Open Journal of Medicinal Chemistry
Vol.2 No.3(2012), Article ID:22916,6 pages DOI:10.4236/ojmc.2012.23009
Synthesis and Evaluation of Anticonvulsant Activity of 6,8-Dimethoxy-3-methyl-1,2,3,4-tetrahydroisoquinoline in PTZ-Induced Seizure Model in Mice
1Graduate School of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand
2Faculty of Pharmaceutical Sciences, Burapha University, Chonburi, Thailand
3Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand
Email: *pploenthip@kku.ac.th.
Received May 18, 2012; revised June 23, 2012; accepted July 5, 2012
Keywords: Anticonvulsant; Pentylenetetrazole; Tetrahydroisoquinoline
ABSTRACT
This study we describe the synthesis of a novel structure of anticonvulsant agent as 6,8-dimethoxy-3-methyl-1,2,3,4-tetrahydroisoquinoline by using GYKI52466, which was the potent anticonvulsant agent, as the lead molecule. Compound IV was synthesized and anticonvulsant effects was evaluated against Pentylenetetrazole (PTZ)-induced seizure model in mice. The acute anticonvulsant effect was tested with a single dose of 25 and 75 µmol/kg of the synthesis compound. Sodium valproate and normal saline were used as the reference standard and control, respectively. All compounds were injected intraperitoneally to each mouse an hour prior to seizure induced by injection of 60 mg/kg PTZ and observed their behavior for 30 minutes. The result showed that the IV at 75 µmol/kg could delay the latency to first twitch and decrease percent mortality compared to control group.
1. Introduction
Epilepsy is a common chronic neurological disorder affecting individuals of all ages. It is defined as the occurrence of at least one epileptic seizure, unprovoked by any immediate identified cause [1]. There are several ways for the treatment of epilepsies, although antiepileptic drugs (AEDs) remain the most widely utilized treatment [2]. 