Open Journal of Biophysics
Vol.3 No.1A(2013), Article ID:28265,11 pages DOI:10.4236/ojbiphy.2013.31A008
Polymer Supported Lipid Bilayers
1Department of Biomedical and Chemical Engineering, Syracuse University, Syracuse, USA
2Physics Department, Syracuse University, Syracuse, USA
Email: mbforstn@syr.edu
Received January 1, 2013; revised February 5, 2013; accepted February 14, 2013
Keywords: Supported Lipid Bilayers; Polymer Supported Bilayers; Membrane Model Systems
ABSTRACT
Lipid bilayers are some of the most fascinating self-assembled structure in living nature. Not only do they serve as the protective boundary of cells and their internal organelles, they also organize and host major parts of the biochemical machinery for cellular communication and transmembrane transport. To study aspects of cellular membranes in a controlled manner, solid supported planar bilayers have served as reliable tools for many decades. They have been used in a large variety of studies ranging from fundamental investigations of membranes and their constituents to the dissection of cellular signaling mechanisms. However, there are limitations to these systems and recently a class of new systems in which the lipid bilayer is supported on a soft, polymer cushion has emerged. Here, we review the different polymer cushioned bilayer systems and discuss their manufacture and advantages.
1. Introduction
For the last three decades functionalization of interfaces with mimics of biological membranes has been an ongoing effort. These model-membrane systems have garnered much attention because they provide a useful and interesting interface between the biological world and man-made materials. Thus, they have great potential for basic membrane and cell-biology research as well as a variety of biotechnological and biomedical applications. The simplest of these membrane mimics is the solid supported lipid bilayer that in many ways behaves similar to free lipid membranes. A thin water layer between the substrate and the bilayer serves as lubricant that enables long range lateral diffusion of the lipids. Thus, they preserve the fluidity of biological membranes that is so central to many cellular functions. Since the first fabrication of a solid supported bilayer via successive deposition of two monolayers by Tamm and McConnell [1], solid supported bilayers have been instrumental in a wide variety of studies. This is in part because proteins or other membrane constituents can be placed on or in the membrane, thus providing a highly controlled environment for experimentation. One of the great advantages of using a planar solid supported lipid membrane as opposed to lipid bilayer vesicles is the ability to bring to bear a number of light based analytical techniques such as; Förster resonance energy transfer (FRET) [2], fluorescence correlation spectroscopy (FCS) [3,4], total internal reflection fluorescence (TIRF) [5,6] or fluorescence recovery after photo-bleaching (FRAP) [7]. By using a thin (~150 µm) support, such as a glass cover slip, means that high magnification optics, with high numerical apertures and small working distances, can be used to bring light to and collect light from those bilayer. For example, supported lipid bilayers have been used to investigate membrane bound signaling events of cells [8,9], study protein-lipid interactions on the single molecule level [5] and develop biosensor platforms [10-12].
However, a significant limitation of such traditional solid supported bilayers can be encountered when trying to incorporate transmembrane proteins into the supported membrane (Figure 1). A typical solid supported bilayer will have an approximately 1 - 3 nm thick hydration cushion between the support and the bilayer. This does not provide sufficient space for the cytosolic domain of most transmembrane proteins and consequently the protein will contact the substrate surface, deform and eventually denature as indicated in Figure 1(b). To overcome this restriction and expand the use of supported lipid bilayers to other research areas and fields, a different type of bilayer support has been developed. In this alternative method a soft polymeric layer is introduced between the solid support and the artificial lipid membrane. The polymer layer provides a low friction interface for the lipid bilayer and any imbedded proteins. The system has proven its adaptability and has been utilized in such diverse applications as; membrane protein binding detection [13], electrophoretic accumulation studies [14,15], cellular cytoskeleton incorporation [16], and electro-
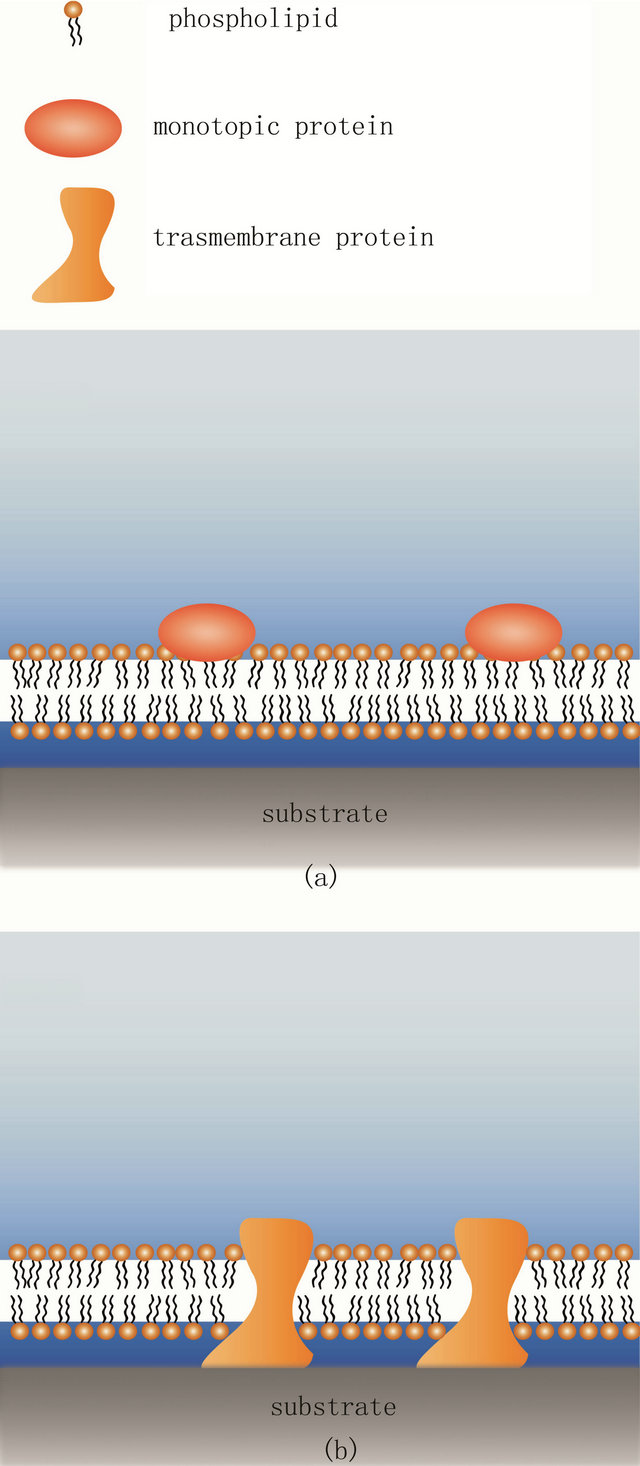
Figure 1. Conventional solid supported lipid bilayer and its limitations. (a) The SLB is able to accommodate monotopic proteins so that they stay functional. Both proteins and lipids are laterally mobile in such a scenario; (b) In contrast, transmembrane proteins with extended cytosolic domains have not enough space between the substrate and the bilayer. Thus, they will contact the substrate, deform and often denature which leads to loss of function. In addition, such proteins are also immobilized.
chemical biosensors [17]. Here, we discuss the options available for polymer bilayer supports and try to underscore the particular strengths and weaknesses of the different systems and methods.
