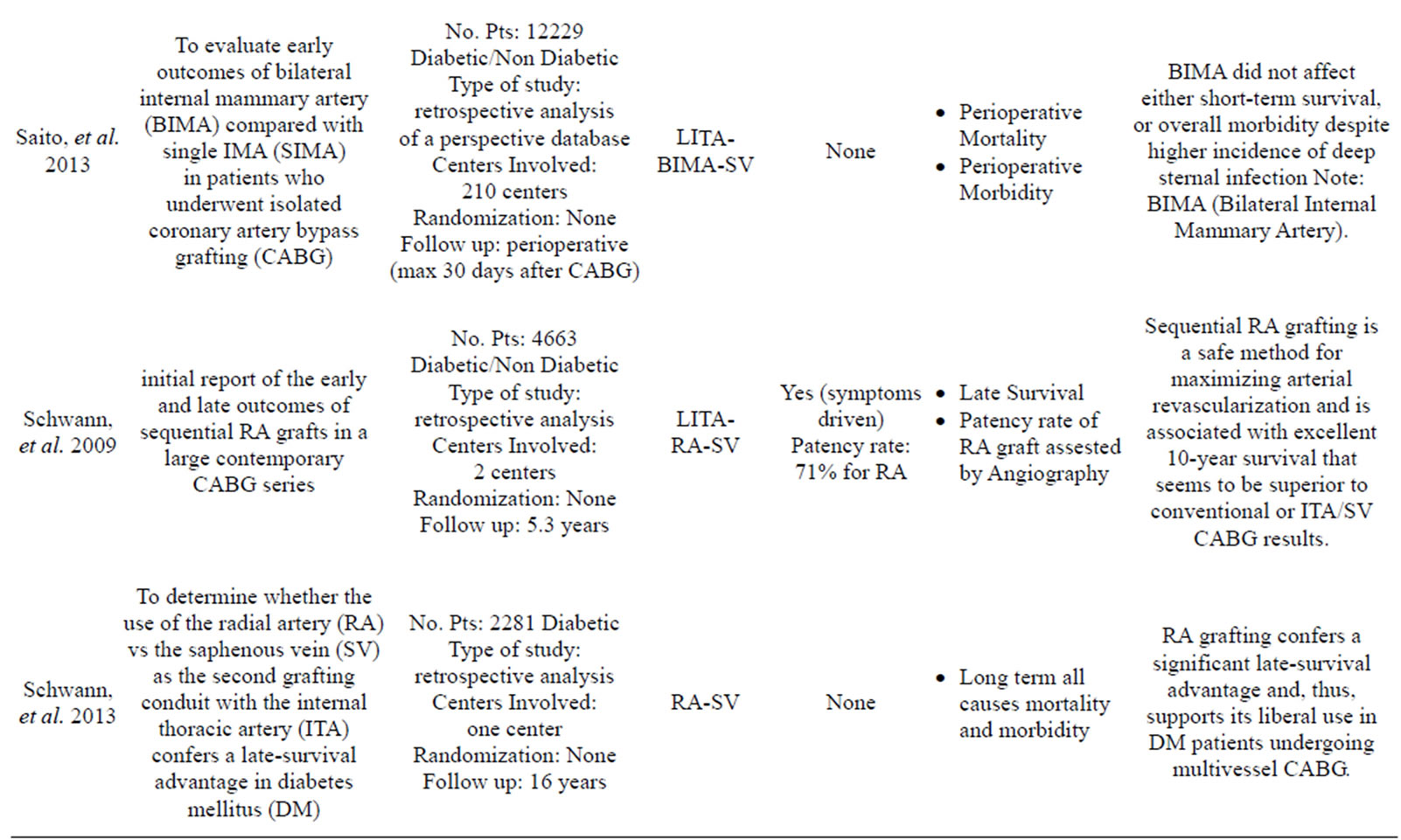
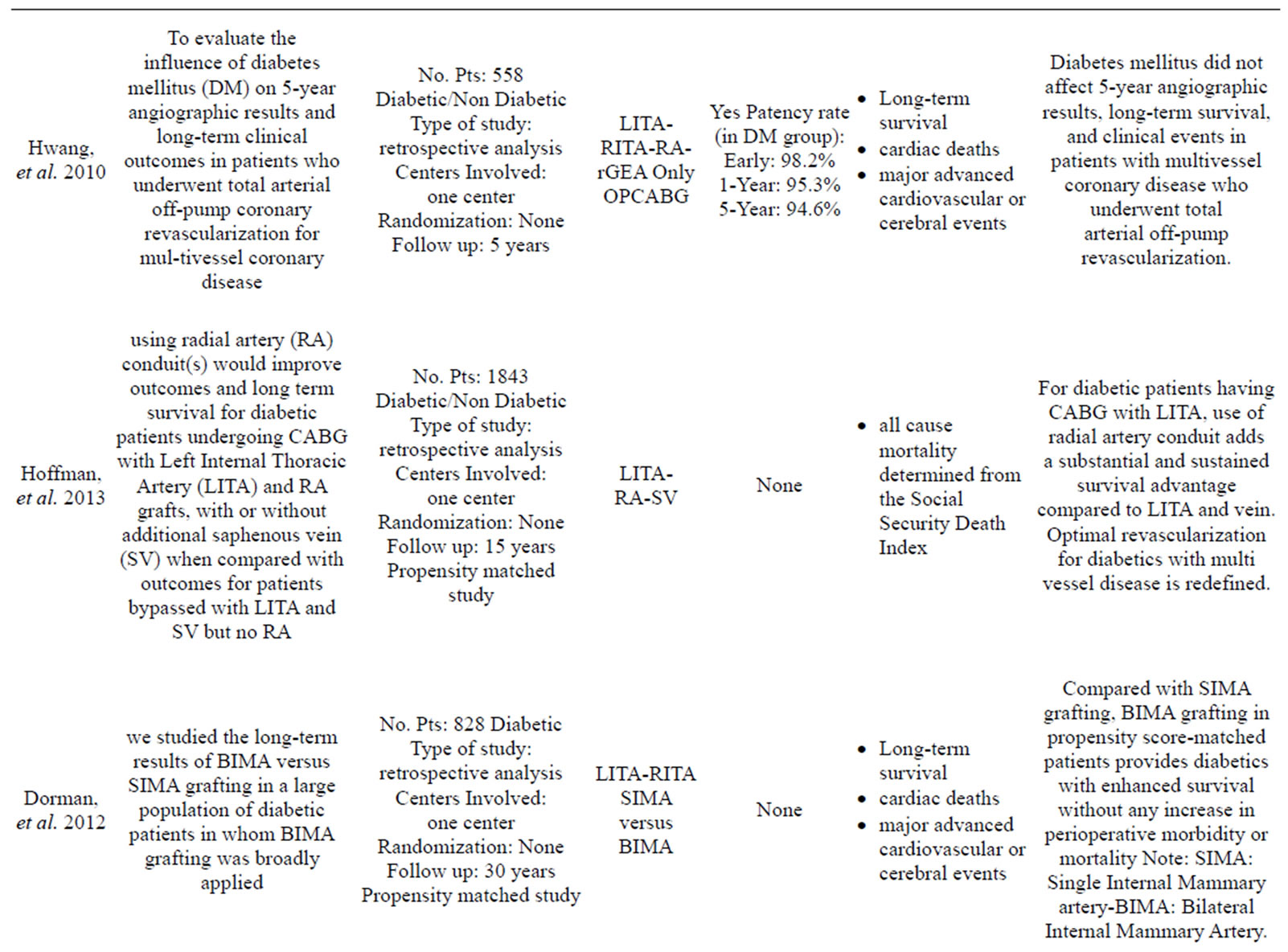
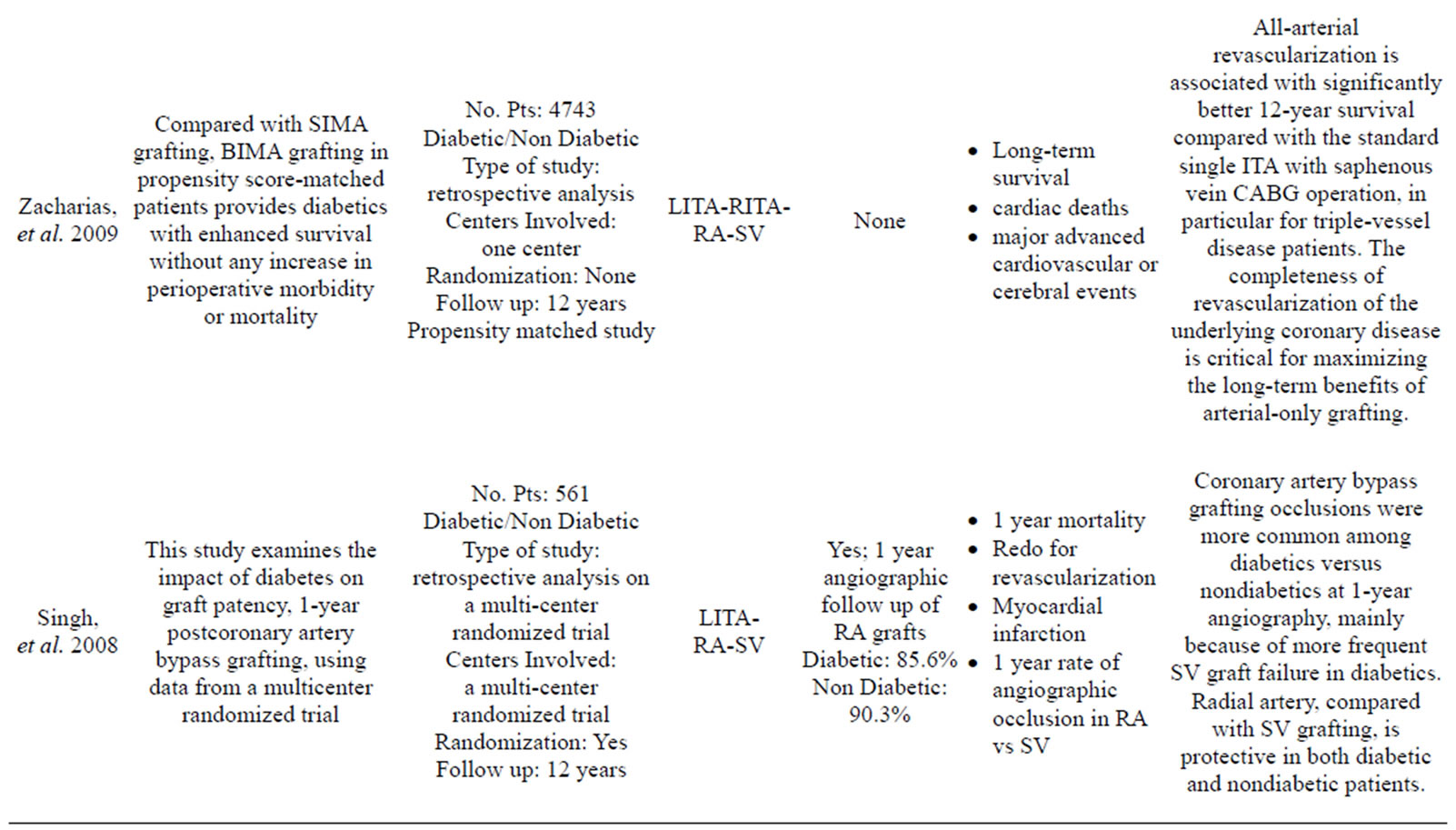
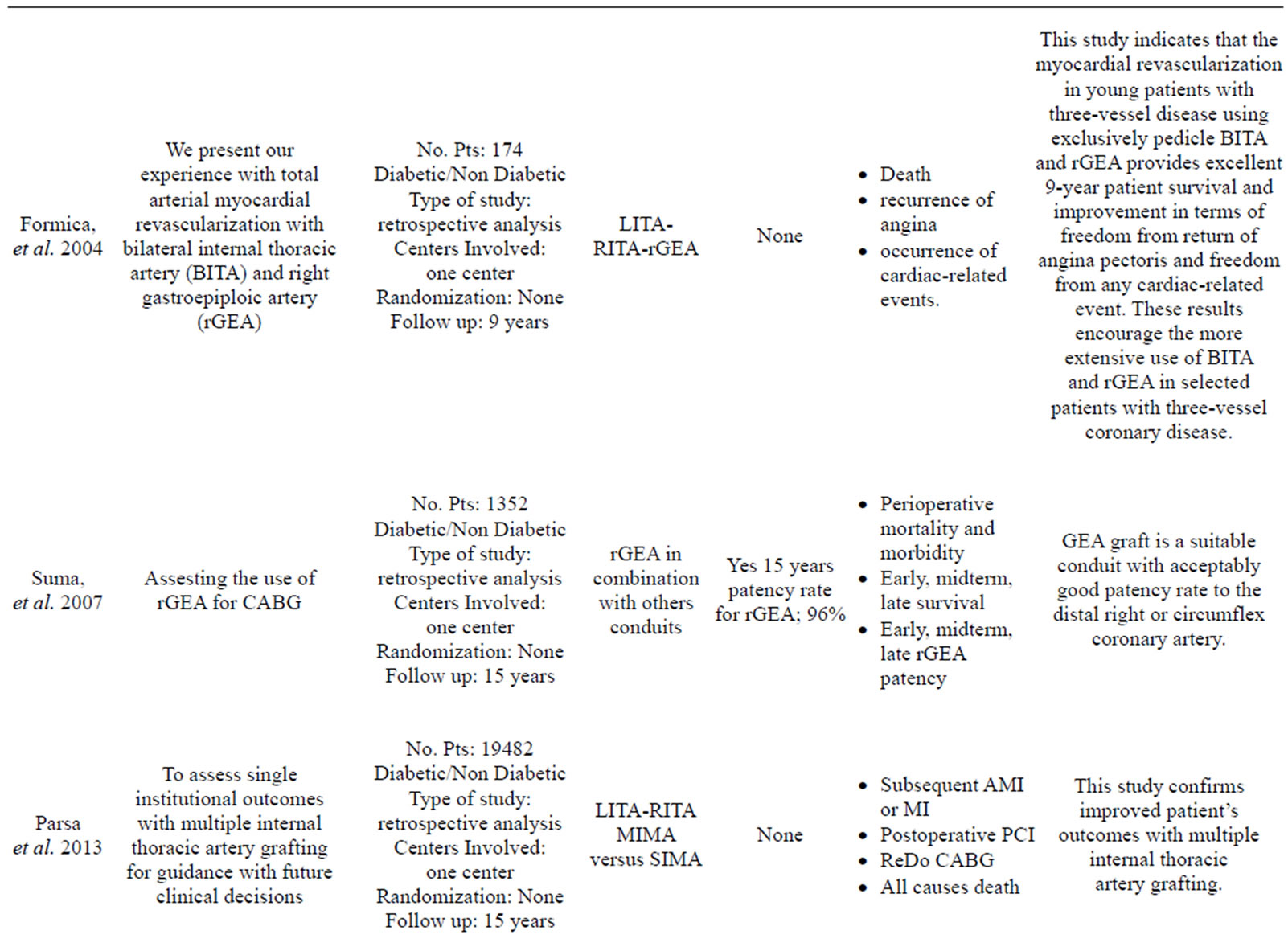
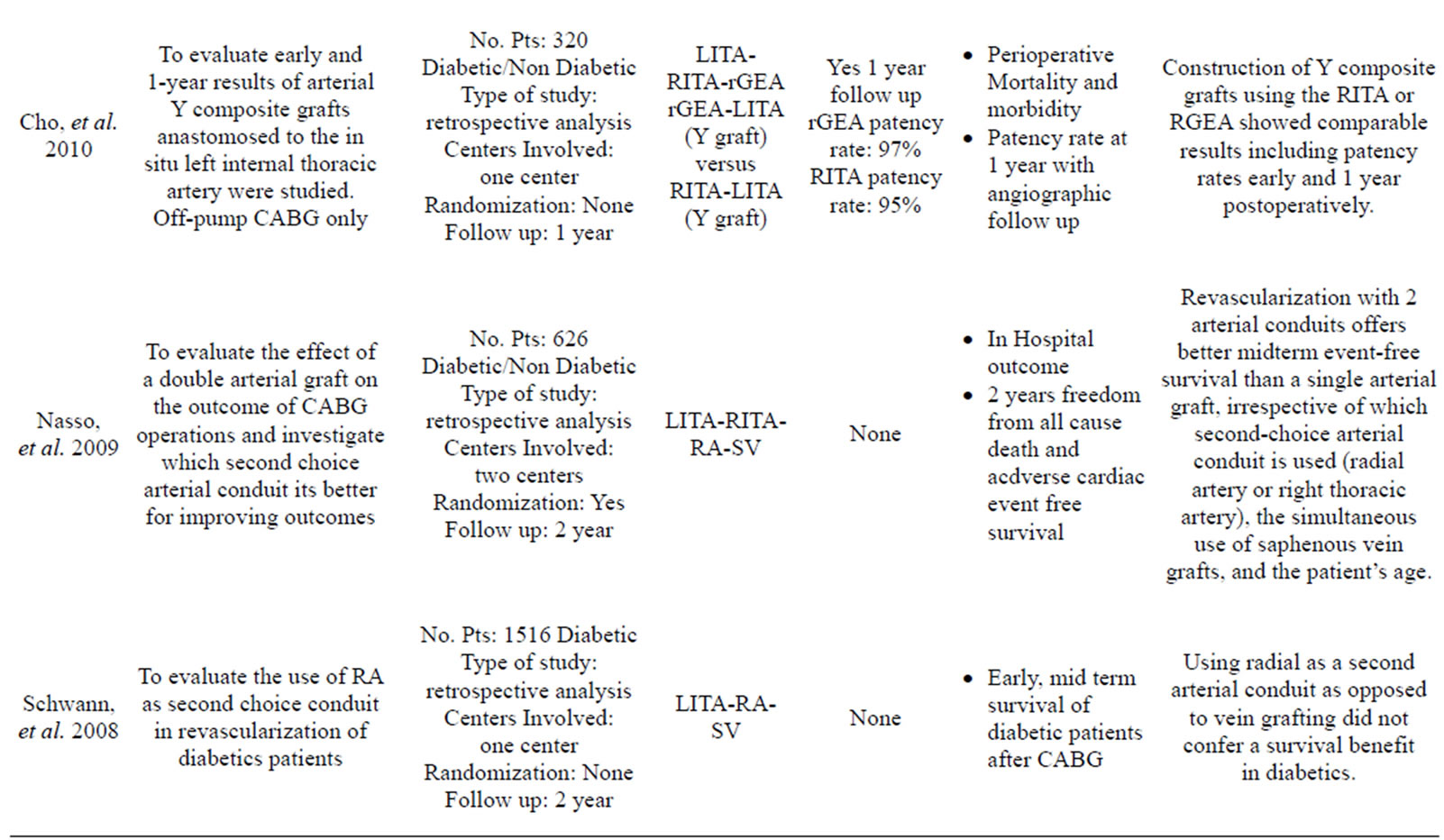
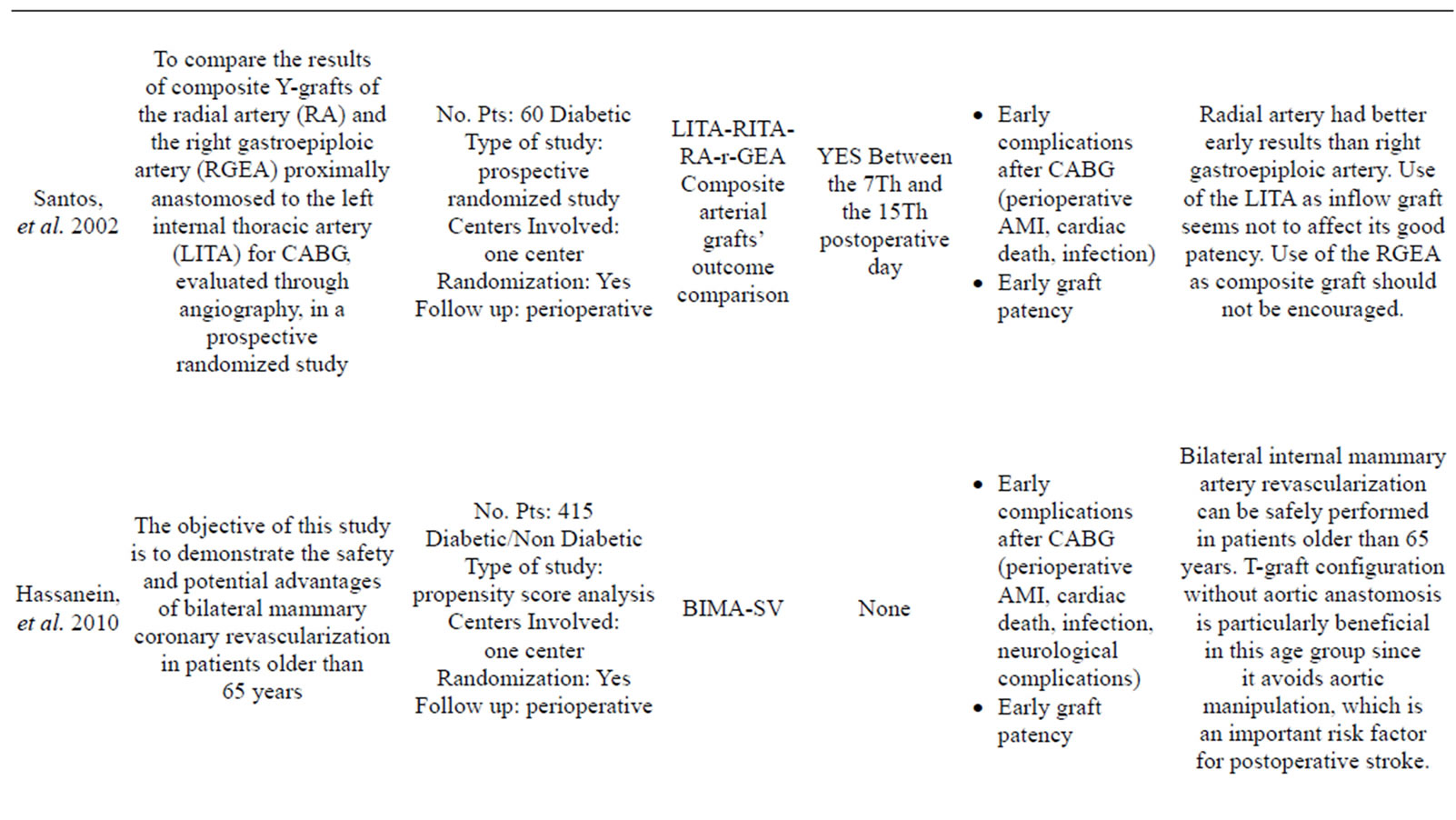