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3- benzodiazepine (GYKI52466, 1) is the first compound showed anticonvulsant that act via noncompetitive-AMPA receptor antagonist and was used as a lead for many studies [3-10]. Subsequently, talampanel (2) and CFM-2 (3) was identified, and showed highly active molecule with good results in various animal seizure models [11- 13]. According to 3D-pharmacophore study of Barreca et al. [11] the simple replacement of diazepine ring with tetrahydropyridine system directed the synthesis toward the 2-acetyl-1-aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolines (4), which mapped well onto the 3D-pharmacophore hypothesis (two hydrophobic groups, hydrogen bond acceptor feature and one aromatic region in a speci- fic three-dimensional arrangement) and shows an anticonvulsant activity more potent than 1 and 2 [14].
According to the structure-activity relationship (SAR) studies, modification by substitute halogens (ex. Cl, Br) at 4’-position on phenyl ring appears to improve the anticonvulsant activity [15-17]. Since there was the study showed that mono-substitution of methoxy showed lower efficacy than the corresponding 6,7-disubstituted derivative as one chemical feature of model is not mapped.
In this study we designed to synthesize IV with the introduction of 6,8-dimethoxy which expect to be well mapped on 3D-pharmacophore hypothesis and 3-methyl for analogy with 2 (Figure 1). Moreover, we introduce Cl at 4’-position on phenyl ring in expected to improve pharmacological and pharmacokinetic of the compound. The aims are to explore if these modification could influent the anticonvulsant effect by primary screen anticonvulsant activity via Pentylenetetrazole (PTZ)-induced seizure model.
2. Result and Discussions
2.1. Chemistry
The synthesis of the study compound was accomplished as shown in Scheme 1. The 3,5-dimethoxybenzaldehyde will
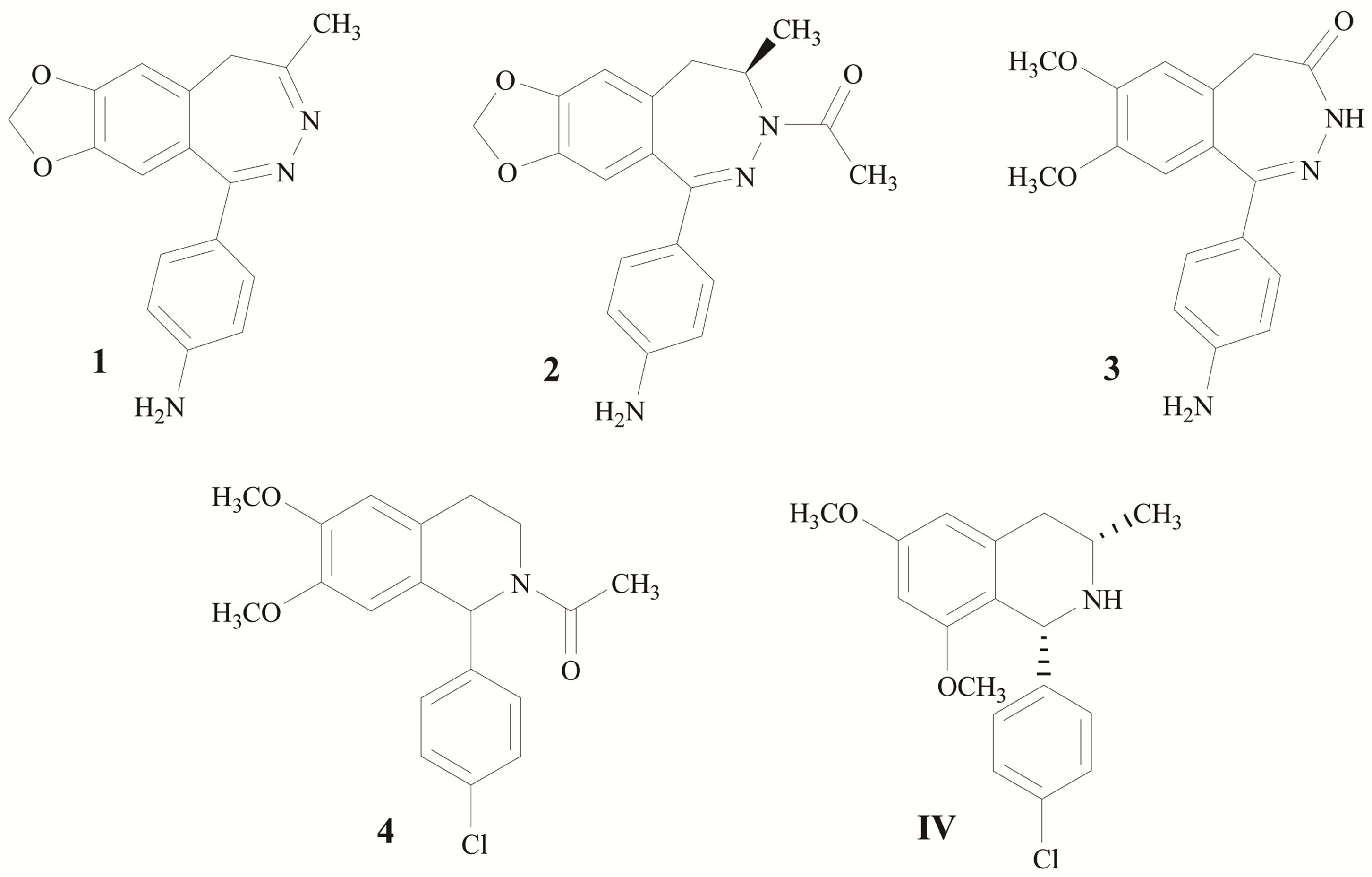
Figure 1. Non-competitive AMPA receptor antagonists.
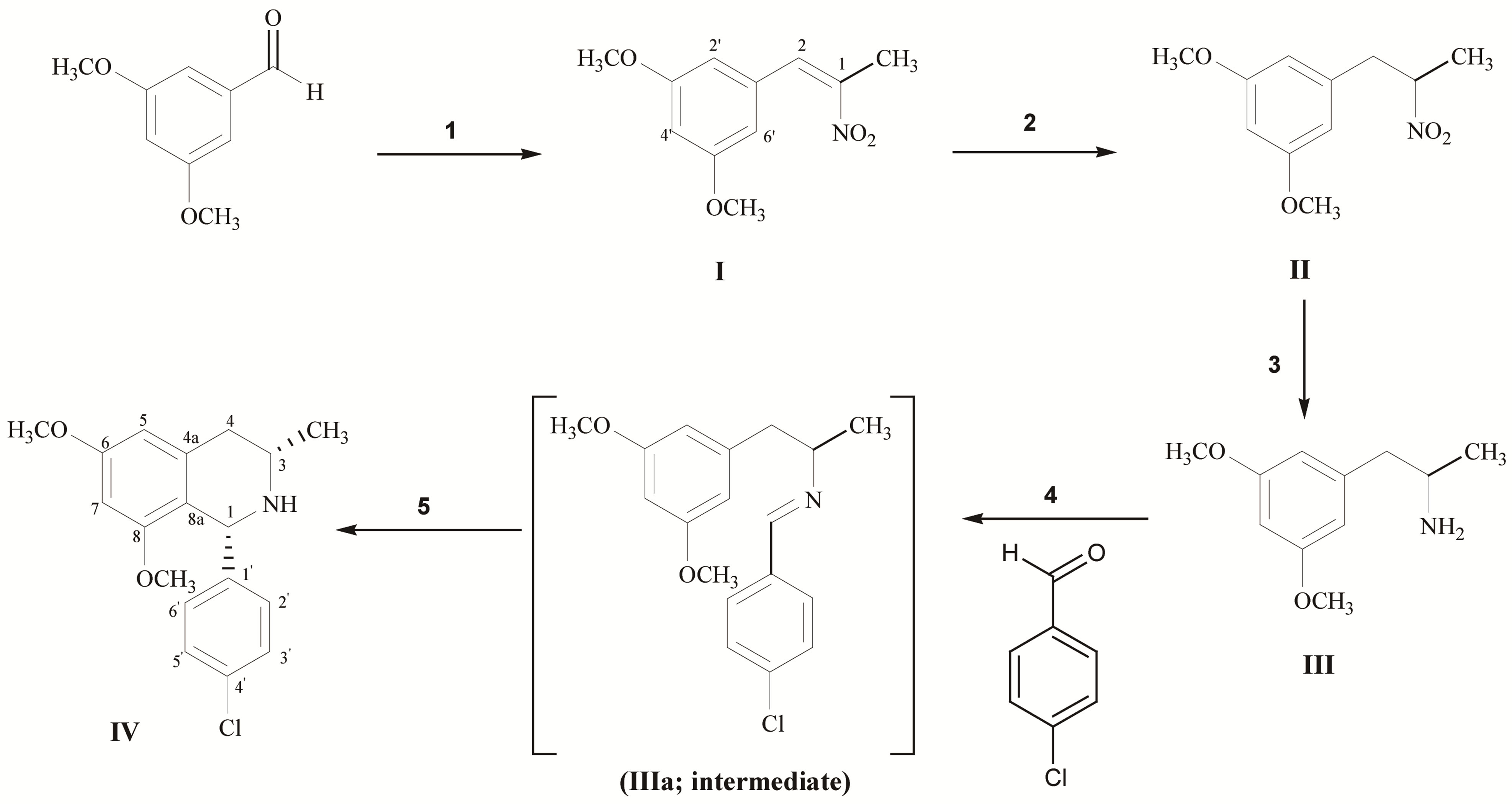
Scheme 1. Reagent and conditions; (1) CH3CH2NO2, NH4OAc, reflux; (2) NaBH4, SiO2, IPA/CHCl3, rt; (3) H2, 10% Pd/C, MeOH; (4) 4-Chlorobenzaldehyde, toluene, reflux; (5) TFA, MeOH, reflux.