2. Polymer Supports for Lipid Bilayers
Most common polymer supports have had their genesis in convenience. Popular biological techniques involve numerous polymerizing substances; consequently some have been adopted for use as membrane cushions. For a successful polymer based lipid bilayer cushion, the polymer must have some few specific characteristics. Firstly, they must be capable of forming a thin layer with surface uniformity suitable for bilayer formation. Secondly, they would ideally have a well-defined elastic modulus that can be replicated at every iteration of the experiment. Thirdly, the polymer must be hydrophilic, and they must be relatively chemically inert so as not to cause unwanted reactions and interactions with the membrane. Due to their hydrophilicity such polymers typically have high water contents and are known as hydrogels. Hydrogels have refractive indices that deviate only slightly from that of the liquid used to hydrate them, this allows for good optical coupling between the hydrogel and the aqueous solution, giving aberration free imaging through the gels. Most other light based measurement techniques such as FCS [18] and FRAP [19] are also compatible with these systems. The nature of self-assembly of amphiphillic molecules such as lipids dictates that there must be water present for the formation of a bilayer. Consequently, to avoid de-wetting of the lipid/polymer interface during or after deposition of the bilayer there can be no strong attractive forces between a substrate and the membrane. Care must be taken when using polymers that have charged or polarized functional groups to ensure the attractive forces between these and the lipids are not too great. Typically polymer wetting ability is characterized by the contact angle of a water droplet on its surface. This can give some indication of a good polymer for a bilayer cushion application. Typical contact angles range from 30 - 70 degrees [20,21].
Polymer supports might be classified with respect to a variety of properties. A first possibility would be a distinction between copolymers (such as styrene-acrylonitrile and nitrile rubber) which are formed using two or more monomer species and homopolymers (such as cellulose, PVC and polyethylene glycol) which consist of only one monomer species. A thorough review of the literature reveals that polymers used for bilayer supports are overwhelmingly of the homopolymer variety although a clear advantage for their use is not obvious per se. Another possible distinction of polymer supported lipid membranes could be made between systems where the polymer layer(s) are formed independently of the bilayer and those that are formed through fusion of vesicles containing lipo-polymers. Another differentiation could be made between polymer supports that attach to the solid support just by adhesion and those polymers that are attached to the solid substrate through an intermediary binding molecule: alkylsilanes for silica and mica substrates [22], or alkylthiols for GaAs or gold substrates [23]. These binding molecules need to have a functionalized domain for polymer attachment and can be either coated over the entire solid substrate when using independent polymer supports [24] or attached to the distal end of each polymer when using lipopolymer supports [25].
Yet, in this review we separated polymer supported bilayers into two main classes: independent polymer to the bilayer, and coupled membrane-polymer systems where all or parts of the polymers are linked to lipids or hydrophobic molecules that integrate into the bilayer (See Figure 2). A short summary of the different polymer systems are given in Table 1, while the chemical structures are summarized in Figure 3.
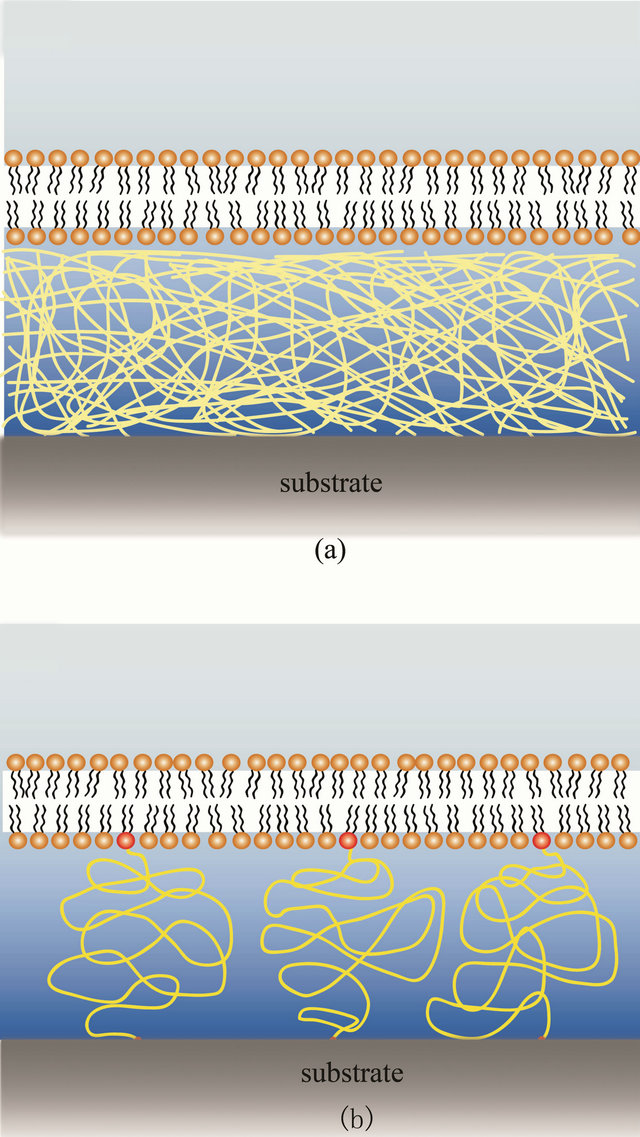
Figure 2. Schematic of the two major classes of polymer supported lipid bilayers. (a) The independent support, without linkage between the bilayer and the polymers; (b) A coupled membrane-polymer systems where the polymer is covalently linked (red dots) to components of the membrane.
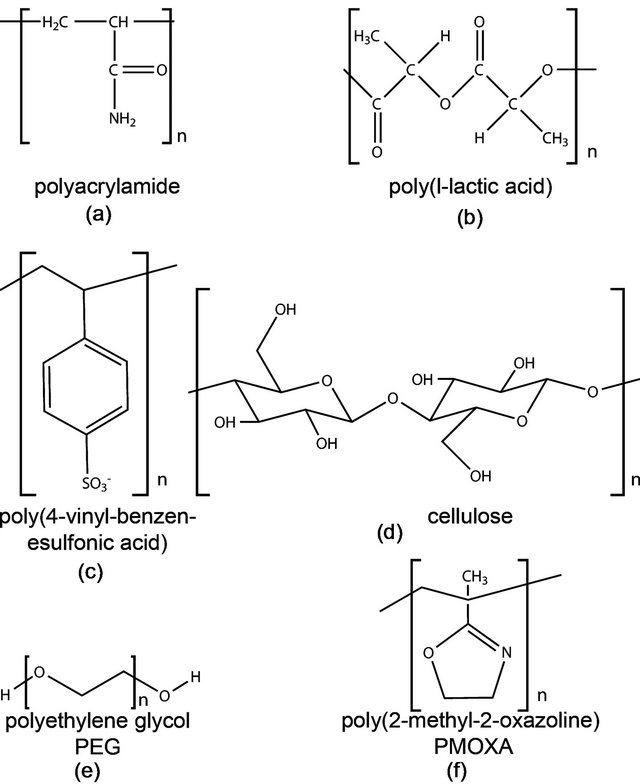
Figure 3. Chemical structure of commonly used polymers for bilayer support. (a) Polyacrylamide; (b) Poylacticacid; (c) The polyelectrolyte poly(4-vinyl-benzen-esulfonic acid); (d) Cellulose; (e) PEG and (f) PMOXA.