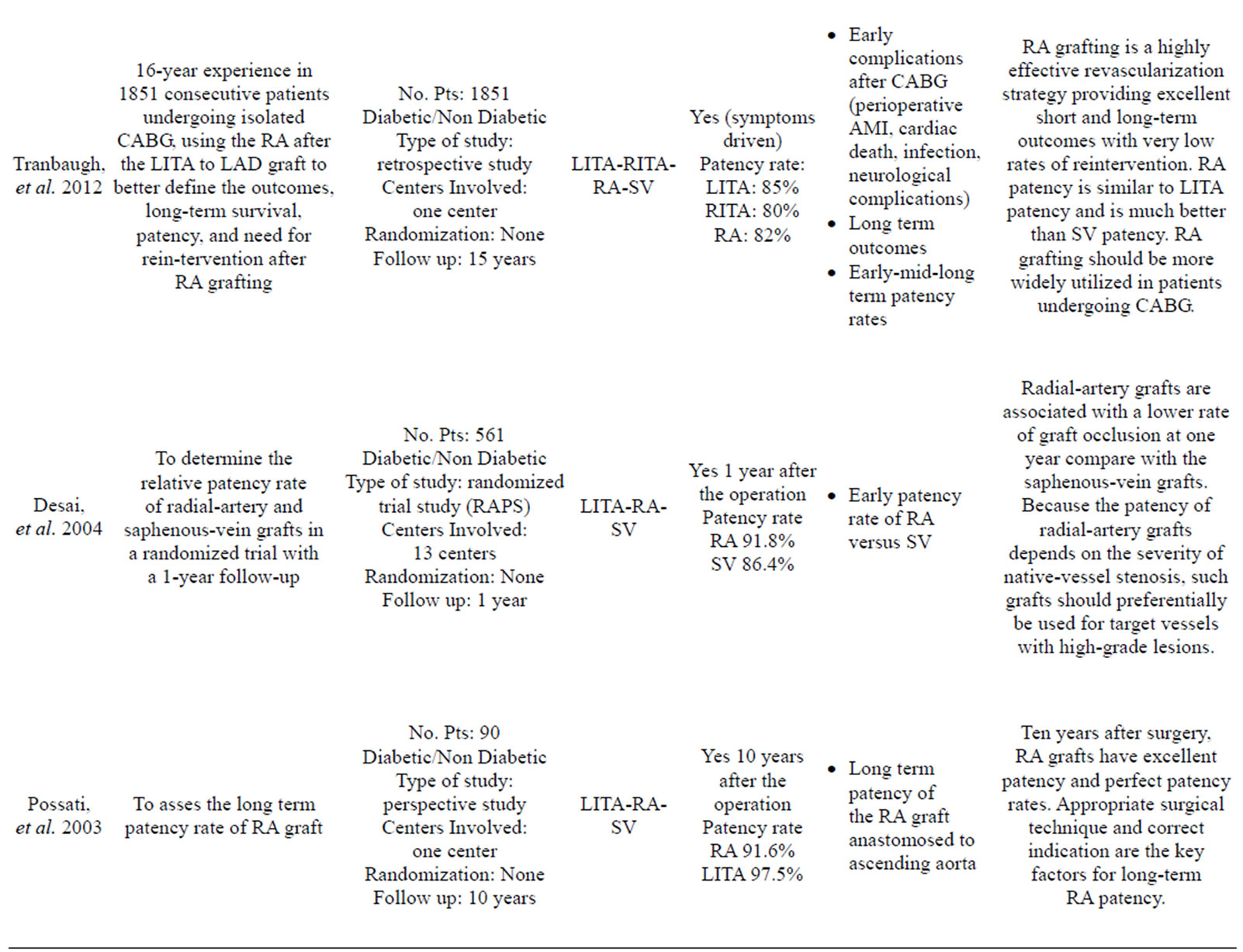
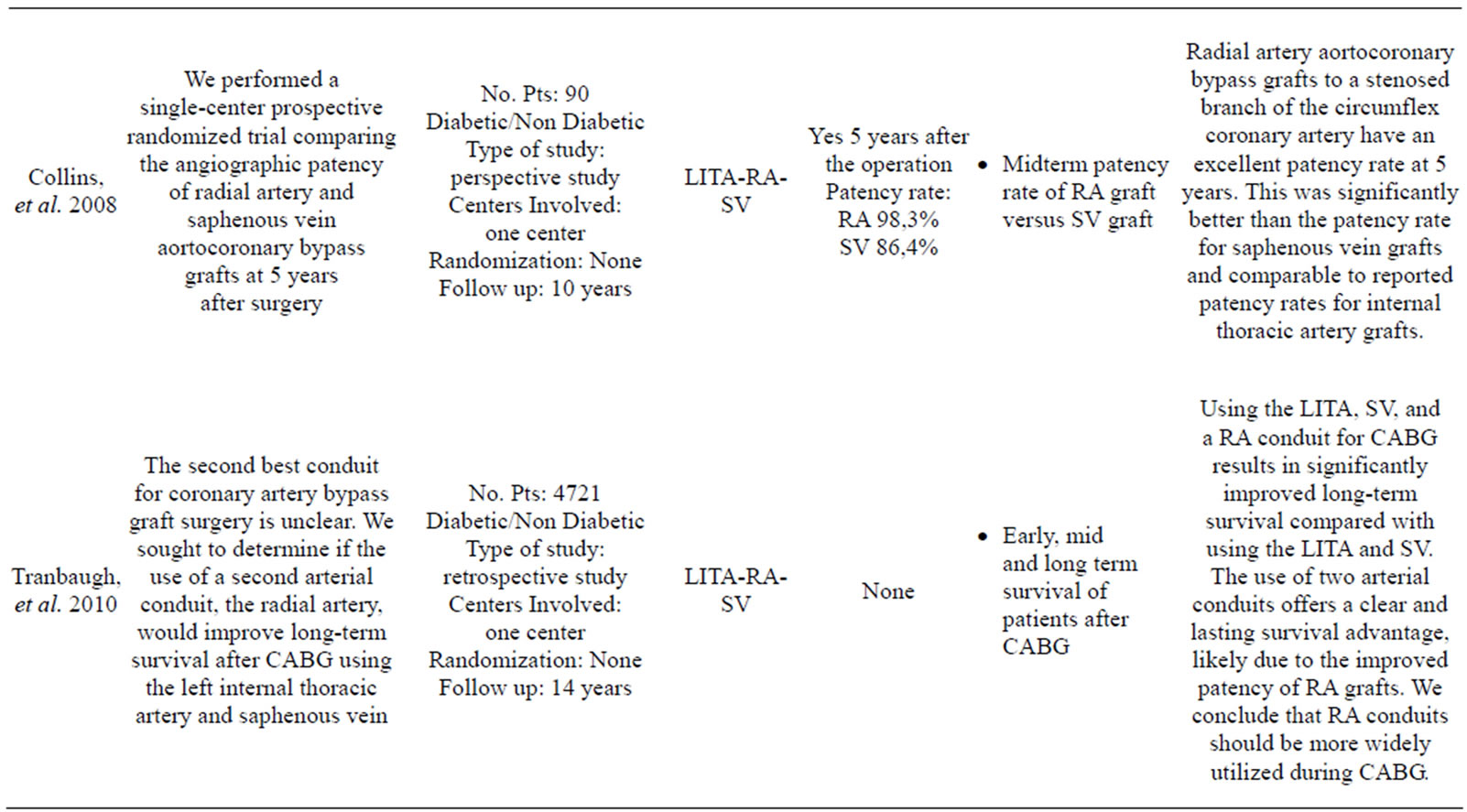
Table 1. List of the selected articles reviewed in our work.
reported similar long-term graft patency [44-47].
Only the Cleveland Clinic reported worse graft patency of the RA than the SV [48].
Possati, et al. reported the long-term (105 ± 9 months) graft patency of RA grafts in a series of 90 consecutive CABG patients [36].
The RA graft patency was 88%, which was less than that of the LITA (96%), but better than that of the SV (53%). Although these results are encouraging the use of the RA as a complementary arterial conduit with the LITA, there are only a few long-term studies assessing RA graft patency in the setting of a randomized controlled trial.