be used as starting material. It was treated with nitroethane and ammonium acetate (1 eq.) in order to obtain I as yellow crystalline solid. The 1H-NMR of the product showed the singlet pattern of methoxy group at δ 3.81 ppm and showed the H-aromatic signal at δ 6.50 - 6.54 ppm. The product was confirmed successful reaction by the singlet signal of proton position 2 showed at δ 8.00 ppm and carbon signal showed at δ 148.5 ppm. The methyl substitute showed proton singlet at δ 2.44 ppm and carbon signal at δ 14.5 ppm. Secondly, it was further reduced by NaBH4 (4 eq.) to give II as yellowish oil. The product was confirmed by multiplet proton signal of position 1 showed at δ 4.75 ppm. The proton signal of position 2 showed doublet of doublet pattern at δ 2.90 (J = 7.6, 13.8 Hz) and 3.22 (J = 6.4, 13.8 Hz) ppm. Moreover, proton signal of methyl group was up-field shifted from δ 2.44 to 1.50 ppm. In 13C-NMR, the carbon signal of position 1 was up field shifted from δ 133.95 ppm to 84.62 ppm and position 2 from 148.55 to 41.75 ppm. Alternation of nitro group to amine group was performed by the hydrogenation under hydrogen gas with 10% Pd/C, III can be obtained as yellow oil. The III was confirmed successful hydrogenation by the up-filed shift of the proton signal at position 1 from δ 4.17 to 3.16 ppm and amine group showed the broad singlet signal at δ 2.09 ppm.
Compound III was further refluxed with 4-chlorobenzaldehyde (5 eq.) in toluene to give yellowish oil product of imine intermediate (Schiff base). The imine intermediate is unstable in the air and high moisture condition. Therefore, the next cyclization step will be continuously performed via Pictet-Spengler synthetic pathway in the presence of TFA (1.5 eq.) to give IV as yellow solid. The IV can be isolated by conventional column chroma- tography. The complete cyclization was confirmed by the 1D and 2D-NMR. The proton signal of position 4 in compound IV at δ 2.6 ppm showed the axial-axial interaction (Jax) between proton at positions 3 and 4 which had coupling constant equal to 10.8 Hz, while represented geminal coupling (Jgem) as 15.6 Hz of both protons at position 4. Furthermore, proton signal of position 4 at δ 2.7 ppm represented the axial-equatorial interaction (Jax) which had coupling constant equal to 2.4 Hz. These difference J values suggested that 3-methyl group of IV should be in equatorial configuration. And stereochemistry of IV was confirmed as cis-isomer by using Nuclear Overhauser effect (NOE) method. The NOE data of IV showed strong exchanges of magnetization among the 3-H and 1-H indicated that these protons should be in a cis-isomer (Figure 2).