2.1. Independent Polymer Support
Independent polymer supports are characterized by the fact that they have no direct linkage with the lipid bilayer. This allows for maximal flexibility with respect to polymer choice as well as deposition and manufacture procedures. The polymer in question can be spin coated on [26], deposited by sequential dipping [27] or, for chemically induced polymerization, polymerized while sandwiched between the substrate and a second solid layer with a nonreactive coating [28]. Following polymer preparation the lipid bilayer is deposited using one of three main techniques: Langmuir-Schaefer, Langmuir-Blodgett, or a hybrid monolayer/vesicle fusion system (vide infra).
2.1.1. Polyacrylamide
Polyacrylamide (see Figure 3(a)) is typically used in gel electrophoresis. In this application the gel structure is controlled by adjusting the ratio of acrylamide monomers and bis cross-linkers in the unpolymerized solution [29]. In an electrophoresis gel this ratio determines the average pore size and if used as membrane support polymer, this ratio can be used to control the elastic modulus of the gel (typically between 1 and 200 kPa [30]). This latter ability made polyacrylamide also very popular as a soft substrate material in studies of cellular biomechanics [31]. To prevent peeling, polyacrylamide requires that the solid substrate be coated with a bonding agent, typically alkylsilane, which covalently binds the cross-linked polymer to the glass [24]. To achieve a smooth surface
Table 1. Polymers used as membrane supports.

*I refers to independent polymer supports (see Section 2.1), C refers to coupled polymer supports (see Section 2.2).
the unpolymerized solution is sandwiched between the activated solid substrate and another surface coated with a special non-reactive coating, typically a short chain silane polymer which renders the surface inert [32]. Once the nonreactive layer is removed the result is a uniform polymer surface suitable for bilayer deposition. This sandwiching technique is only possible because polymerization and crosslinking of the polyacrylamide is induced chemically and occurs over the time of minutes. In contrast to other polymer systems, the thickness of the crosslinked polyacrylamide gel can be easily controlled during production and thickness from tens to hundreds of micrometers can be achieved [32]. In comparison, other techniques give gel thicknesses in the tens to hundreds of nanometers range [29]. This wide range of thicknesses increases the number of potential applications for such a system. However, it should be noted that the acrylamide monomer is a toxin that should be handled and processed with care in particular if live cells are involved in a study.
2.1.2. Poly-L Lactic Acid (PLLA)
PLLA is another commonly used membrane support (Figure 3(b)). It is hydrophilic and quite inert and thus provides a good substrate for biological studies [26]. It has some promise in the medical field due to its biocompatibility and biodegradability making it a good candidate as a scaffold for tissue engineering [33]. PLLA can be formed into a uniform support by spin coating a solution onto a solid substrate. The coating then gets annealed before use to complete the polymerization. This yields layer thicknesses in the 100 nm range [26]. Having polymer layers this thin allows the use of sensitive optical techniques that rely on the use of objectives with high numerical aperture, such as; sum frequency generation vibrational spectroscopy [26], total internal reflection fluorescence and glancing angle illumination [34].
2.1.3. Cellulose
Cellulose has been one of the most widely used polymers in modern history (Figure 3(d)). It is found naturally in plant cell walls and is the main constituent of paper and wood products. Cellulose has a diverse number of common uses from cellophane to wall paper paste to food filler. It is an inert hydrophilic polysaccharide, formed from dehydrated dextrose (the right hand form of glucose). It can be formed into thin layers for bilayer support by first substituting their hydroxyl groups for a hydrophobic side chain; this allows them to be dissolved in organic solvents. Once dissolved they can be spin coated onto a substrate or formed into monolayers on a Langmuir trough and deposited onto a substrate; the thickness can be built up through repeated dipping [19,35]. A variety of cellulose derivatives exist, such as trimethylsilylcellulose (TMSC) and isopentylcellulosecinnamate (IPCC), which provide different properties to the substrate such as solubility in nonpolar solvents and improved surface friction, respectively [19,35,36]. It has been shown that such cellulose derivatives can be modified post deposition via exposure to HCL vapor to create a hydrophilic surface with a hydrophobic core, this can change the electrical resistance of the bilayer which can be useful for ion channel studies [19,35,36]. Cellulose has also been successfully patterned through microcontact printing; here a polydimethylsiloxane (PDMS) stamp is used to transfer patterns of “ink” monolayers onto the cellulose substrate which act as a diffusion barrier to the lipid bilayer. Patterning permits close spatial control of the bilayer contents, and can be used to promote selective cell growth, to study membrane discrimination, or to isolate proteins or channels from each other [37].
2.1.4. Agarose
Agarose is a polysaccharide most commonly found in agar, the gelatinous substance used for bacterial cell culture. It is derived from certain species of red algae and is used in such things as ice cream, the brewing process, as well as a food item in its own right [38]. In biological studies agarose is used to make a porous gel for microorganism motility assays [39]; the concentration of agarose in solution determines the final viscosity of the substance [40]. Agarose has been used as a polymer support for bilayers for the last 15 years [41,42]. It can be deposited on glass by brushing on a solution of agarose type VII in water, this is dried at room temperature, no further modifications are required [43]. This makes agarose arguably the simplest polymer supports to work with.
2.1.5. Polyelectrolyte Cushions
Another polymer cushioning system involves polyelectrolytes. These are polymers whose monomer subunits have an electrolyte group. The electrolyte groups will dissociate when exposed to an aqueous solution leaving the polymer with a net charge. To form a bilayer cushion the polyelectrolyte is deposited onto the substrate (which is typically charged) in a layer by layer fashion [44,45]. The substrate is repeatedly dipped between two polyelectrolyte solutions; one a polycation (such as Poly (diallyldimethylammonium chloride)), one a polyanion (such as poly(4-venyl-benzenesulfonic) acid, Figure 3c) [27]. Each dipping causes a monolayer of polyelectrolyte to be adsorbed on the surface through electrostatic attraction and reverses the charge on the surface leaving it ready for the next layer. This layer by layer method is inexpensive, easy and gives excellent thickness control, down to single nanometer precision [27]. Bilayer deposition is then dependent on the relative charges in the system, for a positively charged final polyelectrolyte layer negatively charged lipids are required to get total coverage. This electrostatic coupling may make polyelectrolyte cushions a poor choice for membrane dynamics studies but a good choice for ion channel studies. Surface patterning can be carried out by making use of the electrostatics to selectively layer certain sections through micro contact printing [27]. This approach has the advantage of providing a chemical contrast as opposed to a topographical contrast for membrane patterning [27].