The Radial Artery Patency Study (RAPS) investigators enrolled 561 patients in 13 centers [49]. In this trial, the RA graft was randomly assigned to bypass in either the right coronary territory or the circumflex coronary territory, with the SV graft used for the opposing territory, which had proximal lesions at least 70% of occlusion diameter narrowing. Angiography for 440 RA grafts and 440 SV grafts was performed in 440 patients in 1 year. They reported that 8.2% of RA grafts and 13.6% of SV grafts were completely occluded (P = 0.009). Diffuse narrowing of the graft (string sign) was present in 7.0% of the RA grafts and only 0.9% of SV grafts (P = 0.001). The absence of severe native vessel stenosis was a risk of graft occlusion and diffuse narrowing of the RA conduit (70% - 89% proximal stenosis: 81.7%; >90% proximal stenosis: 91.5%). Patency of the RA grafts was similar in the right coronary and circumflex arteries. These is compatible with previous reports suggesting that the RA should be limited to grafting to native coronary vessels with a high degree of stenosis (>70%) because of graft sensitivity to competitive flow and diffuse narrowing [50,51].
Diffuse narrowing of the RA graft is thought to be of little or no clinical consequence because the narrowed graft may improve or work well late in the follow-up. However, cardiologists consider the string sign of the RA graft as a failure. As the string sign at 1 year is unfavorable toward a functioning RA or SV graft, 15.2% of RA grafts and 14.5% of SV grafts are occluded or functioning poorly. RA grafting should not be considered in the setting of <75% proximal coronary obstruction, especially in the right coronary branches [52].
The RAPS Investigators also reported that diabetes (RR: 1.45, P = 0.03), female gender (RR: 1.78, P = 0.02), and small target vessel diameter (RR: 2.28, P < 0.01) are multivariate predictors of graft failure [53,54].
Graft occlusion was more common among diabetic patients (14% vs 10%) because of more frequent SV occlusion (19%) than RA occlusion (10%). The RA is protective in the small-sized coronary arteries with diffuse diabetic disease. As regards the gender, RA graft occlusion rate at 1 year was similar in men (8.6%) and women (5.3%) (P = 0.6), whereas SV graft occlusion rates were lower in men (12.0%) than in women (23.3%). A history of peripheral vascular disease was associated with an elevated risk of RA occlusion, but not with SV occlusion. On the contrary, angiographic studies of patients at the Cleveland Clinic found poor graft patency in the RA (51%) compared with the SV (64%). As concerns sex, women had significantly worse RA graft patency (39%) than men (56%) [30].
The Radial Artery Patency and Clinical Outcome (RAPCO) study, was undertaken to compare angiographic patency and cardiac-event-free survival of the RA graft with that of the free RITA and SV during a 10-year period after CABG [55].
The RA was compared with the free RITA in patients <70 years of age and with the SV in patients aged >75 years. The 5-year interim results of this single center trial conducted by Buxton, et al. in Australia reported that there were no differences in angiographic graft failure and cardiac events of the patients with the RA compared with the RITA or SV. The 5-year patency rates between the RA and RITA were 95% vs 100%, respectively, and those between the RA and SV were 87% vs 94%. However, these results were based on a small number of angiographic studies, and SV graft patency was very much better than in previous reports. The final results up to 10 years should clarify the long-term RA graft patency.
The Radial Artery Versus Saphenous Vein Graft Patency (RSVP) trial was a single-center, prospective, randomized clinical trial designed to compare 5-year patency rates of RA and SV aortocoronary grafts to the circumflex coronary artery [56].
At 5 years, 103 patients among 142 enrolled patients underwent angiography. The graft patency of the RA (98.3%) was significantly (P = 0.04) better than that of the SV (86.4%). Graft narrowing occurred in 10% of patent RA grafts and 23% of SV grafts (P = 0.01).
In conclusion, despite the poor first outcomes of the RA graft, the progress of harvesting techniques and pharmacological therapy to prevent the radial spasm had lead to re-discover a useful arterial conduit with wide application opportunities expecially on the left coronary branches with patency rates comparable to the RITA’s ones and superior to those of the SV graft.
3.3. The Right Gastroepiploic Artery (rGEA)
The right GEA represents another interesting option as a conduit for CABG. Several studies demonstrated similarities of this conduit with the internal thoracic artery. Of the other possible arterial conduits, the right gastroepiploic artery (r-GEA) has several advantages, such as providing a comparably sized artery-to-artery anastomosis, necessitating no additional incision in the leg or forearm, and histologic similarity to the ITA, which would suggest long-term patency [57,58].
In 1987, Pym and others authors [59], have independently reported their successful clinical application of the GEA graft for CABG, and now over the last 2 decades, basic research and the clinical application of GEA has widely been undertaken. The GEA graft has a high clinical availability for CABG [60], low incidence of arteriosclerosis [61], and sufficient flow capacity [62].
Its biological and physiological activity have been studied extensively [63,64].
Several investigators have shown that GEA can be used without increased morbidity, particularly in abdominal complications [65,66], and the late survival rate has been excellent in CABG with GEA plus internal thoracic artery grafts [18,56,67,68].
High tendencies to develop vasospasm and competitive flow in moderately stenotic coronary lesions, however, have been indicated as limitations of the in situ RGEA graft, but this tendence to develop spasm seems to be due to the use of pedicled r-GEA, while skeletonized r-GEA appears to have a significantly low rate of spasm [69-72].
Moreover, the lenght of this vessel, if it is used as a composite graft, allows to reach both coronary systems and to achieve a complete arterial revascularization [69].
Suma, et al., in a study published on Circuation [57], a large series of patients who recived a r-GEA graft, demonstrating that r-GEA has a patency rate superior to the SV one.
3.4. The Inferior Epigastric Artery (IEA)
The Inferior Epigastric Artery represents an infrequent arterial conduit used as a free graft for CABG. In our research in PubMed we did not find recent articles or trials about the use of this conduit in CABG so this section of our review is to complete the view of arterial conduits which are used or proposed for total arterial grafting.
For some authors, the artery exhibits favorable physiological vasoreactivity characteristics and its lenght, diameter and location are rather variable. After it has been harvested, it is treated like a radial artery in terms of preparation. It is used to create composite Y graft usually with an anastomosis with the LITA, RITA or SV [73].
Some authors reported good patency rate in early postoperative follow up, even with angiography [74], but the patients’ series and the lack of midand long term follow up can’t encourage a wide use of this conduit and it absolutely requires others clinical investigations on larger series of patients.
4. CONCLUSIONS
In a review of the literature of the last 15 years, it has been reported the superiority of total arterial coronary grafting in CABG, even in the long term follow-up.
The harvesting of this “addictional” conduit expands the operating time, but it has been shown that each of them improve the outcome of the coronaric patients undergoing CABG.
Although diabetes is an independent risk factor for coronary artery disease and graft restenosis, the outcome of the diabetic patient is significantly improved by the use of total arterial grafting.
It is also clear that each arterial vessel we described, especially the radial artery, seems to have particular indications and a preferential anastomosis territory. The wide application and the utilization of these vessels need to be proved by multicenter randomized studies in the future.
Our opinion is that the use of total arterial myocardial revascularization should be encouraged, and cardiac surgery is near to avoid the use of saphenos vein graft in the scheduled coronary patient.