2.2 Anticonvulsant Activity
The anticonvulsant effects were evaluated against PTZinduced seizure model in ICR mice (Table 1). The results were compared with standard anticonvulsant, valproic acid (VPA). The dose for VPA (200 mg/kg) was based on experimental evidence that dose from 100 to 400 mg/kg (i.p.) are effective in animal model [18,19]. PTZ is a convulsant chemical agent frequently use in experimental models for induction of seizure [20]. According to various studies using PTZ-induced seizure model, the dose of PTZ varied from 50 to 125 mg/kg. We decide to
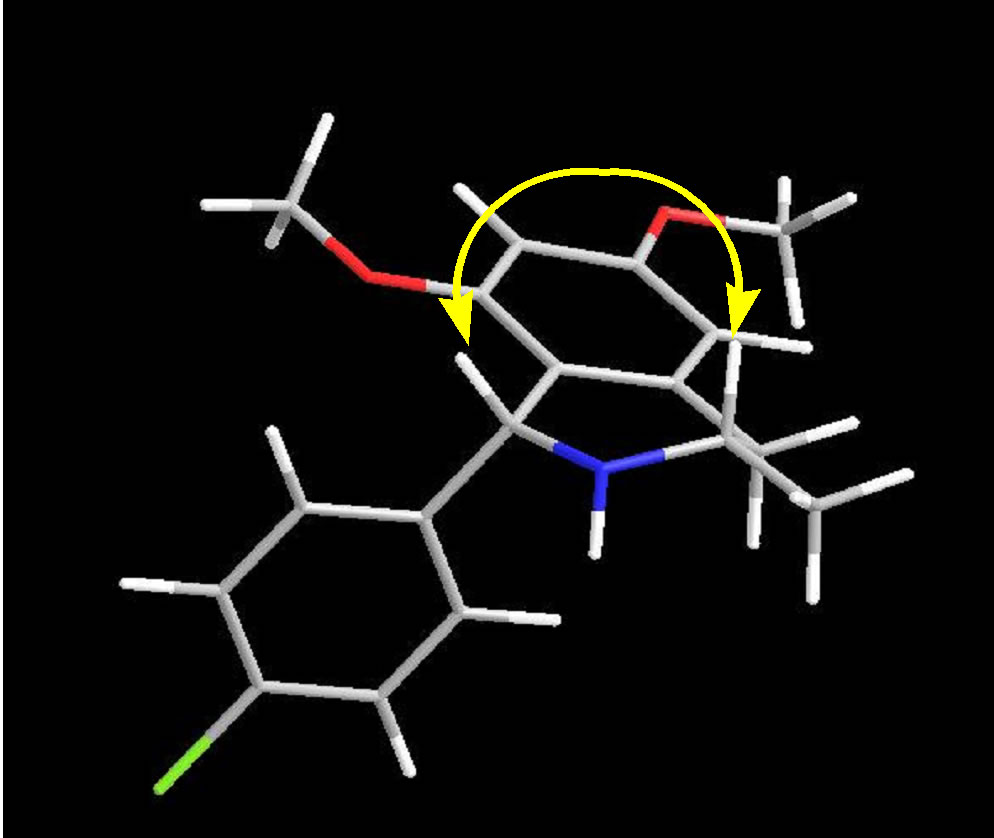
Figure 2. NOE interaction for IV.
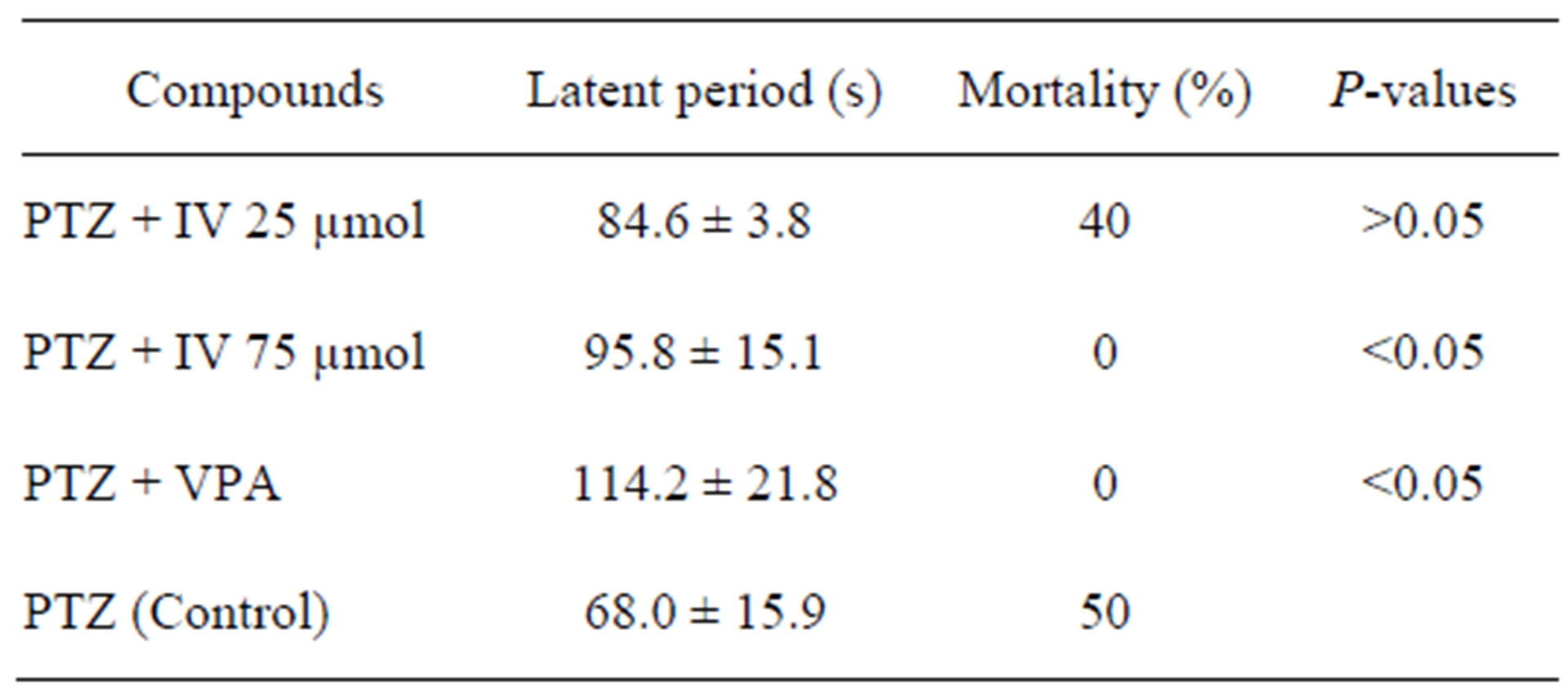
Table 1. Anticonvulsant activity of IV against PTZ-induced seizure model.
use 60 mg/kg PTZ by i.p. injection as it was the dose that produces jerks, myoclonus and clonic generalized seizures in 100% of control animals. Moreover, it was the dose that is between the 50% effective dose (ED50) of 33 mg/kg [21].