2.2. Coupled Membrane Polymer Systems
Coupling between the bilayer and the polymer support is usually achieved by the use of lipopolymers. These are molecules that have a lipid like structure on one end of the polymer chain allowing that part to insert into a lipid bilayer, while the rest of the polymer is free to form the cushion. In order to get full coverage of the membrane with a supporting cushion the distal portion of the lipopolymer needs to have a reactive end domain allowing it to covalently bind to the solid substrate. Without these tethering points the polymers tend to all reside in the upper leaflet of the bilayer and provide no measurable bilayer/substrate spacing [46]. Even in the presence of the covalent bonding of the polymer to the solid support the system tends to segregate into domains with polymer support and bilayer parts that sit right on top of the solid substrate. The concentration of lipopolymers in the bilayer and the length of the polymer chain can be used to fine tune bilayer/substrate distance for each application
2.2.1. Polyethylene Glycol (PEG)
PEG is one of the most ubiquitous polymers used for lipopolymer constructs. It is a polyether that can be linear or branched and carries little to no charge [47] (Figure 3(e)). It is non-toxic and has excellent wetting characteristics making it an ideal choice for a wide range of biological applications: PEG is used as an antifouling coating on biomedical devices due to its “protein repellent” characteristics, i.e. flexibility and hydrophilicity [48-50]. It was first bio-functionalized in the 1970s to aid in drug solubility and stability in immunological studies [51]. The process of covalently attaching a PEG to a biomolecule (known as pegylation) was developed with proteins in mind; however the process is easily adjusted for lipids. The PEG molecule has a hydroxyl group on both ends of the polymer chain that permits hydrogen bonding to a number of end groups suitable for bio reactions such as: amides, esters, and aldehydes. PEGS for lipid studies usually require different reactive groups on either end, some good options include: amine, maleimide, pyridyl disulfide, and carboxylic acids [48]. As with other lipopolymers, the lateral density of pegylated lipids in a supported lipid bilayer determines whether or not the formation of a polymer cushion will be successful. Too few lipopolymer tethers and the bilayer will sag and contact the solid support. Too many PEGs and free diffusion in the bilayer will be impacted [46].
2.2.2. Poly(2-Methyl-2-Oxazoline) (PMOXA)
PMOXA is emerging as an alternative to PEG [52,53] (see Figure 3(f)). It shares many of the same properties as PEG, such as hydrophilicity, protein repellence and its nonionic nature. In contrast to PEG however, PMOXA is lacking PEG’s ether bonds, which are prone to oxidation, thus rendering PMOXA more stable [52,53]. In addition, PMOXA can also be modified during synthesis to include terminal groups for attachment [52,54,55]. This makes it a potentially easier tether to use in lipopolymers, however it has currently nowhere near the commercial availability as PEG.
2.2.3. Protein Coupled Polymer Cushion
Recently researches have started to employ membrane incorporated proteins as anchor points to the polymer cushion. Presently, the only known such system is based on poly(N-(2-hydroxyethyl)acrylamide-co-5-acrylamido-1- carboxypentyl-iminodiacetate-co-4-benzoylphenyl methacrylate) (P(HEAAm-co-NTAAAm-co-MABP)) that has been modified with the nickel chelating nitrilotriacetic acid (NTA) groups. This allows binding of cytochrome c oxidase via a poly histidine-tag to the polymer cushion surface. The bilayer between the proteins is then formed using direct vesicle deposition [56].
This review focused on the popular methods for providing a polymer bilayer support. Other polymer systems such as dextran and polyethyleneimine (PEI) have also been developed as membrane supports, but have so far not seen widespread use [57,58].
3. Bilayer Deposition on Polymer Support
For the deposition of the lipopolymer containing bilayers or of the lipid membrane on the polymer cushion there are three main options that have successfully been used. They all involve the use of a Langmuir film balance to a greater or lesser degree. This is in stark contrast to solid supported bilayers that can often be formed by simple incubation of the clean substrate with small unilamellar vesicles.
The Langmuir-Blodgett technique [59] requires the lipids to be dispersed as a monolayer at the air-water interface of a Langmuir film balance. The Langmuir film balance allows the surface density of the lipid monolayer to be adjusted: eukaryotic cells are thought to have a surface pressure of 32 mN/m [60] and surface pressure anywhere between this and 20 mN/m have been successfully used for membrane deposition. The surface pressure is adjusted using the parallel barriers and the substrate to be coated is drawn from the liquid phase through the lipid monolayer into the air perpendicular to the surface (Figure 4(a)), this deposits the first lipid monolayer onto the polymer substrate. The substrate is then dipped back through the monolayer, again perpendicular to the surface, to deposit the second monolayer creating a bilayer (Figure 4(d)). This technique is suitable for depositing symmetrical as well as asymmetrical bilayers or multilayers.
The second option is the Langmuir-Schäfer technique [61]. This involves the same first step as for LangmuirBlodgett transfer (Figure 4(a)), however this time the second dip is done parallel to the liquid surface (Figure 4(c)), this way lipids are distributed more evenly in the second monolayer as there is no adjustment required to maintain the surface pressure during the dip. Consequently this produces more homogenous bilayers. Langmuir-Schäfer is sometimes regarded as a variant of the Langmuir-Blodgett technique however Langmuir-Schäfer deposition has typically a better success rate.
The third option is a hybrid Langmuir-Blodgett/vesicle fusion technique [62]. Here the first monolayer is deposited using Langmuir-Blodgett transfer (Figure 4(a)) and the upper leaflet is formed by incubation with vesicles of the desired upper leaflet lipid composition (Figure 4(b)).
The preparation of the lipid bilayer in a lipopolymer based support can be achieved using the same techniques as above, but with some small variations. The necessity of binding the polymer to the substrate (typically through
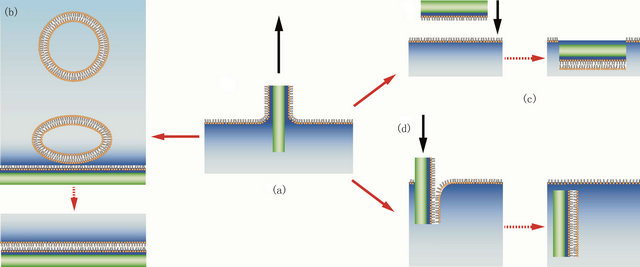
Figure 4. Deposition of a lipid bilayer on a polymer cushion by one of 3 methods. The first step (a) is the same in all schemes: deposition of a monolayer on the substrate via Langmuir-Blodgett transfer. The second monolayer can be created either by incubation with small unilamellar vesicles that fuse to form the top monolayer (b), by horizontal Langmuir-Schäfer transfer (c) or by a second Langmuir-Blodgett transfer.
salinization for glass) means that there are in general two options: Either the molecule that links the solid support to the polymer cushion is part of the polymer already [25] or it is separately deposited as a film over the entire solid support [26]. There are also different options for how to attach the polymers to the bilayer components. The polymers can either be pre-bound to the solid substrate and then have a modified bilayer element to which they bind [63] or they can be pre attached to the lipid monolayer and then deposited on the pretreated surface [25].