REFERENCES
- Fox, C.S., Coady, S., Sorlie, P.D., D’Agostino, R.B., Pencina, M.J., Vasan, R.S., Meigs, J.B., Levy, D. and Savage, P.J. (2007) Increasing cardiovascular disease burden due to diabetes mellitus: The framingham heart study. Circulation, 115, 1544-1550. doi:10.1161/CIRCULATIONAHA.106.658948
- Dorman, M.J., Kurlansky, P.A., Traad, E.A., Galbut, D.L., Zucker, M. and Ebra, G. (2012) Bilateral internal mammary artery grafting enhances survival in diabetic patients: A 30-year follow-up of propensity score-matched cohorts. Circulation, 126, 2935-2942. doi:10.1161/CIRCULATIONAHA.112.117606
- Norhammar, A., Malmberg, K., Diderholm, E., Lagerqvist, B., Lindahl, B., Rydén, L. and Wallentin, L. (2004) Diabetes mellitus: The major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. Journal of the American College of Cardiology, 43, 585-591. doi:10.1016/j.jacc.2003.08.050
- Goraya, T.Y., Leibson, C.L., Palumbo, P.J., Weston, S.A., Killian, J.M., Pfeifer, E.A., Jacobsen, S.J., Frye, R.L. and Roger, V.L. (2002) Coronary atherosclerosis in diabetes mellitus: A population-based autopsy study. Journal of the American College of Cardiology, 40, 946-953. doi:10.1016/S0735-1097(02)02065-X
- Flaherty, J.D. and Davidson, C.J. (2005) Diabetes and Coronary Revascularization. Journal of the American Medical Association, 293, 1501-1508. doi:10.1001/jama.293.12.1501
- Hlatky, M.A., Boothroyd, D.B., Bravata, D.M., Boersma, E., Booth, J., Brooks, M.M., Carrié, D., et al. (2009) Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: A collaborative analysis of individual patient data from ten randomised trials. Lancet, 373, 1190-1197. doi:10.1016/S0140-6736(09)60552-3
- Cariou, B., Bonnevie, L., Mayaudon, H., Dupuy, O., Ceccaldi, B. and Bauduceau, B. (2000) Angiographic characteristics of coronary artery disease in diabetic patients compared with matched non-diabetic subjects. Diabetes, Nutrition & Metabolism, 13, 134-141.
- Jones, R.H., Hannan, E.L., Hammermeister, K.E., Delong, E.R., O’Connor, G.T., Luepker, R.V., Parsonnet, V. and Pryor, D.B. (1996) Identification of preoperative variables needed for risk adjustment of short-term mortality after coronary artery bypass graft surgery. The Working Group Panel on the Cooperative CABG Database Project, 28, 1478-1487.
- Birnbaum, D.E. and Lehle, K. (2006) CPB in high-risk groups: CPB in diabetics. Perfusion, 21, 235-238. doi:10.1191/0267659106pf874oa
- Patel, M.R., Dehmer, G.J., Hirshfeld, J.W., Smith, P.K. and Spertus, J.A. (2009) ACCF/SCAI/STS/AATS/AHA/ ASNC 2009 appropriateness criteria for coronary revascularization: A report of the American college of cardiology foundation appropriateness criteria task force, society for cardiovascular angiography and interventions, society of thoracic surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. Circulation, 119, 1330- 1352. doi:10.1161/CIRCULATIONAHA.108.191768
- Parang, P. and Arora, R. (2009) Coronary vein graft disease: Pathogenesis and prevention. Canadian Journal of Cardiology, 25, e57-e62. doi:10.1016/S0828-282X(09)70486-6
- Loop, F.D., Cosgrove, D.M., Lytle, B.W., Thurer, R.L., Simpfendorfer, C., Taylor, P.C. and Proudfit, W.L. (1979) An 11-year evolution of coronary arterial surgery (1968- 1978). Annals of Surgery, 190, 444-455. doi:10.1097/00000658-197910000-00004
- Kobayashi, J. (2009) Radial artery as a graft for coronary artery bypass grafting. Circulation Journal, 73, 1178- 1183. doi:10.1253/circj.CJ-09-0322
- Cohn, L.H. (2011) Cardiac Surgery in the Adult. McGraw Medical, New York.
- Pick, A.W., Orszulak, T.A., Anderson, B.J. and Schaff, H.V. (1997) Single versus bilateral internal mammary artery grafts: 10-year outcome analysis. The Annals of Thoracic Surgery, 64, 599-605. doi:10.1016/S0003-4975(97)00620-6
- Zacharias, A., Schwann, T.A., Riordan, C.J., Durham, S.J., Shah, A.S. and Habib, R.H. (2009) Late results of conventional versus all-arterial revascularization based on internal thoracic and radial artery grafting. The Annals Thoracic Surgery, 87, 19-26. doi:10.1016/j.athoracsur.2008.09.050
- Hassanein, W., Hegazy, Y.Y., Albert, A., Ennker, I.C., Rosendahl, U., Bauer, S. and Ennker, J. (2010) Short term outcomes of total arterial coronary revascularization in patients above 65 years: A propensity score analysis. Journal of Cardiothoracic Surgery, 5, 25. doi:10.1186/1749-8090-5-25
- Formica, F., Ferro, O., Greco, P., Martino A, Gastaldi D, and Paolini G. (2004) Long-term follow-up of total arterial myocardial revascularization using exclusively pedicle bilateral internal thoracic artery and right gastroepiploic artery. European Journal Cardio-Thoracic Surgery, 26, 1141-1148. doi:10.1016/j.ejcts.2004.08.027
- Rankin, J.S., Tuttle, R.H., Wechsler, A.S., Teichmann, T.L., Glower, D.D. and Califf, R.M. (2007) Techniques and benefits of multiple internal mammary artery bypass at 20 years of follow-up. The Annals of Thoracic Surgery, 83, 1008-1014.
- Nasso, G., Coppola, R., Bonifazi, R., Picone, F., Bozzetti, G. and Speziale, G. (2009) Arterial revascularization in primary coronary artery bypass grafting: Direct comparison of 4 strategies-results of the stand-in-Y mammary study. The Journal of Thoracic and Cardiovascular Surgery, 137, 1093-1100. doi:10.1016/j.jtcvs.2008.10.029
- Parsa, C.J., Shaw, L.K., Rankin, J.S., Daneshmand, M.A., Gaca, J.G., Milano, C.A., Glower, D.D. and Smith, P.K. (2013) Twenty-five-year outcomes after multiple internal thoracic artery bypass. The Journal of Thoracic and Cardiovascular Surgery, 145, 970-975. doi:10.1016/j.jtcvs.2012.11.093
- Locker, C., Shaff, H.V., Dearani, J.A., Joyce, L.D., Park, S.J., Burkhart, H.M., Suri, R.M., et al. (2012) Multiple arterial grafts improve late survival of patients undergoing coronary artery bypass graft surgery. Circulation, 126, 1023-1030. doi:10.1161/CIRCULATIONAHA.111.084624
- Saito, A., Miyata, H., Motomura, N., Ono, M., Takamoto, S. and Japan Cardiovascular Surgery Database Organization. (2013) Propensity-matched analysis of bilateral internal mammary artery vs single internal mammary artery in 7702 cases of isolated coronary artery bypass grafting. European Journal Cardio-Thoracic Surgery, in print. doi:10.1093/ejcts/ezt157
- Grau, J.B., Ferrari, G., Mak, A.W.C., Shaw, R.E., Brizzio, M.E., Mindich, B.P., Strobeck, J. and Zapolanski, A. (2012) Propensity matched analysis of bilateral internal mammary artery versus single left internal mammary artery grafting at 17-year follow-up: Validation of a contemporary surgical experience. European Journal Cardio-Thoracic Surgery, 41, 770-775.
- Kinoshita, T., Asai, T., Suzuki, T., Kambara, A. and Matsubayashi, K. (2011) Off-pump bilateral versus single skeletonized internal thoracic artery grafting in high-risk patients. Circulation, 124, S130-S134. doi:10.1161/CIRCULATIONAHA.110.010892
- Carpentier, A.F., Guermonprez, J.L., Deloche, A., Frechette, C. and DuBost, C. (1973) The aorta-to-coronary radial artery bypass graft: A technique avoiding pathological changes in grafts. The Annals of Thoracic Surgery, 16, 111. doi:10.1016/S0003-4975(10)65825-0
- Newman, R.V. and Lammle, W.G. (2003) Radial artery harvest using endoscopic techniques. Heart Surgery Forum, 6, E194-E195.