In this study, anticonvulsant effect of IV was evaluated after i.p. administration against PTZ-induced seizure in ICR mice. We observed that in control group which mice received 60 mg/kg PTZ in saline, all mice showed seizure and 50% death. Pretreatment of IV 75 µmol/kg could 100% protect the mortality of mice, however mice still have seizure in all stage. While, pretreatment with VPA gave 100% protection. Furthermore, the latency to the first twitch, we found that the latency period was increased in groups pretreatment with IV and VPA. Moreover, a significant increase in the latent period was observed in the dosage of 75 µmol/kg IV (95.8 ± 15.1 as compared to 68.0 ± 15.9 seconds in the control group).
3. Experimentals
Chemicals were purchased from Fluka and Merck from Germany as synthesis grade. Reactions were monitored by TLC Silicagel 60 F254 (Merk, Germany) and were visualized under Ultraviolet light at 254 nm. Iodine vapors were also used for the spot detection. All melting points were recorded on Electrothermal melting point apparatus. Nuclear magnetic resonance (1H-NMR, 13CNMR and 2D-NMR) spectra were recorded in CDCl3 on Varian NMR-400 MHz Spectrometer (Faculty of Science, Khon Kaen University, Khon Kaen, Thailand. Chemical shifts are expressed in δ (ppm) relative to TMS as the internal standard and coupling constants (J) are in hertz.
3.1. Synthesis
2-(3’,5’-Dimethoxyphenyl)-1-methyl-1-nitroethene (I).
Ammonium acetate (2.4 mmol, 207.9 mg) was slowly added to the mixture of 3,5-dimethoxybenzaldehyde (2.4 mmol, 400 mg) in nitroethane (6 mL). The mixture was refluxed for 6 hours. The reaction was worked-up by extracted with water (100 mL) and EtOAc (3 × 100 mL), the organic layer was dried with Na2SO4 anh. and filtered out. The organic layer was then concentrated under rotary evaporator and purification by recrystallization with methanol to afford yellow crystalline solid. Yield: 82%, mp 88.2 - 90.0˚C, Rf = 0.5 in Hexane: EtOAc (5:1). 1H-NMR (δ, ppm): 2.44 (1 H, s, CH3-3), 3.81 (2 × 3 H, s, OCH3-3’ and 5’), 6.50 (1 H, s, H-4’), 6.54 (2 H, s, H-2’ and H-6’), 8.00 (1H, s, H-2). 13C-NMR (δ, ppm): 14.54 (CH3-1), 55.89 (OCH3-3 and 5), 102.13 (C-4), 108.31 (C-2’ and C-6’), 133.95 (C-1), 134.58 (C-1), 148.55 (C-2), 161.36 (C-3’ and C-5’).
2-(3’,5’-Dimethoxyphenyl)-1-methyl-1-nitroethane (II).
The mixture of I (3.64 mmol, 811.2 mg) was dissolved in the mixture of CHCl3: IPA in a ratio of 1:1 (30 mL). Then, silica gel (5 g) was slowly added to the mixture. After that, NaBH4 (14.55 mmol, 550.0 mg) was slowly added over 1 hour in ice-bath. Then, the mixture was stirred at room temperature for 12 hours. The reaction was stopped by slowly added glacial acetic acid until there were no more bubble gases. The silica gel was filtered out. The excess glacial acetic acid was neutralized with 5% NaHCO3. The organic layer was then further washed with water (3 × 100 mL) and dried over Na2SO4 anh. and filtered out. The solution was removed under reduced pressure to give yellow oil product. Yield: 94 %, Rf = 0.4 in Hexane : EtOAc (5:1). 1H-NMR (δ, ppm): 1.50 (3H, d, J = 6.8 Hz, CH3-3), 2.90 (1 H, dd, Jgem = 13.8 Hz, Jvic = 7.6 Hz, H-2), 3.22 (1 H, dd, Jgem = 13.8 Hz, Jvic = 6.4 Hz, H-2), 3.73 (2 × 3 H, s, OCH3-3’ and 5’), 4.72 - 4.77 (1 H, m, H-1), 6.29 (2 H, s, H-2’ and 6’), 6.34 (1 H, s, H-4’). 13C-NMR (δ, ppm): 19.25 (1-CH3), 41.75 (C-2), 55.69 (OCH3-3’ and 5’), 84.62 (C-1), 99.56 (C-4’), 107.48 (C-2’ and C-6’), 138.14 (C-1’), 161.46 (C-3’ and C-5’).
2-(3’,5’-Dimethoxyphenyl)-1-methylethylamine (III).