4. Advantages and Limitations of Polymer Supports
Obviously, creating a lipid bilayer on a polymer support is much more involved and challenging than producing a bilayer on a solid support or most other membrane mimics. Thus, it is worthwhile to briefly discuss the benefits of such an undertaking. Originally, the creation of polymer supported bilayers was driven by the desire to study membrane bound proteins that have substantially sized cytosolic domain (>10 Å, the typical distance between a solid support and a supported lipid bilayer). Studying such large proteins requires that there be no potentially denaturing interactions between the protein and the solid support. Having an inert polymer spacer solves this problem and still permits the protein to diffuse in the bilayer. The introduction of a space between the bilayer and the substrate also means that a reservoir has been created into which ions can flow through ion channels. Therefore with an electrode at the solid support electrophysiological experiments can be conducted in a controlled manner. This makes for an interesting alternative to traditional patch clamping [43] and vertical free-standing black lipid membranes. A further advantage for such studies is the enhanced self-healing seen in polymer supported bilayers. The elimination of bilayer defects increases the electrical resistance across the bilayer; a definite advantage for ion channel characterization.
It has furthermore been shown that similar to solid supported membranes independent polymer supports can also easily be patterned using one of two different techniques: photo mask lithography or micro contact printing. In the lithographic technique the polymer is chosen such that polymerization or crosslinking can be induced by light [64]. This results in a patterned polymer substrate onto which a bilayer can be easily deposited and constrained by a physical corral. The micro contact printing system uses a polydimethylsiloxane (PDMS) master stamp to transfer a patterned monolayer of “ink” onto a substrate through direct contact [65]. The ink is adsorbed to the substrate leaving an ink design with micrometer feature size. A variety of protein inks have been developed (e.g. fibronectin, and albumin [66]) that can be deposited directly onto a polymer substrate providing diffusion barriers to a lipid bilayer. Micro-patterns such as these can be used to do direct side by side comparison of different lipid species without intermixing, or alternatively to apply different stimuli to different parts of the bilayer without intermixing of the lipids.
When using lipids that have a high charges (such as phosphatidylinositol 4,5-bisphosphate (PIP2) which has three negative charges on its head group [67]) there is potentially electrostatic interactions between the supported lipid bilayer and the substrate. In particular since common glass preparation methods such as piranha etching (75% sulfuric acid (H2SO4) and 25% hydrogen peroxide (H2O2)), hydroxylate the surface leaving it hydrophilic and slightly negatively charged [68]. Separating the charged lipid bilayer from the charged solid support through an inert polymer support of at least several dozens of nanometers introduces enough spatial separation to effectively screen any electrostatic interactions between the solid substrate and the bilayer considering that the Debye screening length at physiological conditions (~150 mM NaCL) is about 1 nm [69].
A final advantage of polymer membrane supports is that while they effectively overcome many of the problems inherent to solid supported membranes, they conserve the latter’s compatibility with most of the modern light-based experimental methods. Thus, techniques such as Fluorescence Correlation Spectroscopy (FCS), Föster resonant energy transfer microscopy, fluorescence recovery after photo bleaching or total internal reflection fluorescence microscopy can readily be used with these systems [4,18,70].
The main disadvantage of polymer supported bilayers is the increased complexity of the production process when compared to solid supported bilayers. Many more steps are required for polymer cushion fabrication and lipid deposition presenting many more opportunities for failure of the system. For the independent polymer supports incorporation of the lipid bilayer requires a Langmuir trough. Even in its simplest form this machine requires a moderate outlay in cost and training and requires much more time and resources than vesicle incubation. The total time required for bilayer production with a polymer support is approximately an order of magnitude more than for a solid supported bilayer. This is a severe disadvantage as it increases the personnel cost as well as the materials expenditure.
5. What Lies Ahead for Polymer Cushioned Bilayers?
The clear, distinctive advantage of polymer supports is the ability to incorporate integral membrane proteins into supported lipid bilayers without them losing form or function. The place where this technology advances however will be at the intersection with the techniques discussed above as well as developments that are still ongoing. The combination of polymer supported lipid membranes and semiconductor supports can for example be used as an organic transistor to reliably detect surface charge on a lipid monolayer [71]. Incorporation of this ability with the proper cultured cells could herald new types of cell based biosensors with an electronic output. Patterned polymer supports could be used to develop whole arrays of different biosensors capable of detecting an enormous range of different properties or reagents on an extremely compact surface.
Polymer cushioned bilayers are new meta-materials that have some features similar to the actin-membrane structure of living cells. Actin is a very dynamic biopolymer and it seems natural to utilize polymers for membrane support that have some added functionality. Using such polymers as cushion could turn polymer supported bilayers into rather active surfaces. For example the pH dependent properties of hydrophilic poly(acrylic acid) (PAA) has been recently used to create an active polymer cushion for bilayer support [72]. One could also envision the use of hydrophilic shape memory polymers such as polyethylene terephthalate-polyethylene glycol copolymer [73] or poly(N-isopropylacrylamide) [74,75] to actively change the topography of the polymer support which would allow for interesting studies of the active coupling of membrane composition and curvature.
6. Conclusion
In conclusion, supported lipid bilayers have been an extremely useful tool in membrane characterization. They are limited however when it comes to studying transmembrane proteins. Here we have reviewed some of the options that are available for introducing a polymer cushion to support a lipid bilayer and have discussed the major benefits of such systems. As we reach the limits of what the traditional solid supported lipid bilayer is capable of, we expect greater uptake of polymer cushions and further development of the technology in the coming years.
7. Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. PHY-0955945.
REFERENCES
- L. K. Tamm and H. M. McConnell, “Supported Phospholipid-Bilayers,” Biophysical Journal, Vol. 47, No. 1, 1985, pp. 105-113. doi:10.1016/S0006-3495(85)83882-0
- G. W. Gordon, G. Berry, X. H. Liang, B. Levine and B. Herman, “Quantitative Fluorescence Resonance Energy Transfer Measurements Using Fluorescence Microscopy,” Biophysical Journal, Vol. 74, No. 5, 1998, pp. 2702-2713. doi:10.1016/S0006-3495(98)77976-7
- M. B. Forstner, C. K. Yee, A. N. Parikh and J. T. Groves, “Lipid Lateral Mobility and Membrane Phase Structure Modulation by Protein Binding,” Journal of the American Chemical Society, Vol. 128, No. 47, 2006, pp. 15221- 15227. doi:10.1021/ja064093h
- J. T. Groves, R. Parthasarathy and M. B. Forstner, “Fluorescence Imaging of Membrane Dynamics,” Annual Review of Biomedical Engineering, Vol. 10, No. 1, 2008, pp. 311-338.
- S. Rozovsky, M. B. Forstner, H. Sondermann and J. T. Groves, “Single Molecule Kinetics of Enth Binding to Lipid Membranes,” Journal of Physical Chemistry B, Vol. 116, No. 17, 2012, pp. 5122-5131. doi:10.1021/jp210045r
- D. Axelrod, T. P. Burghardt and N. L. Thompson, “Total Internal-Reflection Fluorescence,” Annual Review of Biophysics and Bioengineering, Vol. 13, No. 1, 1984, pp. 247-268.