- Schwann, T.A., Zacharias, A., Riordan, C.J., Durham, S.J., Shah, A.S. and Habib, R.H. (2008) Does radial use as a second arterial conduit for coronary artery bypass grafting improve long-term outcomes in diabetics? European Journal Cardio-Thoracic Surgery, 33, 914-923. doi:10.1016/j.ejcts.2008.01.062
- Calafiore, A.M., Di Giammarco, G., Teodori, G., D’Annunzio, E., Vitolla, G., Fino, C. and Maddestram N. (1995) Radial artery and inferior epigastric artery in composite grafts: Improved midterm angiographic results. The Annals of Thoracic Surgery, 60, 517-523.
- Zacharias, A., Habib, R.H., Schwann, T.A., Riordan, C.J., Durham, S.J. and Shah, A. (2004) Improved survival with radial artery versus vein conduits in coronary bypass surgery with left internal thoracic artery to left anterior descending artery grafting. Circulation, 109, 1489-1496. doi:10.1161/01.CIR.0000121743.10146.78
- Schwann, T.A., Zacharias, A., Riordan, C.J., Durham, S.J., Shah, A.S. and Habib, R.H. (2009) Sequential radial artery grafts for multivessel coronary artery bypass graft surgery: 10-Year Survival and Angiography Results. The Annals of Thoracic Surgery, 88, 31-39. doi:10.1016/j.athoracsur.2009.03.081
- Mediratta, N., Chalmers, J., Pullan, M., McShane, J., Shaw, M. and Poullis, M. (2013) In-hospital mortality and long-term survival after coronary artery bypass surgery in young patients. European Journal Cardio-Thoracic Surgery, 43, 1014-1021. doi:10.1093/ejcts/ezs459
- Schwann, T.A., Al-Shaar, L., Engoren, M. and Habib, R.H. (2013) Late effects of radial artery vs saphenous vein grafting for multivessel coronary bypass surgery in diabetics: A propensity-matched analysis. European Journal Cardio-Thoracic Surgery, 1-10. doi:10.1093/ejcts/ezt061
- Acar, C., Jebara, V.A., Portoghese, M., Beyssen, B., Pagny, J.Y., Grare, P., Chachques, J.C., Fabiani, J.-N.N., Deloche, A. and Guermonprez, J.L. (1992) Revival of the radial artery for coronary artery bypass grafting. The Annals of Thoracic Surgery, 54, 652-659.
- Amano, A., Hirose, H., Takahashi, A. and Nagano, N. (2001) Coronary artery bypass grafting using the radial artery: Midterm results in a Japanese institute. The Annals of Thoracic Surgery, 72, 120-125. doi:10.1016/S0003-4975(01)02706-0
- Possati, G., Gaudino, M., Prati, F., Alessandrini, F., Trani, C., Glieca, F., Mazzari, M.A., Luciani, N. and Schiavoni, G. (2003) Long-term results of the radial artery used for myocardial revascularization. Circulation, 108, 1350- 1354. doi:10.1161/01.CIR.0000087402.13786.D0
- Da Costa, F.D., Da Costa, I.A., Poffo, R., Abuchaim, D., Gaspar, R., Garcia, L. and Faraco, D.L. (1996) Myocardial revascularization with theradial artery: A clinical and angiographic study. The Annals of Thoracic Surgery, 62, 475-479. doi:10.1016/0003-4975(96)00311-6
- Chen, A.H., Nakao, T., Brodman, R.F., Greenberg, M., Charney, R., Menegus, M., Johnson, M., et al. (1996) Early postoperative angiographicassessment of radial grafts used forcoronary artery bypass grafting. The Journal of Thoracic and Cardiovascular Surgery, 111, 1208-1212. doi:10.1016/S0022-5223(96)70223-4
- Possati, G., Gaudino, M., Alessandrini, F., Luciani, N., Glieca, F., Trani, C., Cellini, C., Canosa, C. and Di Sciascio, G. (2013) Midterm clinical and angiographic results of radial artery grafts used for myocardial revascularization. The Journal of Thoracic and Cardiovascular Surgery, 116, 1015-1021. doi:10.1016/S0022-5223(98)70054-6
- Tranbaugh, R.F., Dimitrova, K.R., Friedmann, P., Geller, C.M., Harris, L.J., Stelzer, P., Cohen, B.M., et al. (2012) Coronary artery bypass grafting using the radial artery: Clinical outcomes, patency, and need for reintervention. Circulation, 126, S170-S175. doi:10.1161/CIRCULATIONAHA.111.083048
- Tranbaugh, R.F., Dimitrova, K.R., Friedmann, P., Geller, C.M., Harris, L.J., Stelzer, P., Cohen, B. and Hoffman, D.M. (2010) Radial artery conduits improve long-term survival after coronary artery bypass grafting. The Annals of Thoracic Surgery, 90, 1165-1172. doi:10.1016/j.athoracsur.2010.05.038
- Hwang, H.Y., Choi, J.S. and Kim, K.B. (2010) Diabetes does not affect long-term results after total arterial offpump coronary revascularization. The Annals of Thoracic Surgery, 90, 1180-1186. doi:10.1016/j.athoracsur.2010.05.021
- Hoffman, D.M., Dimitrova, K.R., DeCastro, H., Friedmann, P., Geller, C.M., Ko, W. and Tranbaugh, R.F. (2013) Improving long term outcome for diabetic patients undergoing surgical revascularization by use of the radial artery conduit: A propensity matched study. Journal of Cardiothoracic Surgery, 8, 27. doi:10.1186/1749-8090-8-27
- Boylan, M.J., Lytle, B.W., Loop, F.D., Taylor, P.C., Borsh, J.A., Goormastic, M. and Cosgrove, D.M. (1994) Surgical treatment of isolated left anterior descending coronary stenosis. Comparison of left internal mammary artery and venous autograft at 18 to 20 years of follow-up. The Journal of Thoracic and Cardiovascular Surgery, 107, 657-662.
- Lemma, M., Mangini, A., Gelpi, G., Innorta, A., Spina, A. and Antona, C. (2004) Is it better to use the radial artery as a composite graft? Clinical and angiographic results of aorto-coronary versus Y-graft. European Journal CardioThoracic Surgery, 26, 110-117. doi:10.1016/j.ejcts.2004.03.020
- Gaudino, M., Alessandrini, F., Pragliola, C., Cellini, C., Glieca, F., Luciani, N., Girola, F. and Possati, G. (2004) Effect of target artery location and severity of stenosis on mid-term patency of aorta-anastomosed vs. internal thoracic artery-anastomosed radial artery grafts. European Journal Cardio-Thoracic Surgery, 25, 424-428. doi:10.1016/j.ejcts.2003.11.027
- Cameron, J., Trivedi, S., Stafford, G. and Bett, J.H.N. (2004) Five-year angiographic patency of radial artery bypass grafts. Circulation, 110, II23- II26.