A solution of II (16.6 mmol, 3.74 g) in ethanol (20 mL) was hydrogenated over 10% Pd/C (10% mass of starting material). After the complete reaction, the catalyst was filtered out by wash with ethanol. The filtrate was removed under reduced pressure and further extracted with EtOAc (100 mL) and 0.5 N HCl (3 × 100 mL). The aqueous layer was basified to pH = 9 - 10 by ammonia solution. After that, the basified solution was extracted with EtOAc (3 × 100 mL). The organic layer was dried over Na2SO4 anh. and removed under reduced pressure to obtain yellow oil product (III). Yield: 56%, Rf = 0.12 in CHCl3: MeOH (10:0.5). 1H-NMR (δ, ppm): 1.11 (3 H, d, J = 6.8 Hz, CH3-3), 2.09 (2H, s, NH2), 2.46 (1 H, dd, Jgem = 13.2 Hz, Jvic = 8.0 Hz, H-2), 2.64 (1 H, dd, Jgem = 13.2 Hz, Jvic = 5.2 Hz, H-2), 3.14-3.19 (1 H, m, H-1), 3.75 (2 × 3H, s, OCH3-3’ and 5’), 6.30 (2 H, s, H-2’ and 6’), 6.32 (1H, s, H-4’). 13C-NMR (δ, ppm): 23.98 (1-CH3), 47.33 (C-2), 48.71 (C-1), 55.65 (OCH3-3’ and 5’), 98.54 (C-4’), 107.65 (C-2’ and C-6’), 142.48 (C-1’), 161.18 (C-3’ and C-5’).
N-(4-Chlorobenzylidene)-2-(3’,5’-dimethoxyphenyl)-1-methylethylamine (IIIa).
4-Chlorobenzaldehyde (6.65 mmol, 931 mg) was added to a solution of III (1.33 mmol, 259 mg) in toluene (20 mL). The reaction mixture was refluxed under DeanStark separator for 6 hours. The solvent was removed under rotary evaporator apparatus to give yellowish oil product (IIIa) as intermediate. The compound was then continuously processed in the next step.
1-(4’-Chlorophenyl)-6,8-dimethoxy-3-methyl-1,2,3,4-tetrahydroisoquinoline (IV).
Trifluoroacetic acid (1.5 eq) was added to IIIa in methanol (10 mL) and refluxed for 2.5 hours. After that, the reaction was cooled down at room temperature and extracted with water (100 mL) and EtOAc (3 × 100 ml). Finally, dried the organic layer over Na2SO4 anh. and concentrated under reduced pressure. Purification by column chromatography to obtain yellow solid. Yield: 57%, mp: 80.5˚C - 82.0˚C, Rf = 0.53 in Hexane: EtOAc (1:1). 1H-NMR (CDCl3) δ: 1.20 (3H, d, J = 6 Hz, CH3-3), 1.25 (1 H, bs, NH), 2.60 (1 H, dd, Jgem =15.6 Hz, Jax = 10.8 Hz, Hax-4), 2.70 (1 H, dd, Jgem = 15.6 Hz, Jeq = 2.8 Hz, Heq-4), 2.98 - 3.07 (1 H, m, H-3), 3.38 (3 H, s, OCH3-8), 3.79 (3 H, s, OCH3-6), 5.13 (1 H, s, H-1), 6.22 (1 H, s, H-7), 6.28 (1 H, s, H-5), 7.12 - 7.21 (4 H, Aromatic-H). 13C-NMR (δ, ppm): 21.77 (CH3-3), 38.91 (C-4), 48.97 (C-3), 55.00 (OCH3-8), 55.24 (OCH3-6), 58.38 (C-1), 97.20 (H-7), 104.49 (C-5), 118.39 (C-8a), 128.40 (C-3’ and 5’), 128.84 (C-2’ and 6’), 132.08 (C-4’), 138.45 (C-4a), 144.60 (C-1’), 157.55 (C-8), 159.28 (C-6).
3.2. Anticonvulsant Activity
Anticonvulsant activity was performed on five-week male ICR mice weigh 30 g - 35 g (Mahidol University, Thailand). The animal were acclimatized in the laboratory in a ventilated room at the ambient temperature of 25˚C on a natural light/dark cycle for at least one week prior to the experiments. Food and water were provided. Experiments were completed within a week to minimize the effect of increasing age on seizure susceptibility. In all experiments, each animal was used for only once and the experiments were carried out between 9.00 a.m.-6.00 p.m. The experiment was approved by Animals Ethics Committee of Khon Kaen University based on the Ethic of Animal Experimentation of National Research Council of Thailand.
3.3. Anticonvulsant Activity against PTZ-Induced Seizure Model
All compounds were fleshly prepared. VPA and PTZ were was dissolved in 15% tween 80 and normal saline, respectively. The method was modified from Agarwal et al. [22]. PTZ in a dose 60 mg/kg was intraperitoneally injected after 45 minutes administration of normal saline, IV in a dose of 25, 75 µmol/kg or valproic acid 200 mg/kg. All compounds were injected in a volume of 0.01 mL/g body weight. In the control, PTZ was injected 45 minutes after the first administration of saline. The behavior of the mice was observed and recorded for 30 minutes after PTZ injection and subsequently analyzed. Time latencies for generalized clonus was measured. The absence of seizures within 30 min, the latency time was taken as 1800 sec. Latencies were calculated as the time between PTZ injections to the onset of these stages. Generalizes clonus was described by the involvement of all four limbs and tail, rearing, wild running and jumping, sudden loss of upright posture and autonomic signs [23].