- D. Axelrod, D. E. Koppel, J. Schlessinger, E. Elson and W. W. Webb, “Mobility Measurement by Analysis of Fluorescence Photobleaching Recovery Kinetics,” Biophysical Journal, Vol. 16, No. 9, 1976, pp. 1055-1069. doi:10.1016/S0006-3495(76)85755-4
- K. D. Mossman, G. Campi, J. T. Groves and M. L. Dustin, “Altered Tcr Signaling from Geometrically Repatterned Immunological Synapses,” Science, Vol. 310, No. 5751, 2005, pp. 1191-1193. doi:10.1126/science.1119238
- K. Salaita, P. M. Nair, R. S. Petit, R. M. Neve, D. Das, J. W. Gray, “Restriction of Receptor Movement Alters Cellular Response: Physical Force Sensing by Epha2,” Science, Vol. 327, No. 5971, 2010, pp. 1380-1385. doi:10.1126/science.1181729
- Y. Shao, Y. D. Jin, J. L. Wang, L. Wang, F. Zhao and S. J. Dong, “Conducting Polymer Polypyrrole Supported Bilayer Lipid Membranes,” Biosensors & Bioelectronics, Vol. 20, No. 7, 2005, pp. 1373-1379. doi:10.1016/j.bios.2004.06.001
- M. Tanaka and E. Sackmann, “Supported Membranes as Biofunctional Interfaces and Smart Biosensor Platforms,” Physica Status Solidia-Applications and Materials Science, Vol. 203, No. 14, 2006, pp. 3452-3462.
- M. Trojanowicz and A. Mulchandani, “Analytical Applications of Planar Bilayer Lipid Membranes,” Analytical and Bioanalytical Chemistry, Vol. 379, No. 3, 2004, pp. 347-350. doi:10.1007/s00216-004-2611-4
- M. M. Baksh, M. Jaros and J. T. Groves, “Detection of Molecular Interactions at Membrane Surfaces through Colloid Phase Transitions,” Nature, Vol. 427, No. 6970, 2004, pp. 139-141. doi:10.1038/nature02209
- M. Stelzle, R. Miehlich and E. Sackmann, “2-Dimensional Microelectrophoresis in Supported Lipid Bilayers,” Biophysical Journal, Vol. 63, No. 5, 1992, pp. 1346-1354. doi:10.1016/S0006-3495(92)81712-5
- A. van Oudenaarden and S. G. Boxer, “Brownian Ratchets: Molecular Separations in Lipid Bilayers Supported on Patterned Arrays,” Science, Vol. 285, No. 5430, 1999, pp. 1046-1048. doi:10.1126/science.285.5430.1046
- M. Fischer, A. Bacher, I. Haase, M. Tristl and E. Sackmann, “Design of Biofunctional Assemblies on Solids through Recombinant Spherical Bacterial Protein Lumazine Synthase,” ChemPhysChem, Vol. 2, No. 10, 2001, pp. 623-627. doi:10.1002/1439-7641(20011015)2:10<623::AID-CPHC623>3.0.CO;2-R
- V. Borisenko, T. Lougheed, J. Hesse, E. Fureder-Kitzmuller, N. Fertig and J. C. Behrends, “Simultaneous Optical and Electrical Recording of Single Gramicidin Channels,” Biophysical Journal, Vol. 84, No. 1, 2003, pp. 612- 622. doi:10.1016/S0006-3495(03)74881-4
- Y. F. Dufrene and M. F. Garcia-Parajo, “Recent Progress in Cell Surface Nanoscopy: Light and Force in the Near- Field,” Nano Today, Vol. 7, No. 5, 2012, pp. 390-403. doi:10.1016/j.nantod.2012.08.002
- M. Tanaka, J. Hermann, I. Haase, M. Fischer and S. G. Boxer, “Frictional Drag and Electrical Manipulation of Recombinant Proteins in Polymer-Supported Membranes,” Langmuir, Vol. 23, No. 10, 2007, pp. 5638-5644. doi:10.1021/la0628219
- R. J. Good, “Contact-Angle, Wetting, and Adhesion—A Critical-Review,” Journal of Adhesion Science and Technology, Vol. 6, No. 12, 1992, pp. 1269-1302. doi:10.1163/156856192X00629
- P. F. Rios, H. Dodiuk, S. Kenig, S. McCarthy and A. Dotan, “The Effect of Polymer Surface on the Wetting and Adhesion of Liquid Systems,” Journal of Adhesion Science and Technology, Vol. 21, No. 3-4, 2007, pp. 227- 241. doi:10.1163/156856107780684567
- J. Piehler, A. Brecht, R. Valiokas, B. Liedberg and G. Gauglitz, “A High-Density Poly(Ethylene Glycol) Polymer Brush for Immobilization on Glass-Type Surfaces,” Biosensors & Bioelectronics, Vol. 15, No. 9-10, 2000, pp. 473-481. doi:10.1016/S0956-5663(00)00104-4
- E. Sackmann and M. Tanaka, “Supported Membranes on Soft Polymer Cushions: Fabrication, Characterization and Applications,” Trends in Biotechnology, Vol. 18, No. 2, 2000, pp. 58-64. doi:10.1016/S0167-7799(99)01412-2
- M. Kuhner, R. Tampe and E. Sackmann, “Lipid Monoand Bilayer Supported on Polymer Films: Composite Polymer-Lipid Films on Solid Substrates,” Biophysical Journal, Vol. 67, No. 1, 1994, pp. 217-226. doi:10.1016/S0006-3495(94)80472-2
- M. Wagner and L. Tamm, “Tethered Polymer-Supported Planar Lipid Bilayers for Reconstitution of Integral Membrane Proteins: Silane-Polyethyleneglycol-Lipid as a Cushion and Covalent Linker,” Biophysical Journal, Vol. 79, 2000, pp. 1400-1414. doi:10.1016/S0006-3495(00)76392-2
- T. Wang, D. Li, X. Lu, A. Khmaladze, X. Han and S. Ye, “Single Lipid Bilayers Constructed on Polymer Cushion Studied by Sum Frequency Generation Vibrational Spectroscopy,” Journal of Physical Chemistry C, Vol. 115, No. 15, 2011, pp. 7613-7620.
- N. Kohli, S. Vaidya, R. Ofoli, R. Worden and I. Lee, “Arrays of Lipid Bilayers and Liposomes on Patterned Polyelectrolyte Templates,” Journal of Colloid and Interface Science, Vol. 301, No. 2, 2006, pp. 461-469.
- Y. L. Wang and R. J. Pelham, “Preparation of a Flexible, Porous Polyacrylamide Substrate for Mechanical Studies of Cultured Cells,” Molecular Motors and the Cytoskeleton, Vol. 298, 1998, pp. 489-496.