- Khot, U.N., Friedman, D.T., Pettersson, G.B., Smedira, N.G., Li, J. and Ellis, S.G. (2004) Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation, 109, 2086-2091. doi:10.1161/01.CIR.0000127570.20508.5C
- Desai, N.D., Cohen, E.A., Naylor, C.D., Fremes, S.E. (2004) A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. The New England Journal of Medicine, 351, 2302-2309. doi:10.1056/NEJMoa040982
- Royse, A.G., Royse, C.F., Tatoulis, J., Grigg, L.E., Shah, P., Hunt, D., Better, N. and Marasco, S.F. (2000) Postoperative radial artery angiography for coronary artery bypass surgery. European Journal Cardio-Thoracic Surgery, 17, 294-304. doi:10.1016/S1010-7940(99)00364-4
- Maniar, H.S., Sundt, T.M., Barner, H.B., Prasad, S.M., Peterson, L., Absi, T. and Moustakidis, P. (2002) Effect of target stenosis and location on radial artery graft patency. The Journal of Thoracic and Cardiovascular Surgery, 123, 45-52. doi:10.1067/mtc.2002.118686
- Nakajimaa, H., Kobayashi, J., Funatsu, T., Shimahara, Y., Kawamura, M., Kawamura, A., Yagihara, T. and Kitamura, S. (2007) Predictive factors for the intermediateterm patency of arterial grafts in aorta no-touch off-pump coronary revascularization. European Journal CardioThoracic Surgery, 32, 711-717. doi:10.1016/j.ejcts.2007.07.025
- Singh, S.K., Desai, N.D., Petroff, S.D., Deb, S., Cohen, E.A., Radhakrishnan, S., Schwartz, L., Dubbin, J., Fremes, S.E. (2008) The impact of diabetic status on coronary artery bypass graft patency: Insights from the radial artery patency study. Circulation, 118, S222- S225. doi:10.1161/CIRCULATIONAHA.107.757161
- Desai, N.D., Naylor, C.D., Kiss, A., Cohen, E.A., FederElituv, R., Miwa, S., Radhakrishnan, S., Dubbin, J., Schwartz, L. and Fremes, S.E. (2007) Impact of patient and target-vessel characteristics on arterial and venous bypass graft patency: Insight from a randomized trial. Circulation, 115, 684-691. doi:10.1161/CIRCULATIONAHA.105.567495
- Buxton, B.F., Raman, J.S., Ruengsakulrach, P., Gordon, I., Rosalion, A., Bellomo, R., Horrigan, M. and Hare, D.L. (2003) Radial artery patency and clinical outcomes: Fiveyear interim results of a randomized trial. The Journal of Thoracic and Cardiovascular Surgery, 125, 1363- 1371. doi:10.1016/S0022-5223(02)73241-8
- Collins, P., Webb, C.M., Chong, C.F. and Moat, N.E. (2008) Radial artery versus saphenous vein patency randomized trial: Five-year angiographic follow-up. Circulation, 117, 2859-2864. doi:10.1161/CIRCULATIONAHA.107.736215
- Suma, H., Tanabe, H., Takahashi, A., Horii, T., Isomura, T., Hirose, H. and Amano, A. (2007) Twenty years experience with the gastroepiploic artery graft for CABG. Circulation, 116, I188-I191. doi:10.1161/CIRCULATIONAHA.106.678813
- Mills, N.L. and Everson, C.T. (1989) Right gastroepiploic artery: A third arterial conduit for coronary artery bypass. The Annals of Thoracic Surgery, 47, 706-711. doi:10.1016/0003-4975(89)90122-7
- Suma, H., Fukumoto, H. and Takeuchi, A. (1987) Coronary artery bypass grafting by utilizing in situ right gastroepiploic artery: Basic study and clinical application. The Annals of Thoracic Surgery, 44, 394-397. doi:10.1016/S0003-4975(10)63799-X
- [61] Saito, T., Suma, H., Terada, Y., Wanibuchi, Y., Fukuda, S. and Furuta, S. (1992) Availability of the in situ right gastroepiploic artery for coronary artery bypass. The Annals of Thoracic Surgery, 53, 266-268. doi:10.1016/0003-4975(92)91330-C
- [62] Suma, H., Wanibuchi, Y., Furuta, S., Isshiki, T., Yamaguchi, T. and Takanashi, R. (1991) Comparative study between the gastroepiploic and the internal thoracic artery as a coronary bypass graft. size, flow, patency, histology. European Journal Cardio-Thoracic Surgery, 5, 244-247. doi:10.1016/1010-7940(91)90171-F
- [63] Kusukawa, J., Hirota, Y., Kawamura, K., Suma, H., Takeuchi, A., Adachi, I. and Akagi, H. (1989) Efficacy of coronary artery bypass surgery with gastroepiploic artery. Assessment with thallium 201 myocardial scintigraphy. Circulation, 80, I135- I140.
- [64] Ochiai, M., Ohno, M., Taguchi, J., Hara, K., Suma, H., Is-shiki, T., Yamaguchi, T. and Kurokawa, K. (1992) Responses of human gastroepiploic arteries to vasoactive substances: Comparison with responses of internal mammary arteries and saphenous veins. The Journal of Thoracic and Cardiovascular Surgery, 104, 453-458.
- [65] Takayama, T., Suma, H., Wanibuchi, Y., Tohda, E., Matsunaka, T. and Yamashita, S. (1992) Physiological and pharmacological responses of arterial graft flow after coronary artery bypass grafting measured with an implantable ultrasonic Doppler miniprobe. Circulation, 86, II217-II223.
- [66] Suma, H., Wanibuchi, Y., Furuta, S. and Takeuchi, A. (1991) Does use of gastroepiploic artery graft increase surgical risk? The Journal of Thoracic and Cardiovascular Surgery,101, 121-125.
- [67] Hirose, H., Amano, A., Takanashi, S. and Takahashi, A. (2002) Coronary artery bypass grafting using the gastroepiploic artery in 1,000 patients. The Annals of Thoracic Surgery, 73, 1371-1379. doi:10.1016/S0003-4975(02)03416-1
- [68] Nishida, H., Tomizawa, Y., Endo, M., Koyanagi, H. and Kasanuki, H. (2001) Coronary artery bypass with only in situ bilateral internal thoracic arteries and right gastroepiploic artery. Circulation, 104, I76-I80. doi:10.1161/hc37t1.094812
- [69] Tavilla, G., Kappetein, A.P., Braun, J., Gopie, J., Tjien, A.T.J. and Dion, R.A.E. (2004) Long-term follow-up of coronary artery bypass grafting in three-vessel disease using exclusively pedicled bilateral internal thoracic and right gastroepiploic arteries. The Annals of Thoracic Surgery, 77, 794-799.
- [70] Cho, K.R., Hwang, H.Y., Kim, J.-S., Jeong, D.S. and Kim, K.-B. (2010) Comparison of right internal thoracic artery and right gastroepiploic artery Y grafts anastomosed to the left internal thoracic artery. The Annals of Thoracic Surgery, 90, 744-750.
- [71] He, G.-W. (1999) Arterial grafts for coronary artery bypass grafting: Biological characteristics, functional classification, and clinical choice. The Annals of Thoracic Surgery, 67, 277-284. doi:10.1016/S0003-4975(98)01207-7
- [72] Glineur, D., D’hoore, W., Khoury, E.l.G., Sondji, S., Kalscheuer, G., Funken, J.-C., Rubay, J., et al. (2008) Angiographic predictors of 6-month patency of bypass grafts implanted to the right coronary artery : A prospective randomized comparison of gastroepiploic artery and saphenous vein grafts. Journal of the American College of Cardiology, 51, 120-125. doi:10.1016/j.jacc.2007.09.030
- [73] Santos, G.G., Stolf, N.A.G., Moreira, L.F.P., Haddad, V.L.S., Simões, R.M.C., Carvalho, S.R.V., Salgado, A.A. and Avelar, S.F. (2002) Randomized comparative study of radial artery and right gastroepiploic artery in composite arterial graft for CABG. European Journal CardioThoracic Surgery, 21, 1009-1014. doi:10.1016/S1010-7940(02)00180-X
- [74] Ayabe, T., Fukushima, Y., Yoshioka, M. and Onizuka, T. (2003) Clinical outcome of the coronary arterial bypass graft with inferior epigastric artery as a composite graft. Kyobu Geka, 56, 731-737.
- [75] Kamata, S., Kasegawa, H., Shimokawa, T., Kasahara, K., Matsushita, Y., Abe, Y., Kitanaka, Y., et al. (1998) Coronary artery bypass grafting with arterial graft alone: Radial artery and inferior epigastric artery used in combination. Kyobu Geka, 51, 393-397.