3.4. Statistical Analysis
The data are expressed as means ± standard error (SE). The correlations were carried out by ANOVA followed by Dunnett’s test and Kruskal-Wallis nonparametric oneway analysis of variance. SigmaStat 3.11 software was used for statistical analysis and probability value of less than 0.05 was accepted as statistically significant.
4. Conclusion
We prepared a new core structure of anticonvulsant agent as 6,8-dimethoxy-3-methyl-1,2,3,4-tetrahydroisoquinoline bearing a pharmacophoric fragment of GYKI52466. The data revealed that the compound IV in the dosage of 75 µmol/kg was able to increase the latency period and decrease mortality in preliminary screening PTZ-induced seizure model in mice. On the basis of the results obtained with the new compound, IV could present new core structure for further chemical modification for anticonvulsant agent.
5. Acknowledgements
Financial supports for this work were provided by the SubCluster of Integrated Multidisciplinary Researches in Health Sciences (MIH-2554-M-12, MIH-55-MR-11) and Graduate Research Fund Academic Year 2011 (54211125) from Khon Kaen University, Thailand. The authors grateful to Prof. Naoki Saito and Prof. Akinori Kubo, Meiji Pharmaceutical University, Japan, for supporting 3,5-dimethoxybenzaldehyde and Pd/C and Department of Pharmaceutical chemistry, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand, for supporting Parr hydrogenation apparatus in the preparation of compound III.
REFERENCES
- R. S. Fisher, W. van Emde Boas, W. Blume, C. Elger, P. Genton, P. Lee and J. J. Engel, “Epileptic Seizures and Epilepsy: Definitions Proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE),” Epilepsia, Vol. 46, No. 4, 2005, pp. 470- 472. doi:10.1111/j.0013-9580.2005.66104.x
- A. C. Gerlach and J. L. Krajewski, “Antiepileptic Drug Discovery and Development: What Have We Learned and Where Are We Going?” Pharmaceuticals, Vol. 3, No. 9, 2010, pp. 2884-2899. doi:10.3390/ph3092884
- A. Chimirri, G. De Sarro, A. De Sarro, R. Gitto, S. Grasso, S. Quartarone, M. Zappalà, P. Giusti, V. Libri, A. Constanti and A. G. Chapman, “1-Aryl-3,5-dihydro- 4H-2,3-benzodiazepin-4-ones: Novel AMPA Receptor Antagonists,” Journal of Medicinal Chemistry, Vol. 40, No. 8, 1997, pp. 1258-1269. doi:10.1021/jm960506l
- A. Chimirri, G. De Sarro, A. De Sarro, R. Gitto, S. Quartarone, M. Zappalà, A. Constanti and V. Libri, “3,5- Dihydro-4H-2,3-benzodiazepine-4-thiones: A New Class of AMPA Receptor Antagonists,” Journal of Medicinal Chemistry, Vol. 41, No. 18, 1998, pp. 3409-3416. doi:10.1021/jm9800393
- M. Zappalà, R. Gitto, F. Bevacqua, S. Quartarone, A. Chimirri, M. Rizzo, G. D. Sarro and A. D. Sarro, “Synthesis and Evaluation of Pharmacological and Pharmacokinetic Properties of 11H-[1,2,4]triazolo[4,5-c][2,3] benzodiazepin-3(2H)-ones,” Journal of Medicinal Chemistry, Vol. 43, No. 25, 2000, pp. 4834-4839. doi:10.1021/jm001012y
- M. Zappalà, G. Postorino, N. Micale, S. Caccamese, N. Parrinello, G. Grazioso, G. Roda, F. S. Menniti, G. D. Sarro and S. Grasso, “Synthesis, Chiral Resolution, and Enantiopharmacology of a Potent 2,3-benzodiazepine Derivative as Noncompetitive AMPA Receptor Antagonis,” Journal of Medicinal Chemistry, Vol. 49, No. 2, 2006, pp. 575-581. doi:10.1021/jm050552y
- Y. Wang, C. S. Konkoy, V. I. Ilyin, K. E. Vanover, R. B. Carter, E. Weber, J. F. Keana, R. M. Woodward and S. X. Cai, “Synthesis of 7,8-(Methylenedioxy)-1-phenyl-3,5-dihydro-4H-2,3-benzodiazepin-4-ones as Novel and Potent Noncompetitive AMPA Receptor Antagonists,” Journal of Medicinal Chemistry, Vol. 41, No. 14, 1998, pp. 2621- 2625. doi:10.1021/jm980168j
- G. Abrahám, S. Sólyom, E. Csuzdi, P. Berzsenyi , I. Ling, I. Tarnawa, T. Hámori, I. Pallagi, K. Horváth, F. Andrási, G. Kapus, L. G. J. Hársing, I. Király, M. Patthy and G. Horváth, “New Noncompetitive AMPA Antagonists,” Bioorganic & Medicinal Chemistry, Vol. 8, No. 8, 2000, pp. 2127-2143 doi:10.1016/S0968-0896(00)00133-4