- H. Towbin, T. Staehelin and J. Gordon, “Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets—Procedure and Some Applications,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 76, No. 9, 1979, pp. 4350- 4354. doi:10.1073/pnas.76.9.4350
- E. C. Muniz and G. Geuskens, “Compressive Elastic Modulus of Polyacrylamide Hydrogels and Semi-Ipns with Poly(N-Isopropylacrylamide),” Macromolecules, Vol. 34, No. 13, 2001, pp. 4480-4484. doi:10.1021/ma001192l
- C. E. Kandow, P. C. Georges, P. A. Janmey and K. A. Beningo, “Polyacrylamidc Hydrogels for Cell Mechanics: Steps toward Optimization and Alternative Uses,” Cell Mechanics, Vol. 83, 2007, pp. 29-46. doi:10.1016/S0091-679X(07)83002-0
- J. Tse and A. Engler, “Preparation of Hydrogel Substrates with Tunable Mechanical Properties,” Current Protocols in Cell Biology, Vol. 10, No. 16, 2010, pp. 1-16.
- F. Yang, R. Murugan, S. Ramakrishna, X. Wang, Y. X. Ma and S. Wang, “Fabrication of Nano-Structured Porous Plla Scaffold Intended for Nerve Tissue Engineering,” Biomaterials, Vol. 25, No. 10, 2004, pp. 1891-1900.
- Y. Duan, J. Liu, H. Sato, J. Zhang, H. Tsuji and Y. Ozaki, “Molecular Weight Dependence of the Poly(L-Lactide)/ Poly(D-Lactide) Stereocomplex at the Air-Water Interface,” Biomacromolecules, Vol. 7, No. 10, 2006, pp. 2728- 2735.
- F. Rehfeldt and M. Tanaka, “Hydration Forces in Ultrathin Films of Cellulose,” Langmuir, Vol. 19, 2003, pp. 1467-1473.
- H. Hillebrandt, G. Wiegand, M. Tanaka and E. Sackmann, “High Electric Resistance Polymer/Lipid Composite Films on Indium-Tin-Oxide Electrodes,” Langmuir, Vol. 15, 1999, pp. 8451-8459.
- J. Groves, L. Mahal and C. Bertozzi, “Control of Cell Adhesion and Growth with Micropatterned Supported Lipid Membranes,” Langmuir, Vol. 17, No. 17, 2001, pp. 5129- 5133.
- R. Falshaw, R. H. Furneaux and D. E. Stevenson, “Agars from Nine Species of Red Seaweed in the Genus Curdiea (Gracilariaceae, Rhodophyta),” Carbohydrate Research, Vol. 308, No. 1-2, 1998, pp. 107-115. doi:10.1016/S0008-6215(98)00049-4
- N. K. Jerne and A. A. Nordin, “Plaque Formation in Agar by Single Antibody-Producing Cells,” Science, Vol. 140, No. 356, 1963, p. 405. doi:10.1126/science.140.3565.405
- W. Y. Gu, H. Yao, C. Y. Huang and H. S. Cheung, “New Insight into Deformation-Dependent Hydraulic Permeability of Gels and Cartilage, and Dynamic Behavior of Agarose Gels in Confined Compression,” Journal of Biomechanics, Vol. 36, No. 4, 2003, pp. 593-598. doi:10.1016/S0021-9290(02)00437-2
- H. Yuan, A. Leitmannova-Ottova and H. Ti Tien, “An Agarose-Stabilized Blm: A New Method for Forming Bilayer Lipid Membranes,” Materials Science and Engineering C, Vol. 4, No. 1, 1996, pp. 35-38.
- X. Lu, A. Leitmannova-Ottova and H. Tien, “Biophysical Aspects of Agar-Gel Supported Bilayer Lipid Nembranes: A New Method for Forming and Studying Planar Bilayer Lipid Membranes,” Bioelectroehemistry and Bioenergetics, Vol. 39, No. 2, 1996, pp. 285-289.
- T. Ide and T. Yanagida, “An Artificial Lipid Bilayer Formed on an Agarose-Coated Glass for Simultaneous Electrical and Optical Measurement of Single Ion Channels,” Biochemical and Biophysical Research Communications, Vol. 265, No. 2, 1999, pp. 595-599.
- K. Katagiri and F. Caruso, “Monodisperse Polyelectrolyte-Supported Asymmetric Lipid-Bilayer Vesicles,” Advanced Materials, Vol. 17, No. 6, 2005, pp. 738-743. doi:10.1002/adma.200401441
- G. Lee, Y. Lee and B. Kyung, “Layer-by-Layer Assembly of Zeolite Crystals on Glass with Polyelectrolytes as Ionic Inkers,” Journal of the American Chemical Society, Vol. 123, No. 40, 2001, pp. 9769-9779. doi:10.1021/ja010517q
- E. B. Watkins, R. J. El-Khouri, C. E. Miller, B. G. Seaby, J. Majewski and C. M. Marques, “Structure and Thermodynamics of Lipid Bilayers on Polyethylene Glycol Cushions: Fact and Fiction of Peg Cushioned Membranes,” Langmuir, Vol. 27, No. 22, 2011, pp. 13618-13628. doi:10.1021/la200622e
- J. Jimenez, A. Heim, G. Matthews and N. Alcantar, “Construction and Characterization of Soft-Supported Lipid Bilayer Membranes for Biosensors Application,” IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Vol. 1, No. 1, 2006, pp. 4119-4122.
- S. M. Ryan, G. Mantovani, X. X. Wang, D. M. Haddleton and D. J. Brayden, “Advances in Pegylation of Important Biotech Molecules: Delivery Aspects,” Expert Opinion on Drug Delivery, Vol. 5, No. 4, 2008, pp. 371-383. doi:10.1517/17425247.5.4.371
- N. Ngadi, J. Abrahamson, C. Fee and K. Morison, “Are Peg Molecules a Universal Protein Repellent?” International Journal of Biological and Life Sciences, Vol. 5, No. 3, 2009, pp. 106-110.
- G. Pasut and F. Veronese, “State of the Art in Pegylation: The Great Versatility Achieved after Forty Years of Research,” Journal of Controlled Release, Vol. 161, No. 161, 2012, pp. 461-472. doi:10.1016/j.jconrel.2011.10.037
- F. F. Davis, “Commentary—The Origin of Pegnology,” Advanced Drug Delivery Reviews, Vol. 54, No. 4, 2002, pp. 457-458. doi:10.1016/S0169-409X(02)00021-2
- R. Konradi, B. Pidhatika, A. Muhlebach and M. Textor, “Poly-2-Methyl-2-Oxazoline: A Peptide-Like Polymer for Protein-Repellent Surfaces,” Langmuir, Vol. 24, No. 3, 2008, pp. 613-616.
- R. Konradi, B. Pidhatika, Q. Li and M. Textor, “Poly(2- Methyl-2-Oxazoline): Protein-Like Polymer for the Fabrication of Functional Non-Fouling Surface Coatings,” European Cells and Materials, Vol. 14, No. 3, 2007, p. 131.