- T. Hámore, S. Sólyom, P. Berzsenyi, F. Andrási and I. Tarnawa, “Structural Analogues of Some Highly Active Non-Competitive AMPA Antagonists,” Bioorganic & Medicinal Chemistry Letters, Vol. 10, No. 9, 2000, pp. 899- 902. doi:10.1016/S0960-894X(00)00117-7
- S. Grasso, G. De Sarro, A. De Sarro, N. Micale, M. Zappalà, G. Puia, M. Baraldi and C. D. Micheli, “Synthesis and Anticonvulsant Activity of Novel and Potent 2,3- benzodiazepine AMPA/Kainate Receptor Antagonists,” Journal of Medicinal Chemistry, Vol. 42, 1999, pp. 4414- 4421. doi:10.1021/jm991086d
- J. J. Luszczki, “Third-Generation Antiepileptic Drugs: Mechanisms of Action, Pharmacokinetics and Interactions,” Pharmacological Reports, Vol. 61, No. 2, 2009, pp. 197-216
- M. L. Barreca, R. Gitto, S. Quartarone, D. L. Luca, G. D. Sarro and A. Chimirri, “Pharmacophore Modeling as an Efficient Tool in the Discovery of Novel Noncompetitive,” Journal of Chemical Information and Modeling, Vol. 43, No. 2, 2003, pp. 651-655. doi:10.1021/ci025625q
- J. F. Howes and C. Bell, “Talampanel,” Neurotherapeutics, Vol. 4, No. 1, 2007, pp. 126-129. doi:10.1016/j.nurt.2006.11.001
- R. Gitto, R. Caruso, V. Orlando, S. Quartarone, M. L. Barreca, G. Ferreri, E. Russo, G. D. Sarro and A. Chimirri, “Synthesis and Anticonvulsant Properties of Tetrahydroisoquinoline Derivative,” Farmaco, Vol. 59, No. 1, 2004, pp. 7-12. doi:10.1016/j.farmac.2003.10.003
- R. Gitto, R. Caruso, B. Pagano, L. D. Luca, R. Citraro, E. Russo, G. D. Sarro and A. Chimirri, “Novel Potent Anticonvulsant Agent Containing a Tetrahydroisoquinoline Kkeleton,” Journal of Medicinal Chemistry, Vol. 49, No. 18, 2006, pp. 5618-5622. doi:10.1021/jm060411b
- R. Gitto, B. Pagano, R. Citrato, F. Scicchitano, G. D. Sarro and A. Chimirri, “Solution-Phase Parallel Synthesis and Evaluation of Anticonvulsant Activity of N-Substituted- 3,4-dihydroisoquinoline-2(1H)-carboxamides,” European Journal of Medicinal Chemistry, Vol. 44, No. 3, 2009, pp. 1349-1354. doi:10.1016/j.ejmech.2008.02.025
- L. De Luca, R. Gitto, M. L. Barreca, R. Caruso, S. Quartarone, R. Citraro, G. D. Sarro and A. Chimirri, “3D Pharmacophore Models for 1,2,3,4-Tetrahydroisoquinoline Derivatives Acting as Anticonvulsant Agents,” Archiv der Pharmazie Chemistry in Life Sciences, Vol. 339, No. 7, 2006, pp. 388-400.
- K. J. Bough and D. A. Engel, “Comparison of the Anticonvulsant Efficacies and Neuroroxic Effects of Valproic Acid, Phehytoin, and the Ketogenic Diet,” Epilepsia, Vol. 42, No. 10, 2001, pp. 1345-1353. doi:10.1046/j.1528-1157.2001.08901.x
- J. B. Monent, I. Jorquera, I. Mazzucchelli, A. Depaulis, E. Perucca, Y. Ben-Ari and A. Represa, “Fetal Exposure to GABA-Acting Antiepileptic Drugs Generates Hippocampal and Cortical Dyspkasias,” Epilepsia, Vol. 48, No. 4, 2007, pp. 684-693. doi:10.1111/j.1528-1167.2007.01056.x
- V. B. Brito, V. Folmer, G. O. Puntel, R. Fachinetto, J. C. Soares, G. Zeni, C. W. Nogueira and J. B. Rocha, “Diphenyl Diselenide and 2,3-Dimercaptopropanol Increase the PTZ-Induced Chemical Seizure and Mortality in Mice,” Brain Research Bulletin, Vol. 68, No. 6, 2006, pp. 414- 418.
- A. Ilhan, M. A. Aladag, A. Kocer, A. Boluk, A. Gurel and F. Armutcu, “Erdosteine Ameliorates PTZ-Induced Oxidative Stress in Mice Seizure Model,” Brain Research Bulletin, Vol. 30, No. 6, 2005, pp. 495-499. doi:10.1016/j.brainresbull.2005.02.027
- N. B. Agarwal, S. Jain, D. Nagpal, N. K. Agarwal, P. K. Mediratta and K. K. Sharma, “Liposomal Formulation of Curcumin Attenuates Seizures in Different Experimental Models of Epilepsy in Mice,” Fundamental & Clinical Pharmacology, 2011. doi:10.1111/j.1472-8206.2011.01002.x
- A. Zandieh, F. Maleki, A. Hajimirzabeigi, B. Zandieh, O. Khalilzadeh and A. R. Dehpour, “Anticonvulsant Effect of Celecoxib on Pentylenetetrazole-Induced Convulsion: Modulation by NO Pathway,” Acta Neurobiologiae Experimentalis, Vol. 70, No. 4, 2010, pp. 390-397.
NOTES
*Corresponding author.