- O. Purrucker, A. Fortig, R. Jordan and M. Tanaka, “Supported Membranes with Well-Defined Polymer TethersIncorporation of Cell Receptors,” ChemPhysChem, Vol. 5, No. 3, 2004, pp. 327-335. doi:10.1002/cphc.200300863
- C. A. Naumann, O. Prucker, T. Lehmann, J. Ruhe, W. Knoll and C. W. Frank, “The Polymer-Supported Phospholipid Bilayer: Tethering as a New Approach to Substrate-Membrane Stabilization,” Biomacromolecules, Vol. 3, No. 1, 2002, pp. 27-35. doi:10.1021/bm0100211
- A. Kibrom, R. F. Roskamp, U. Jonas, B. Menges, W. Knoll and H. Paulsen, “Hydrogel-Supported Protein-Tethered Bilayer Lipid Membranes: A New Approach toward Polymer-Supported Lipid Membranes,” Soft Matter, Vol. 7, No. 1, 2011, pp. 237-246. doi:10.1039/c0sm00618a
- J. Majewski, J. Y. Wong, C. K. Park, M. Seitz, J. N. Israelachvili and G. S. Smith, “Structural Studies of Polymer-Cushioned Lipid Bilayers,” Biophysical Journal, Vol. 75, No. 5, 1998, pp. 2363-2367. doi:10.1016/S0006-3495(98)77680-5
- K. Adlkofer, M. Tanaka, H. Hillebrandt, G. Wiegand, E. Sackmann and T. Bolom, “Electrochemical Passivation of Gallium Arsenide Surface with Organic Self-Assembled Monolayers in Aqueous Electrolytes,” Applied Physics Letters, Vol. 76, No. 22, 2000, pp. 3313-3315. doi:10.1063/1.126636
- Y. G. Jin, Y. X. Qiao and X. P. Hou, “The Effects of Chain Number and State of Lipid Derivatives of Nucleosides on Hydrogen Bonding and Self-Assembly through the Investigation of Langmuir-Blodgett Films,” Applied Surface Science, Vol. 252, No. 22, 2006, pp. 7926-7929. doi:10.1016/j.apsusc.2005.09.073
- [61] H. L. Brockman, M. M. Momsen, J. R. Knudtson, S. T. Miller, G. Graff and J. M. Yanni, “Interactions of Olopatadine and Selected Antihistamines with Model and Natural Membranes,” Ocular Immunology and Inflammation, Vol. 11, No. 4, 2003, pp. 247-268. doi:10.1076/ocii.11.4.247.18261
- [62] A. V. Hughes, J. R. Howse, A. Dabkowska, R. A. L. Jones, M. J. Lawrence and S. J. Roser, “Floating Lipid Bilayers Deposited on Chemically Grafted Phosphatidylcholine Surfaces,” Langmuir, Vol. 24, No. 5, 2008, pp. 1989-1999. doi:10.1021/la702050b
- [63] E. T. Castellana and P. S. Cremer, “Solid Supported Lipid Bilayers: From Biophysical Studies to Sensor Design,” Surface Science Reports, Vol. 61, No. 10, 2006, pp. 429- 444. doi:10.1016/j.surfrep.2006.06.001
- [64] Y. Lin, D. Minner, V. Herring and C. Naumann, “Physisorbed Polymer-Tethered Lipid Bilayer with Lipopolymer Gradient,” Materials, Vol. 5, No. 3, 2012, pp. 2243- 2257. doi:10.3390/ma5112243
- [65] K. Morigaki, T. Baumgart, A. Offenhausser and W. Knoll, “Patterning Solid-Supported Lipid Bilayer Membranes by Lithographic Polymerization of a Diacetylene Lipid,” Angewandte Chemie-International Edition, Vol. 40, No. 1, 2001, pp. 172-174. doi:10.1002/1521-3773(20010105)40:1<172::AID-ANIE172>3.0.CO;2-G
- [66] J. T. Groves, L. K. Mahal and C. R. Bertozzi, “Control of Cell Adhesion and Growth with Micropatterned Supported Lipid Membranes,” Langmuir, Vol. 17, No. 17, 2001, pp. 5129-5133. doi:10.1021/la010481f
- [67] C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, “Geometric Control of Cell Life and Death,” Science, Vol. 276, No. 5317, 1997, pp. 1425-1428. doi:10.1126/science.276.5317.1425
- [68] I. M. Thornell, J. P. Wu, X. F. Liu and M. O. Bevensee, “Pip2 Hydrolysis Stimulates the Electrogenic Na+-Bicarbonate Cotransporter Nbce1-B and -C Variants Expressed in Xenopus Laevis Oocytes,” Journal of Physiology-London, Vol. 590, No. 23, 2012, pp. 5993-6011. doi:10.1113/jphysiol.2012.242479
- [69] K. J. Seu, A. P. Pandey, F. Haque, E. A. Proctor, A. E. Ribbe and J. S. Hovis, “Effect of Surface Treatment on Diffusion and Domain Formation in Supported Lipid Bilayers,” Biophysical Journal, Vol. 92, No. 7, 2007, pp. 2445-2450. doi:10.1529/biophysj.106.099721
- [70] D. Trebotich, G. H. Miller and M. D. Bybee, “A Penalty Method to Model Particle Interactions in DNA-Laden Flows,” Journal of Nanoscience and Nanotechnology, Vol. 8, No. 7, 2008, pp. 3749-3756.
- [71] K. P. N. Bruns, L. M. Bergeron, T. A. Whitehead and D. S. Clark, “Mechanical Nanosensor Based on Fret within a Thermosome for Damage-Reporting Polymeric Materials,” Angewante Chemie Internatioanl Edition, Vol. 48, No. 31, 2009, pp. 5666-5669.
- [72] H. Hillebrandt, M. Tanaka and E. Sackmann, “A Novel Membrane Charge Sensor: Sensitive Detection of Surface Charge at Polymer/Lipid Composite Films on Indium Tin Oxide Electrodes,” Journal of Physical Chemistry B, Vol. 106, No. 2, 2002, pp. 477-486. doi:10.1021/jp011693o
- [73] R. J. El-Khouri, D. A. Bricarello, E. B. Watkins, C. Y. Kim, C. E. Miller, T. E. Patten, “Ph Responsive Polymer Cushions for Probing Membrane Environment Interactions,” Nano Letters, Vol. 11, No. 5, 2011, pp. 2169-2172. doi:10.1021/nl200832c
- [74] J. S. Leng, X. Lan, Y. J. Liu and S. Y. Du, “Shape-Memory Polymers and Their Composites: Stimulus Methods and Applications,” Progress in Materials Science, Vol. 56, No. 7, 2011, pp. 1077-1135. doi:10.1016/j.pmatsci.2011.03.001
- [75] P. T. Mather, X. F. Luo and I. A. Rousseau, “Shape Memory Polymer Research,” Annual Review of Materials Research, Vol. 39, No. 1, 2009, pp. 445-471. doi:10.1146/annurev-matsci-082908-145419
- [76] K. A. Davis, K. A. Burke, P. T. Mather and J. H. Henderson, “Dynamic Cell Behavior on Shape Memory Polymer Substrates,” Biomaterials, Vol. 32, No. 9, 2011, pp. 2285- 2293. doi:10.1016/j.biomaterials.2010.12.006

